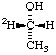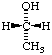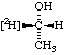Section H: Isotopically Modified Compounds
(Recommendations 1978)
Introduction and H-1, Symbols, Definitions and Formulae
CONTENTS
INTRODUCTION
These rules provide a general system of nomenclature for organic compounds whose isotopic nuclide (ref. 1) composition deviates from that occurring in nature. Comparative examples of the application of these rules are given in the Appendix.
Note:
For a discussion of the meaning of 'natural composition', see ref. 2. In any context where the accuracy requires it, the natural nuclidic composition used shall be stated.
There is one other general system in use for describing isotopically modified compounds. It is based on an extension of the principles proposed by Boughton (ref. 3) for designating compounds containing hydrogen isotopes and is currently in use mainly in the Chemical Abstracts Service index nomenclature system. For a description of its current use, see ref. 4.
The system codified in these present rules provides for recognition of various types of isotopic modification and thus was chosen over the system based on the Boughton principles.
H-1. SYMBOLS, DEFINITIONS, AND FORMULAE
Rule H-1.1. Symbols
1.11 - Nuclide symbols.
The symbol for denoting a nuclide in the formula or name of an isotopically modified compound consists of the atomic symbol for the element and an Arabic numeral in the left superscript position which indicates the mass number of the nuclide (ref. 5a).
1.12 - Atomic symbols.
The atomic symbols used in the nuclide symbol are those given in the IUPAC Inorganic Nomenclature Rules (ref. 5b). In the nuclide symbol, the atomic symbol is printed in Roman type, italicized atomic symbols being reserved for letter locants, as is customary in organic chemical nomenclature (cf. Rule C-814.4, ref. 6).
Note:
For the hydrogen isotopes protium, deuterium, and tritium, the nuclide symbols 1H, 2H, and 3H, respectively, are used. The symbols D and T for 2H and 3H, respectively, may be used, but not when other modifying nuclides are also present because this may cause difficulties in the alphabetic ordering of the nuclide symbols in the isotopic descriptor. Although the symbols d and t have been and are still used in place of 2H and 3H in names formed according to the Boughton system (see Introduction), in no other cases are lower-case letters used as atomic symbols. Therefore, the use of d and t in chemical nomenclature outside of the Boughton system is not recommended.
Rule H-1.2. Definitions and formulae of various types of isotopic modification
1.21 - An isotopically unmodified compound has a macroscopic composition such that its constituent nuclides are present in the proportions occurring in nature. Its formula and name are written in the customary manner.
Examples:
| 1. | CH4 | Methane |
| 2. | CH3-CH2-OH | Ethanol |
1.22 - An isotopically modified compound has a macroscopic composition such that the isotopic ratio of nuclides for at least one element deviates measurably from that occurring in nature. It is either an isotopically substituted compound or an isotopically labeled compound.
1.23 - An isotopically substituted compound has a composition such that essentially all the molecules of the compound have only the indicated nuclide(s) at each designated position. For all other positions, the absence of nuclide indication means that the nuclide composition is the natural one.
The formula of an isotopically substituted compound is written as usual except that appropriate nuclide symbols are used. When different isotopes of the same element are present at the same position, common usage is to write their symbols in order of increasing mass number.
Examples (for names see Rule H-2.11):
| 1. | 14CH4 | (14C)Methane |
| 2. | 12CHCl3 | (12C)Chloroform |
| 3. | CH3-CH2H-OH
not
CH3-C2HH-OH | (1-2H1)Ethanol |
1.24 - An isotopically labeled compound is a mixture of an isotopically unmodified compound with one or more analogous isotopically substituted compound(s).
Note:
Although an isotopically labeled compound is really a mixture as far as chemical identity is concerned (in the same way as is an unmodified compound), such mixtures are called "isotopically labeled compounds" for nomenclature purposes.
1.25 - An isotopically labeled compound is designated as specifically labeled when a unique isotopically substituted compound is formally added to the analogous isotopically unmodified compound. In such a case, both position(s) and number of each labeling nuclide are defined.
The structural formula of a specifically labeled compound is written in the usual way, but with the appropriate nuclide symbol(s) and multiplying subscript, if any, enclosed in square brackets. Other principles used in writing the formula are described in Rule H-1.23.
Examples:
| Isotopically
substituted
compound | when
added
to | Isotopically
unmodified
compound | gives
rise
to | Specifically
labeled
compound |
|---|
| 1. | 14CH4 | | CH4 | | [14C]H4 |
| 2. | CH22H2 | | CH4 | | CH2[2H2] |
| 3. | CH3-CH2-18OH | | CH3-CH2-OH | | CH3-CH2-[18O]H |
| 4. | CH2H2-CH2-O2H | | CH3-CH2-OH | | CH[2H2]-CH2-O[2H] |
| 5. | 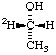 | | 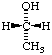 | | 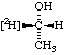 |
Note:
Although the formula for a specifically labeled compound does not represent the composition of the bulk material, which usually consists overwhelmingly of the isotopically unmodified compound, it does indicate the presence of the compound of chief interest, the isotopically substituted compound.
A specifically labeled compound is (a) singly labeled when the isotopically substituted compound has only one isotopically modified atom, e.g., CH3-CH[2H]-OH; (b) multiply labeled when the isotopically substituted compound has more than one modified atom of the same element at the same position or at different positions, e.g., CH3-C[2H2]-OH and CH2[2H]-CH[2H]-OH; or (c) mixed labeled when the isotopically substituted compound has more than one kind of modified atom, e.g., CH3-CH2-[18O][2H].
1.26 - An isotopically labeled compound is designated as selectively labeled when a mixture of isotopically substituted compounds is formally added to the analogous isotopically unmodified compound in such a way that the position(s) but not necessarily the number of each labeling nuclide is defined. A selectively labeled compound may be considered as a mixture of specifically labeled compounds.
A selectively labeled compound may be (a) multiply labeled when in the unmodified compound there is more than one atom of the same element at the position where the isotopic modification occurs, e.g., H in CH4, or there are several atoms of the same element at different positions where the isotopic modification occurs, e.g., C in C4H8O; or (b) mixed labeled when there is more than one labeling nuclide in the compound, e.g., C and O in CH3-CH2-OH.
Note:
When there is only one atom of an element that can be modified in a compound, only specific labeling can result (see Rule H-1.25).
A selectively labeled compound cannot be described by a unique structural formula; therefore it is represented by inserting the nuclide symbols preceded by any necessary locant(s) (letters and/or numbers) but without multiplying subscripts, enclosed in square brackets directly before the usual formula or, if necessary, before parts of the formula that have an independent numbering. Identical locants are not repeated.
When different nuclides are present, the nuclide symbols are written in alphabetic order according to their symbols, or when the atomic symbols are identical, in order of increasing mass number (see Rules H-2.81 and H-2.82).
Examples:
| | Mixture of
isotopically
substituted
compounds | when
added
to | Isotopically
unmodified
compound | gives
rise
to | Selectively
labeled
compound |
|---|
| 1. | CH32H, CH22H2,
CH2H3, C2H4
or any two or
more of the above | | CH4 | | [2H]CH4 |
| 2. | CH3-CH2-CH2H-CO2H
CH3-CH2-C2H2-CO2H | | CH3-CH2-CH2-CO2H | | [2-2H]CH3-CH2-CH2-CO2H |
| 3. | CH3-14CH2-14CH2-CO2H
CH3-14CH2-CH2-CO2H
CH3-CH2-14CH2-CO2H
or any two of the
above | | CH3-CH2-CH2-CO2H | | [2,3-14C]CH3-CH2-CH2-CO2H |
| 4. | CH3-14CH2-OH
CH3-CH2-18OH
CH3-14CH2-18OH
or any two of
the above | | CH3-CH2-OH | | [1-14C,18O]CH3-CH2-OH |
| 5. | 14CH3-CH2-CO2-CH3
CH3-CH2-CO2-14CH3
14CH3-CH2-CO2-14CH3
or any two of the
above | | CH3-CH2-CO2-CH3 | | [3-14C]CH3-CH2-CO2-[14C]CH3 |
1.27 - In a selectively labeled compound formally arising from mixing several known isotopically substituted compounds with the analogous isotopically unmodified compound, the number or the possible number of labeling nuclide(s) for each position may be indicated by subscripts to the atomic symbol(s). Two or more subscripts referring to the same nuclide symbol are separated by a semicolon. For a multiply labeled or mixed labeled compound (see Rule H-1.26), the subscripts are written successively in the same order as the various isotopically substituted compounds are considered. The subscript zero is used to indicate that one of the isotopically substituted compounds is not modified at the indicated position.
Examples:
| | Mixture of
isotopically
substituted
compounds | when
added
to | Isotopically
unmodified
compound | gives
rise
to | Selectively
labeled
compound |
|---|
| 1. | CH22H-CH2-OH
CH2H2-CH2-OH | | CH3-CH2-OH | | [2-2H1;2]CH3-CH2-OH |
| 2. | CH2H2-CH2-OH
CH2H2-CH2-18OH | | CH3-CH2-OH | | [2-2H2;2,18O0;1]CH3-CH2-OH * |
| 3. | CH3-CH2-18OH
CH2H2-CH2-OH | | CH3-CH2-OH | | [2-2H0;2,2-18O1;0]CH3-CH2-OH |
| 4. | CH3-CH2H-OH
CH2H2-CH2-OH | | CH3-CH2-OH | | [1-2H1;0,2-2H0;2]CH3-CH2-OH |
* Repetition of locants is not necessary as it may lead to ambiguity. Therefore, they have been omitted from this edition and a subscript added.
1.28 - An isotopically labeled compound is designated as nonselectively labeled when the position(s) and the number of the labeling nuclide(s) are both undefined.
In such cases the labeling is indicated by inserting the nuclide symbol, enclosed in square brackets, directly before the usual line formula with no locants or subscripts.
Example
[14C]CH3-CH2-CH2-CO2H
1.29 - An isotopically labeled compound may be designated as isotopically deficient when the isotopic content of one or more elements has been depleted, i.e., when one or more nuclide(s) is(are) present in less than the natural ratio. Such an isotopically modified compound is denoted in the formula by adding the italicized syllable def immediately preceding, without a hyphen, the appropriate nuclide symbol.
Example:
[def13C]CHCl3
Note:
According to one's viewpoint, one may also use [12C]CHC13.
References
1. I.U.P.A.C. Manual of Symbols and Terminology for Physicochemical Quantities and Units, 1973 Edition, Butterworths, London, 1975, Rules 7.1 and 7.2, p. 24.
2. I.U.P.A.C. Commission on Atomic Weights, Pure and Applied Chemistry, 37, 59l-603 (1974).
3. (a) W. A. Boughton, Naming Hydrogen Isotopes, Science, 79, 159-60 (1934); (b) E. J. Crane, Nomenclature of the Hydrogen Isotopes and Their Compounds, Science, 80, 86-9 (1934); (c) American Chemical Society, Report of Committee on Nomenclature, Spelling, and Pronunciation, Nomenclature of the Hydrogen isotopes and Their Compounds, Ind. Eng. Chem. (News Ed.), 13, 200-1 (1935).
4. "The Naming and Indexing of Chemical Substances for Chemical Abstracts During the Ninth Collective Period (1972-1976)", ¶ 220, p. 111I, a reprint of Section IV of the Introduction to the Chemical Abstracts Volume 76 Index Guide.
5. I.U.P.A.C. Nomenclature of Inorganic Chemistry, 2nd Edition (1970), Butterworths, London. 1971: (a) Rule 1.31, p. 11; (b) Rule 1.1, p. 10.
6. I.U.P.A.C. Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H (1979 edition), Pergamon Press, Oxford. 1979.
Continued with Rules H-2, Names for Isotopically Modified Compounds.
Return to Section H: Isotopic Modified Compounds home page.