Nomenclature of Carbohydrates (Recommendations 1996)
2-Carb-37
Continued from 2-Carb-36
Contents
- 2-Carb-37. Oligosaccharides
- 37.1. Oligosaccharides without a free hemiacetal group
- 37.2. Oligosaccharides with a free hemiacetal group
- 37.3. Branched oligosaccharides
- 37.4. Cyclic oligosaccharides
- 37.5. Oligosaccharide analogues
- Reference for this section
2-Carb-37. Higher oligosaccharides
2-Carb-37.1. Oligosaccharides without a free hemiacetal group
Trisaccharides (for example) are named as glycosylglycosyl glycosides or glycosyl glycosylglycosides as appropriate. A choice between the two residues linked through their anomeric positions for citation as the 'glycoside' portion can be made on the basis of 2-Carb-2.1. Alternatively, a sequential (end-to-end) naming approach may be used, regardless of 2-Carb-2.1. The names are formed by the preferred method of naming disaccharides (see 2-Carb-36.3): the locant of the anomeric carbon atom, an arrow, and the locant of the connecting oxygen of the next monosaccharide unit are set in parentheses between the names of the residues concerned.
Examples:
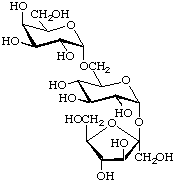
β-D-Fructofuranosyl α-D-galactopyranosyl-(1![[arrow right]](../greek/ARROWR.GIF) 6)-α-D-glucopyranoside
6)-α-D-glucopyranoside
(glucose preferred to fructose for citation as 'glycoside')
or α-D-galactopyranosyl-(1![[arrow right]](../greek/ARROWR.GIF) 6)-α-D-glucopyranosyl β-D-fructofuranoside
6)-α-D-glucopyranosyl β-D-fructofuranoside
(sequential method)
[α-D-Galp-(1![[arrow right]](../greek/ARROWR.GIF) 6)-α-D-Glcp-(1
6)-α-D-Glcp-(1![[2headed arrow]](../greek/ARROWB.GIF) 2)β-D-Fruf ]
2)β-D-Fruf ]
(trivial name raffinose)
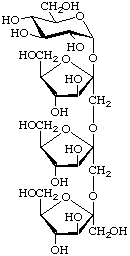
β-D-Fructofuranosyl-(2![[arrow right]](../greek/ARROWR.GIF) 1)-β-D-fructofuranosyl-(2
1)-β-D-fructofuranosyl-(2![[arrow right]](../greek/ARROWR.GIF) 1)-β-D-fructofuranosyl α-D-glucopyranoside
1)-β-D-fructofuranosyl α-D-glucopyranoside
[β-D-Fruf-(2![[arrow right]](../greek/ARROWR.GIF) 1)-β-D-Fruf-(2
1)-β-D-Fruf-(2![[arrow right]](../greek/ARROWR.GIF) 1)-β-D-Fruf-(2
1)-β-D-Fruf-(2![[2headed arrow]](../greek/ARROWB.GIF) 1)-α-D-Glcp ]
1)-α-D-Glcp ]
(trivial name nystose)
If derivatives are to be named on the basis of the trivial name, the component cited last in the systematic name receives locants with no primes, the preceding component singly-primed locants, etc. However, naming of trisaccharide and higher oligosaccharide derivatives systematically is preferred, to avoid ambiguity.
2-Carb-37.2. Oligosaccharides with a free hemiacetal group
An oligosaccharide of this class is named as a glycosyl[glycosyl]nglycose, i.e. the reducing sugar is the parent. Anomeric descriptors and locants are given as described in 2-Carb-37.1. The conventional depiction has the reducing sugar (glycose residue) on the right and the non-reducing end (glycosyl group) on the left. Internal sugar units are called glycosyl residues (the term 'anhydrosugar unit' is misleading and its use is discouraged). As the reducing end is often converted into the corresponding alditol, aldonic acid or glycoside derivative, the more general term 'downstream end' has been proposed for this end of the molecule.
Examples:
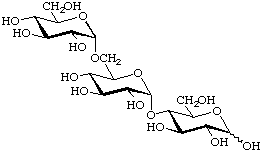
α-D-Glucopyranosyl-(1![[arrow right]](../greek/ARROWR.GIF) 6)-α-D-glucopyranosyl-(1
6)-α-D-glucopyranosyl-(1![[arrow right]](../greek/ARROWR.GIF) 4)-D-glucopyranose
4)-D-glucopyranose
(trivial name panose)
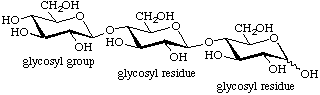
β-D-Glucopyranosyl-(1![[arrow right]](../greek/ARROWR.GIF) 4)-β-D-glucopyranosyl-(1
4)-β-D-glucopyranosyl-(1![[arrow right]](../greek/ARROWR.GIF) 4)-D-glucopyranose
4)-D-glucopyranose
(trivial name cellotriose)
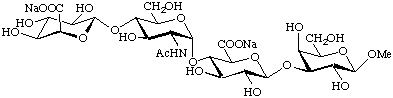
Methyl (sodium α-L-idopyranosyluronate)-(1![[arrow right]](../greek/ARROWR.GIF) 4)-(2-acetamido2-deoxy-α-D-glucopyranosyl)-(1
4)-(2-acetamido2-deoxy-α-D-glucopyranosyl)-(1![[arrow right]](../greek/ARROWR.GIF) 4)-(sodium β-D-glucopyranosyluronate)-(1
4)-(sodium β-D-glucopyranosyluronate)-(1![[arrow right]](../greek/ARROWR.GIF) 3)-β-D-galactopyranoside
3)-β-D-galactopyranoside
{Na2[α-L-IdopA-(1![[arrow right]](../greek/ARROWR.GIF) 4)-α-D-GlcpNAc-(1
4)-α-D-GlcpNAc-(1![[arrow right]](../greek/ARROWR.GIF) 4)-β-D-GlcpA-(1
4)-β-D-GlcpA-(1![[arrow right]](../greek/ARROWR.GIF) 3)-β-D-GalpOMe]}
3)-β-D-GalpOMe]}
Higher oligosaccharides are named systematically in the same way. However, it is often preferable to give their structures by use of the symbolic approach outlined in 2-Carb-38).
Trivial names for linear oligosaccharides consisting only of (1![[arrow right]](../greek/ARROWR.GIF) 4)-linked α-D-glucopyranosyl residues are maltotriose, maltotetraose etc. Similar names, based on the component sugar, are convenient for referring to other homo-oligosaccharides (e.g. xylobiose, galactotetraose), but such names should be used sparingly. Locants for naming substituted derivatives may be obtained by assigning roman numerals to the residues in ascending order starting from the reducing end.
4)-linked α-D-glucopyranosyl residues are maltotriose, maltotetraose etc. Similar names, based on the component sugar, are convenient for referring to other homo-oligosaccharides (e.g. xylobiose, galactotetraose), but such names should be used sparingly. Locants for naming substituted derivatives may be obtained by assigning roman numerals to the residues in ascending order starting from the reducing end. 
Example:
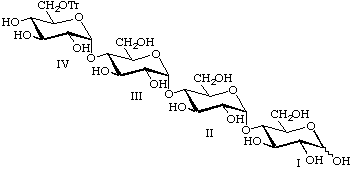
6IV-O-Tritylmaltotetraose
Arabic numerals have also been used in this context, but confusion may result when component sugar residues have structural modifications (e.g. chain branches) requiring superscript locant numbers. The present recommendation follows long-established usage in glycolipids [21].
2-Carb-37.3. Branched oligosaccharides
Terms designating branches should be enclosed in square brackets. In a branched chain, the longest chain is regarded as the parent. If two chains are of equal length the one with lower locants at the branch point is preferred, although some oligosaccharides are traditionally depicted otherwise, such as the blood group A trisaccharide exemplified below.
Examples:
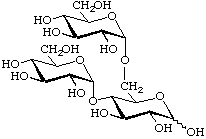
α-D-Glucopyranosyl-(1![[arrow right]](../greek/ARROWR.GIF) 4)-[α-D-glucopyranosyl-(1
4)-[α-D-glucopyranosyl-(1![[arrow right]](../greek/ARROWR.GIF) 6)]-D-glucopyranose
6)]-D-glucopyranose
[or 4,6-di-O-(α-D-glucopyranosyl)-D-glucopyranose]
(trivial name isopanose)
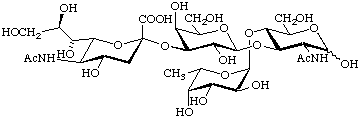
(5-Acetamido-3,5-dideoxy-D-glycero-α-D-galacto-non2-ulopyranosylonic acid)-(2![[arrow right]](../greek/ARROWR.GIF) 3)-β-D-galactopyranosyl-(1
3)-β-D-galactopyranosyl-(1![[arrow right]](../greek/ARROWR.GIF) 3)-[α-L-fucopyranosyl-(1
3)-[α-L-fucopyranosyl-(1![[arrow right]](../greek/ARROWR.GIF) 4)]-
4)]-
2-acetamido-2-deoxy-D-glucopyranose
or 5-N-acetyl-α-neuraminyl-(2![[arrow right]](../greek/ARROWR.GIF) 3)-β-D-galactopyranosyl-(1
3)-β-D-galactopyranosyl-(1![[arrow right]](../greek/ARROWR.GIF) 3)-
3)-
[α-L-fucopyranosyl-(1![[arrow right]](../greek/ARROWR.GIF) 4)]-2-acetamido-2-deoxy-D-glucopyranose
4)]-2-acetamido-2-deoxy-D-glucopyranose
{α-Neup5Ac-(2![[arrow right]](../greek/ARROWR.GIF) 3)-β-D-Galp-(1
3)-β-D-Galp-(1![[arrow right]](../greek/ARROWR.GIF) 3)-[α-L-Fucp-(1
3)-[α-L-Fucp-(1![[arrow right]](../greek/ARROWR.GIF) 4)]-D-GlcpNAc}
4)]-D-GlcpNAc}
(sialyl-Lea trisaccharide)
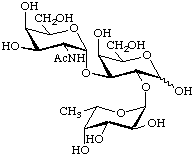
2-Acetamido-2-deoxy-α-D-galactopyranosyl-(1![[arrow right]](../greek/ARROWR.GIF) 3)-[α-L-fucopyranosyl-(1
3)-[α-L-fucopyranosyl-(1![[arrow right]](../greek/ARROWR.GIF) 2)]-
2)]-
D-galactopyranose
{α-D-GalpNAc-(1![[arrow right]](../greek/ARROWR.GIF) 3)-[α-L-Fucp-(1
3)-[α-L-Fucp-(1![[arrow right]](../greek/ARROWR.GIF) 2)]-D-Galp} (blood group A trisaccharide)
2)]-D-Galp} (blood group A trisaccharide)
2-Carb-37.4. Cyclic oligosaccharides
2-Carb-37.4.1. Semisystematic names
Cyclic oligosaccharides composed of a single type of oligosaccharide unit may be named semisystematically by citing the prefix 'cyclo', followed by terms indicating the type of linkage [e.g. 'malto' for α-(1![[arrow right]](../greek/ARROWR.GIF) 4)-linked glucose units], the number of units (e.g. 'hexa' for six) and the termination '-ose'. The trivial names α-cyclodextrin (α-CD) for cyclomaltohexaose, β-cyclodextrin (β-CD) for cyclomaltoheptaose and γ-cyclodextrin (γ-CD) for cyclomaltooctaose are well established.
4)-linked glucose units], the number of units (e.g. 'hexa' for six) and the termination '-ose'. The trivial names α-cyclodextrin (α-CD) for cyclomaltohexaose, β-cyclodextrin (β-CD) for cyclomaltoheptaose and γ-cyclodextrin (γ-CD) for cyclomaltooctaose are well established.
Example:
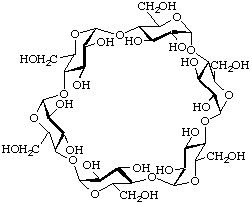
Cyclomaltohexaose (α-cyclodextrin, α-CD)
Structures with linkages other than (1![[arrow right]](../greek/ARROWR.GIF) 4) should be named systematically (see 2-Carb-37.4.2).
4) should be named systematically (see 2-Carb-37.4.2).
Note. The cyclic oligosaccharides arising from enzymic transglycosylation of starch have been referred to as Schardinger dextrins. These names (and those of the cyclohexaamylose type) are not recommended, but the abbreviation CD is tolerated.
Derivatives with the same substitution pattern on each residue can be named semisystematically by assigning a single multiplicative prefix (e.g. hexakis, heptakis etc.) to the substituent prefixes as a group.
Example:
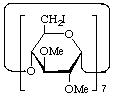
Heptakis(6-deoxy-6-iodo-2,3-di-O-methyl)cyclomaltoheptaose
Derivatives with different substitution patterns on the various residues can be named by the method of 2-Carb-37.2, assigning a roman numeral to each residue.
Example:
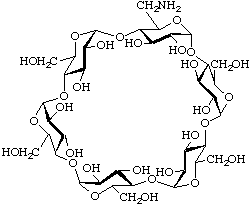
6I-Amino-6I-deoxycyclomaltohexaose
2-Carb-37.4.2. Systematic names
Cyclic oligosaccharides composed of a single type of residue can be named by giving the systematic name of the glycosyl residue, preceded by the linkage type in parentheses, preceded in turn by 'cyclo-' with a multiplicative suffix (i.e. 'cyclohexakis-' etc.)
Examples:
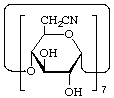
Cycloheptakis-(1![[arrow right]](../greek/ARROWR.GIF) 4)-(6-deoxy-α-D-gluco-heptopyranosylurononitrile)
4)-(6-deoxy-α-D-gluco-heptopyranosylurononitrile)
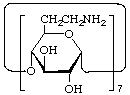
Cycloheptakis-(1![[arrow right]](../greek/ARROWR.GIF) 4)-(7-amino-6,7-dideoxy-α-D-gluco-heptopyranosyl)
4)-(7-amino-6,7-dideoxy-α-D-gluco-heptopyranosyl)
The 1![[arrow right]](../greek/ARROWR.GIF) 6 isomer of cyclomaltohexaose should be named cyclohexakis-(1
6 isomer of cyclomaltohexaose should be named cyclohexakis-(1![[arrow right]](../greek/ARROWR.GIF) 6)-α-D-glucosyl, rather than cycloisomaltohexaose.
6)-α-D-glucosyl, rather than cycloisomaltohexaose.
2-Carb-37.5. Oligosaccharide analogues
Structures in which the linking glycosidic oxygen is replaced by -CH2- may be named by use of the replacement prefix 'carba-' (cf. 2-Carb-34.2) for emphasis of homomorphic relationships. The oxygen replaced is given the locant of the carbon atom to which it is attached in the residue with the lower roman numeral (cited as superscript) (cf. 2-Carb-37.2), with suffix 'a'.
Example:
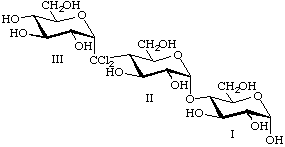
4IIa,4IIa-Dichloro-4IIa-carba-αmaltotriose
If the glycosidic oxygen link is replaced by -O-NH-, normal amino sugar nomenclature can be employed.
Example:
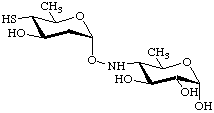
4-(2,6-Dideoxy-4-thio-α-D-arabino-hexopyranosyloxyamino)-4,6-dideoxy-α-D-glucopyranose
Reference
21. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN), The nomenclature of lipids (Recommendations 1976), Eur. J. Biochem., 79, 11-21 (1977); Hoppe-Seyler's Z. Physiol. Chem., 358, 617-631 (1977); Lipids, 12, 455-468 (1977); Mol. Cell. Biochem., 17, 157-171 (1977); Chem. Phys. Lipids, 21, 159-173 (1978); J. Lipid Res., 19, 114-40728 (1978); Biochem. J., 171, 21-35 (1978); ref. 2, pp. 180-191.
Continue to the next section with 2-Carb-38 of Nomenclature of Carbohydrates.
Return to Carbohydrates home page.
1)-β-D-fructofuranosyl-(2
1)-β-D-fructofuranosyl α-D-glucopyranoside
1)-β-D-Fruf-(2
1)-β-D-Fruf-(2
1)-α-D-Glcp ]