Nomenclature of Carbohydrates (Recommendations 1996)
2-Carb-6 and 2-Carb7
Continued from 2-Carb-5
Contents
- 2-Carb-6. Anomeric forms; use of α and β
- 6.1. The anomeric centre
- 6.2. The anomeric reference atom and the anomeric configurational symbol
- 6.3. Mixtures of anomers
- 6.4. Use of α and β
- 2-Carb-7. Conformation of cyclic forms
- 7.1. The conformational descriptor
- 7.2. Notation of ring shape
- 7.3. Notation of variants
- 7.4. Enantiomers
- References for this section
2-Carb-6 Anomeric forms; use of α and β
2-Carb-6.1. The anomeric centre
The new centre of chirality generated by hemiacetal ring closure is called the anomeric centre. The two stereoisomers are referred to as anomers, designated α or β according to the configurational relationship between the anomeric centre and a specified anomeric reference atom.
2-Carb-6.2. The anomeric reference atom and the anomeric configurational symbol (α or β)
The anomeric reference atom is the configurational atom (see 2-Carb-4.2 and 2-Carb-4.3) of the parent, unless multiple configurational prefixes (see 2-Carb-8.3) are used. If multiple configurational prefixes are used, the anomeric reference atom is the highest-numbered atom of the group of chiral centres next to the anomeric centre that is involved in the heterocyclic ring and specified by a single configurational prefix. In the α anomer, the exocyclic oxygen atom at the anomeric centre is formally cis, in the Fischer projection, to the oxygen attached to the anomeric reference atom; in the β anomer these oxygen atoms are formally trans.
The anomeric symbol α or β, followed by a hyphen, is placed immediately before the configurational symbol D or L of the trivial name or of the configurational prefix denoting the group of chiral carbon atoms that includes the anomeric reference atom.
Examples:
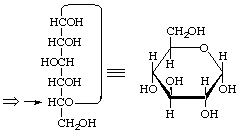
α-D-gluco
α-D-Glucopyranose
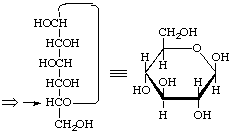
β-D-gluco
β-D-Glucopyranose
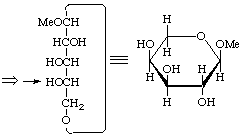
α-L-arabino
Methyl α-L-arabinopyranoside

β-L-threo
Methyl β-L-threofuranoside
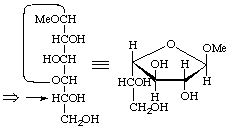
β-D-galacto
Methyl β-D-galactofuranoside
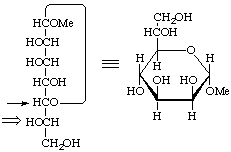
L-glycero-α-D-manno
Methyl L-glycero-α-D-manno-heptopyranoside
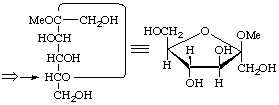
β-D-arabino
Methyl β-D-fructofuranoside
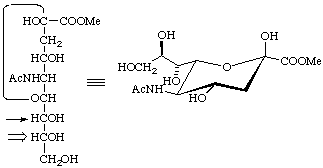
D-glycero-β-D-galacto
Methyl 5-acetamido-3,5-dideoxy-D-glycero-β-D-galacto-non-2-ulopyranosonate (see 2-Carb-14.2)
![[arrow right]](../greek/ARROWR.GIF) denotes the anomeric reference atom;
denotes the anomeric reference atom; ![[retrosynthetic arrow]](../greek/RETARR.GIF) denotes the configurational atom.
denotes the configurational atom.
Note. For simple aldoses up to aldohexoses, and ketoses up to hept-2-uloses, the anomeric reference atom and the configurational atom are the same.
2-Carb-6.3. Mixtures of anomers
In solution, most simple sugars and many of their derivatives occur as equilibrium mixtures of tautomers. The presence of a mixture of two anomers of the same ring size may be indicated in the name by the notation α,β-, e.g. α,β-D-glucose. In formulae, the same situation can be expressed by separating the representation of the ligands at the anomeric centre from the α and β bonds [see examples (a) and (c)], or by use of a wavy line [(b) and (d)] (particularly if hydrogen atoms are omitted).
Examples:
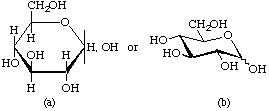
α,β-D-Glucopyranose
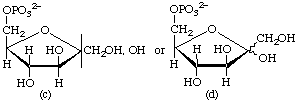
α,β-D-Fructofuranose 6-phosphate
2-Carb-6.4. Use of α and β
The Greek letters α and β are applicable only when the anomeric carbon atom has a lower locant than the anomeric reference atom. In the case of dialdoses (cf. 2-Carb-9), some diketoses (cf. 2-Carb-11) and aldoketoses (cf. 2-Carb-12), ring closure is also possible in the other direction, i.e. of a carbonyl group with a higher locant than the reference carbon atom with a hydroxy group having a lower locant. In this case, the configuration of the anomeric carbon atom is indicated by the appropriate symbol R or S according to the sequence rule (cf. Section E in [13]).
Examples:
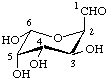
(6R)-D-gluco-Hexodialdo-6,2-pyranose
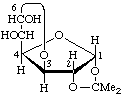
(6S)-1,2-O-Isopropylidene-α-D-gluco-hexodialdo-1,4:6,3-difuranose
Note that locant numerals (potential carbonyl first) may be needed before the ring-size suffix in such cases.
2-Carb-7. Conformation of cyclic forms
This is an abridged version of the document 'Conformational Nomenclature for Five- and Six-membered Ring Forms of Monosaccharides and their Derivatives. Recommendations 1980 [3].
2-Carb-7.1. The conformational descriptor
The conformation, i.e. the (approximate) spatial arrangement of the ring atoms of a monosaccharide in the cyclic form, may be indicated by an italic capital letter designating the type of ring shape, and numerals, distinguishing the variants. The conformational descriptor is joined to the end of the name of the monosaccharide by a hyphen.
Example:
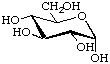
α-D-Glucopyranose-4C1
Table 1. Conformations and their notations; some examples are shown in Chart III
| Type of sugar | Conform-
ation | Atoms of
reference plane | Above plane | Below plane | Notation | Example |
|---|
| Aldofuranose | envelope | O-4,C-1,C-3,C-4 | - | C-2 | E2 | 1 |
| Aldofuranose | envelope | C-1,C-2,C-4,O-4 | C-3 | - | 3E | 2 |
| Aldofuranose | twist | C-1,O-4,C-4 | C-3 | C-2 | 3T2 | 3 |
| Aldofuranose | twist | C-3,C-4,O-4 | C-2 | C-1 | 2T1 | 4 |
| Aldopyranose | chair | C-2,C-3,C-5,O-5 | C-4 | C-1 | 4C1 | 5 |
| Pyranoid lactone | chair | C-2,C-3,C-5,O-5 | C-1 | C-4 | 1C4 | 6 |
| Aldopyranose | boat | O-5,C-1,C-3,C-4 | C-2,C-5 | - | 2,5B | 7 |
| Aldopyranose | boat | C-2,C-3,C-5,O-5 | - | C-1,C-4 | B1,4 | 8 |
| Aldopyranose | skew | C-2,C-4,C-5,O-5 | C-1 | C-3 | 1S3 | 9 |
| Aldopyranose | skew | C-1,C-3,C-4,C-5 | C-2 | O-5 | 2SO | 10 |
| Aldopyranose | half-chair | C-1,C-2,C-3,C-4 | C-5 | S-5 | 5HS | 11 |
| Pyranoid lactone | envelope | C-1,C-2,C-3,C-4,O-5 | C-5 | - | 5E | 12 |
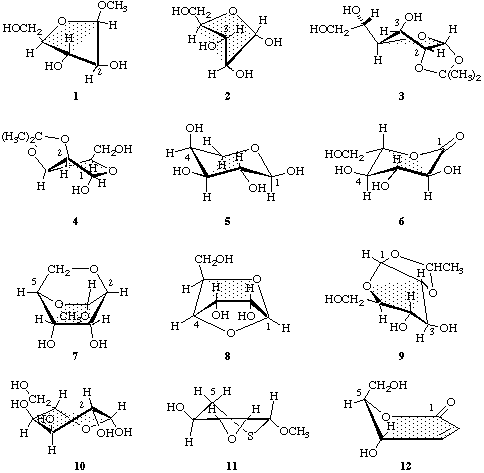
1 Methyl β-D-arabinofuranoside-E2
2 α-D-Arabinofuranose-3E
3 1,2-O-Isopropylidene-β-L-idofuranose-3T2
4 2,3-O-Isopropylidene-α-D-lyxofuranose-2T1
5 α-L-Arabinopyranose-4C1
6 L-Glucono-1,5-lactone-1C4
7 Methyl 2,6-anhydro-α-D-altropyranoside-2,5B
8 1,4-Anhydro-α-D-allopyranose-B1,4
9 1,2-O-Ethylidene-α-D-glucopyranose-1S3
10 β-L-Altropyranose-2SO
11 Methyl 2,3-anhydro-5-thio-β-L-lyxopyranoside-5HS 
12 2,3-Dideoxy-D-erythro-hex-2-enono-1,5-lactone-5E
Chart III. Drawings of the conformations listed in Table 1. The reference plane is stippled.
2-Carb-7.2. Notation of ring shape
The appropriate letters are as follows. Five-membered rings: E for envelope and T for twist; six-membered rings: C for chair, B for boat, S for skew, H for half-chair, and E for envelope. Examples are given in Chart III.
2-Carb-7.3. Notation of variants
The variants are distinguished by the locants of those ring atoms that lie outside a reference plane (defined below) and are listed for some examples in Table 1. The locants of ring atoms that lie on the side of the reference plane from which numbering appears clockwise (i.e. the upper side in the normal Haworth representation of furanoses and pyranoses) are written as superscripts and precede the letter; those that lie on the other side are written as subscripts and follow the letter. Heteroatoms (e.g. O, S) are indicated by their subscript or superscript atomic symbols. Table 1 gives the notations and Chart III some examples.
Six-membered rings
Chairs. The reference plane is defined by two parallel ring sides, so chosen that the lowest-numbered carbon atom in the ring is exoplanar (examples 5 and 6).
Boats. The reference plane is defined by the two parallel 'sides' of the boat (examples 7 and 8).
Skews. Each skew form has two potential reference planes, containing three adjacent atoms and the remaining non-adjacent atom. The reference plane is so chosen that the lowest-numbered carbon atom in the ring, or the atom numbered next above it, is exoplanar, in that order of preference (examples 9 and 10).
Half-chairs. The reference plane is defined by the four adjacent coplanar atoms (example 11).
Envelopes. The reference plane is defined by the five adjacent coplanar atoms (example 12).
Five-membered rings
Envelopes. The reference plane is defined by the four adjacent coplanar atoms (examples 1 and 2).
Twists. The reference plane is defined by three adjacent ring-atoms, so chosen that the exoplanar atoms lie on opposite sides of the plane (examples 3 and 4).
Note 1. Many of the possible conformations are not likely to contribute significantly to the chemistry of a particular monosaccharide, but must be stabilized by formation of additional rings, as in anhydrides or other derivatives. Some others may occur as transition-state intermediates.
Note 2. A more precise specification of conformation can be achieved by use of the Cremer-Pople puckering parameters [22].
2-Carb-7.4. Enantiomers
The conformational symbols for enantiomers are different. It is therefore important to state in the context whether the D or the L form is under consideration. Enantiomers have the same reference plane (see 2-Carb-7.3), and it should be noted that the mirror image of α-D-glucose-4C1 is α-L-glucose-1C4.
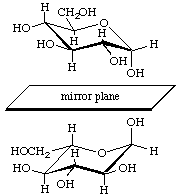
Mirror images: α-D-glucopyranose-4C1 (upper) and α-L-glucopyranose-1C4 (lower)
References
3. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN), Conformational nomenclature for five- and six-membered ring forms of monosaccharides and their derivatives (Recommendations 1980), Eur.J.Biochem., 111, 295-298 (1980); Arch. Biochem. Biophys., 207, 469-472 (1981); Pure Appl. Chem., 53, 1901-1905 (1981); ref. 2, pp. 158-161.
13. IUPAC Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 Edition, Pergamon Press, Oxford, U.K. Sections E and F are reprinted in ref. 2, pp. 1-18 and 19-26, respectively.
22. D. Cremer and J.A. Pople, J. Am. Chem. Soc., 97, 1354-1358 (1975): J.C.A. Boeyens, J. Cryst. Mol. Struct., 8, 317-320 (1978); P. Köll, H.-G. John, and J. Kopf, Liebigs Ann. Chem., 626-638 (1982).
Continue to the next section with 2-Carb-8 and 2-Carb-9 of Nomenclature of Carbohydrates.
Return to Carbohydrates home page.