IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN)
http://www.chem.qmw.ac.uk/iupac/misc/noGreek/toc.html
World Wide Web version prepared by G. P. Moss
Department of Chemistry, Queen Mary and Westfield College,
Mile End Road, London, E1 4NS, UK
e-mail G.P.Moss@QMW.AC.UK
These Rules are as close as possible to the published version [see Arch. Biochem. Biophys., 1982, 218, 347-348; Eur. J. Biochem., 1982, 123, 473-475; Mol. Cell. Biochem., 1982, 49, 183-185; Pure Appl. Chem., 1982, 54, 1507-1510; Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pages 239-241; Copyright IUPAC and IUBMB; reproduced with the permission of IUPAC and IUBMB]. If you need to cite these rules please quote this reference as their source.
Any comments should be sent to any member of the Committee
This version of the document uses graphics to display Greek letters. For a version using the font symbol click here. Not all computers and/or browser software support the font symbol.
Recommendations for the nomenclature of the vitamins, including the tocopherols, were published in 1966 [1]; in that document the configuration of the natural -tocopherol, 2R,4'R,8'R, is mentioned in a footnote, followed by the sentence 'The designation of other stereoisomers is under consideration'. The work of Isler and his co-workers [2-5] provided a description of the configuration of all compounds of this class, but it proved difficult to devise a system for their nomenclature, because some of the important tocopherols are mixtures of diastereoisomers. Nevertheless recommendations, based on drafts prepared by W. Klyne and O. Hoffmann-Ostenhof after wide consultations, were published in 1974 [6], to replace section M-3 of the 1966 recommendations [1], and these use the RS system for designating stereoisomers [8]. The present recommendations take into account points raised about the previous ones [6]; although they contain no new recommendations they explain more fully the relation of present designations (especially on stereochemistry in section 12) to older ones. They incorporate agreements reached in 1976 between representatives of the predecessor of JCBN and of the Commision 1/I of the International Union of Nutritional Sciences (IUNS); they are in agreement with the IUNS Recommendations of 1976 [10].
RECOMMENDATIONS
The term vitamin E should be used as the generic descriptor for all tocol and tocotrienol derivatives exhibiting qualitatively the biological activity of -tocopherol. This term should be used in derived terms such as vitamin E deficiency, vitamin E activity, vitamin E antagonist.
The term tocol is the trivial designation for 2-methyl-2-(4,8,12-trimethyltridecyl)chroman-6-ol (I, R1 = R2 = R3 = H)
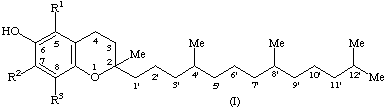
The term tocopherol(s) should be used as a generic descriptor for all mono, di, and trimethyltocols. Thus, this term is not synonymous with the term vitamin E.
2. Compound I (R1 = R2 = R3 = Me), known as -tocopherol, is designated
-tocopherol (note 1) or 5,7,8-trimethyltocol. For the designation of the configuration of
-tocopherol see Recommendations 11 and 12 (note 2).
3. Compound I (R1 = R3 = Me; R2 = H), known as, -tocopherol, is designated,
-tocopherol (note 1) or 5,8-dimethyltocol.
4. Compound I (R1 = H; R2 = R3 = Me), known as -tocopherol, is designated
-tocopherol (note 1) or 7,8-dimethyltocol.
5. Compound I (R1 = R2 = H; R3 = Me), known as -tocopherol, is designated
-tocopherol (note 1) or 8-methyltocol (note 3).
6. Compound II (R1 = R2 = R3 = H), 2-methyl-2-(4,8,12-trimethyltrideca-3,7,11-trienyl)chroman-6-ol, is designated tocotrienol [only the all-trans (E,E)-tocotrienol has been found occurring in nature].
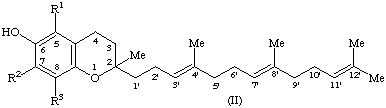
7. Compound II (R1 = R2 = R3 = Me), formerly known as 1 or
2-tocopherol, is designated 5,7,8-trimethyltocotrienol (note 3) or
-tocotrienol (note 1). The name tocochromanol-3 (cf. paragraph 3.2 in [11]) has also been used.
8. Compound II (R1 = R2 = Me; R2 = H), formerly known as -tocopherol, is designated 5,8-dimethyltocotrienol (note 3) or
-tocotrienol (note 1).
9. Compound II (R1 = H; R2 = R3 = Me), formerly known as -tocopherol, is designated 7,8-dimethyltocotrienol (note 3) or
-tocotrienol (note 1). The name plastochromanol-3 (cf. paragraph 3.2 in [11]) has also been used.
10. Compound II (R1 = R2 = H; R3 = Me) is designated 8-methyltocotrienol (note 3) or -tocotrienol (note 1).
11. The only naturally occurring stereoisomer of -tocopherol hitherto discovered (III) has the configuration 2R,4'R,8'R according to the sequence-rule system [8]. Its semisystematic name is therefore (2R,4'R,8'R)-
-tocopherol. The same system can be applied to all other individual stereoisomers of tocopherols.
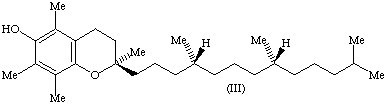
12. Trivial designations are sometimes desirable to indicate briefly the configuration of important stereoisomers of -tocopherol and especially of mixtures of such stereoisomers. Some of these materials are of considerable commercial and therapeutic importance. The use of the following trivial designations for the most important materials of this class is recommended.
a) The above-mentioned -tocopherol with the configuration 2R,4'R,8'R, formerly known as d-
-tocopherol, should be called RRR-
-tocopherol.
b) The diastereoisomer of RRR--tocopherol, formerly known as l-
-tocopherol, being the epimer of RRR-
-tocopherol at C-2 with the configuration 2S,4'R,8'R, should be called 2-epi-
-tocopherol.
c) A mixture of RRR--tocopherol and 2-epi-
-tocopherol (obtained by synthesis using phytol (note 4) and the appropriate achiral hydroquinone derivative), should be called 2-ambo-
-tocopherol (notes 5 and 6): this mixture was formerly known as dl-
-tocopherol until the optical activity of phytol was recognized when dl-
-tocopherol was restricted to all-rac-
-tocopherol (see 12e). It is probable that the asymmetric reaction involved in this partial synthesis would only by chance lead to the formation of equimolar proportions of the two possible epimers [12]. However, 2-ambo-
-tocopherol obtained by this method closely approaches equimolar proportions [13-16]. The acetate of 2-ambo-
-tocopherol acetate) was the former international standard vitamin E activity [17].
d) The reduction product of natural 5,7,8-tocotrienol, in which the double bonds at 3', 7' and 11' are hydrogenated and two new asymmetric centres are created at C-4' and C-8', is a mixture in unspecified proportions of four diastereoisomeric -tocopherols, having the configurations 2R,4'R,8'R: 2R,4'S,8'R; 2R,4'S,8'S; and 2R,4'R,8'S. The material should be called 4'-ambo,8'-ambo-
-tocopherol
e) The totally synthetic vitamin E, obtained without any control of stereochemistry, is a mixture in unspecified proportions (in preparations examined the proportions closely approached equimolar [13-16]) of four racemates or pairs of enantiomers (i.e. eight diastereoisomers). It should be called all-rac--tocopherol (it was formerly known as dl-
-tocopherol, although this designation was previously used for 2-ambo-
-tocopherol, see 12c).
13. Esters of tocopherols and tocotrienols should be called tocopheryl esters and tocotrienyl esters, respectively (e.g. -tocopheryl acetate,
-tocotrienyl acetate).
Recommended names are listed in the Table.
Table 1. List of the trivial names for some -tocopherols
| Description of material | Configuration | Recommended trivial name | |
|---|---|---|---|
| 1. | The compound having the configuration shown in the next column, exemplified by the only isomer of | 2R,4'R,8'R | RRR- |
| 2. | The isomer epimeric only at C-2 with RRR- | 2S,4'R,8'R | 2-epi- |
| 3. | 2R,4'R,8'R and 2S,4'R,8'R a mixture, not necessarily equimolar | 2-ambo- | |
| 4. | Synthetic | a mixture, not necessarily equimolar, of all four possible racemates (i.e. of all the four pairs of enantiomers) | all-rac- |
| 5. | Synthetic | a mixture, not necessarily equimolar, of the four isomers 2R,4'R,8'R; 2R,4'S,8'R; 2R,4'R,8'S; 2R,4'S,8'S | 4'-ambo-8'-ambo- |
1. Recommended for nutritional usage by IUNS [10].2. The name
-tocopherol should never be used without a stereochemical designation when referring to a specific compound.
3. Recommended for chemical usage.
4. The name 'phytol' designates the suhstance with 7R,11R-configuration.
5. From 'ambo', Latin for 'both'.
6. A more complete name would be 2-ambo-(4'R,8'R)-
-tocopherol but in order to keep trivial names short it is to be assumed that the configuration at each centre in the tocopherols is R unless stated otherwise. The term
-tocopherol, by itself a generic descriptor without stereochemical implication, thus obtains a stereochemical meaning when preceded by a stereochemical prefix like ambo or epi.
References
1. IUPAC-IUB Commission on Biochemical Nomenclature (CBN) Tentative rules, section on Trivial names of miscellaneous compounds of importance in biochemistry. 1964, Arch. Biochem. Biophys. 118, 505-507 (1967), Biochem. J. 102, 15-16 (1967), Biochem. Biophys. Acta, 107, l-4 (1965), Bull. Soc. Chim. Biol. (in French) 44, 332-335 (1967), Eur. J. Biochem. 2, l-2 (1967), Hoppe-Seyler's Z. Physiol. Chem. (in German) 348, 266-268 (1967). IUPAC Inf. Bull. 25, 19-23 (1966), and J. Biol. Chem. 241, 2987-2988 (1966).
2. Schudel. P., Mayer, H., Metzger. J., Ruegg, R. & Isler, O. (1963) Helv. Chim. Acta, 46, 333-343.
3. Schudel. P., Mayer, H., Metzger, J., Ruegg. R. & Isler, O. (1963) Helv. Chim. Acta, 46, 636-649.
4. Mayer, H., Schudel, P., Ruegg, R. & Isler, O. (1963) Helv. Chim. Acta, 46, 650-671.
5. Mayer, H., Schudel, P., Ruegg, R. & Isler, O. (1963) Helv. Chim. Acta. 46, 963-982.
6. IUPAC-IUB Commission on Biochemical Nomenclature (CBN) Nomenclature of tocopherols and related compounds. Recommendations 1973, Arch. Biochem. Biophys. 165, 6-8 (1974), Biochem. J. 147, 11-14 (1975), Eur. J. Biochem. 46, 217-219 (1974), and IUPAC Inf. Bull., Append. Provisional Nomencl. Symb. Units Stand. No. 47 (1975); also on pages 171-173 in [7].
7. International Union of Biochemistry (1978) Biochemical Nomenclature and Related Documents, The Biochemical Society, London. [Now as the 1992 edition]
8. IUPAC Commission on Nomenclature of Organic Chemistry (1976) Rules for the nomenclature of organic chemistry, Section E: Stereochemistry (Recommendations 1974) Pure Appl. Chem. 45, 11-30; also on pages 1-18 in [7] and section E in [9].
9. International Union of Pure and Applied Chemistry (1979) Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, Pergamon Press, Oxford.
10. International Union of Nutritional Sciences, Committee 1/I. Nomenclature (1978) Nutr. Abstr. Rev. 48A, 831-835.
11. IUPAC-IUB Commission on Biochemical Nomenclature (CBN) Nomenclature of quinones with isoprenoid side-chains, Recommendations 1973, Arch. Biochem. Biophys. 165, 1-5 (1974), Biochem. J. 147, 15-19 (1975), Biochim. Biophys. Acta, 387, 397-401 (1975), Eur. J. Biochem. 53, 15-18 (1975), and Pure Appl. Chem. 38, 439-447 (1974); also on pages 154-157 in [7].
12. Morrison, J. D. & Mosher, H. S. (1971) Asymmetric Organic Reactions, Prentice Hall, Englewood Cliffs, New Jersey.
13. Ames, S. R. (1979) J. Nutr. 109, 2198-2204.
14. Slover, H. T. & Thompson. R. H., Jr (1981) Lipids, 16, 268-275.
15. Weiser, H. & Vecchi, M. (1981) Int. J. Vitam. Nutr. Res. 51, 100-113.
16. Cohen, N., Scott, C. G., Neukom, C., Lopresti, R. J., Weber, G. & Saucy, G. (1981) Helv. Chim. Acta. 64, 1158-1173.
17. Hume, E. M. (1941) Nature (Lond.) 148, 472-473; see also World Health Organ. Tech. Rep. Ser. 259, 58 (1963).