Nomenclature of Carbohydrates (Recommendations 1996)
2-Carb-14 and 2-Carb-15
Continued from 2-Carb-13
Contents
2-Carb-14. Amino sugars
2-Carb-14.1. General principles
The replacement of an alcoholic hydroxy group of a monosaccharide or monosaccharide derivative by an amino group is envisaged as substitution of the appropriate hydrogen atom of the corresponding deoxy monosaccharide by the amino group. The stereochemistry at the carbon atom carrying the amino group is expressed according to 2-Carb-8.2, with the amino group regarded as equivalent to OH.
Some examples of N-substituted derivatives are given here; for a detailed treatment see 2-Carb-25.
2-Carb-14.2. Trivial names
Accepted trivial names are as follows.
D-Galactosamine for 2-amino-2-deoxy-D-galactose
D-Glucosamine for 2-amino-2-deoxy-D-glucose
D-Mannosamine for 2-amino-2-deoxy-D-mannose
D-Fucosamine for 2-amino-2,6-dideoxy-D-galactose
D-Quinovosamine for 2-amino-2,6-dideoxy-D-glucose
Neuraminic acid for 5-amino-3,5-dideoxy-D-glycero-D-galacto-non-2-ulosonic acid
Muramic acid for 2-amino-3-O-[(R)-1-carboxyethyl]-2-deoxy-D-glucose.
In the last two cases the trivial name refers specifically to the D enantiomer. (See also the alphabetical listing of trivial names in the Appendix.)
Such names as 'bacillosamine' for 2,4-diamino-2,4,6-trideoxy-D-glucose and 'garosamine' for 3-deoxy-4-C-methyl-3-methylamino-L-arabinose are not recommended, as they imply replacement of OH by NH2 in a nonexistent parent sugar.
Examples:
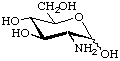
2-Amino-2-deoxy-D-glucopyranose (D-glucosamine).
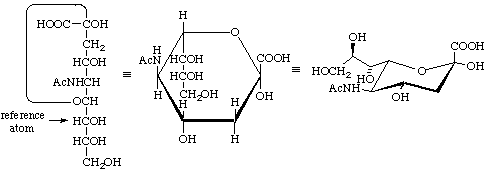
5-Acetamido-3,5-dideoxy-D-glycero-α-D-galacto-non-2-ulopyranosonic acid
(N-acetyl-α-neuraminic acid, α-Neu5Ac), drawn in three ways (note that C-7 is the anomeric reference atom)
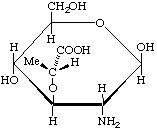
2-Amino-3-O-[(R)-1-carboxyethyl]-2-deoxy-β-D-glucopyranose (β-muramic acid)
For examples with nitrogen in the ring, see 2-Carb-34.1.
2-Carb-14.3. Systematic names
The compounds are named by use of a combination of 'deoxy-' and 'amino-' prefixes. When the complete name of the derivative includes other prefixes, 'deoxy-' takes its place in the alphabetical order of detachable prefixes.
Examples:
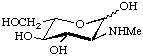
2-Deoxy-2-methylamino-L-glucopyranose
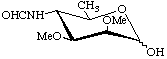
4,6-Dideoxy-4-formamido-2,3-di-O-methyl-D-mannopyranose
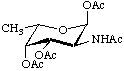
2-Acetamido-1,3,4-tri-O-acetyl-2,6-dideoxy-α-L-galactopyranose
When the amino group is at the anomeric position, the compound is normally named as a glycosylamine (see 2-Carb-33.6).
2-Carb-15. Thio sugars and other chalcogen analogues
Replacement of a hydroxy oxygen atom of an aldose or ketose, or of the oxygen atom of the carbonyl group of the acyclic form of an aldose or ketose, by sulfur is indicated by placing the prefix 'thio', preceded by the appropriate locant, before the systematic or trivial name of the aldose or ketose.
Replacement of the ring oxygen atom of the cyclic form of an aldose or ketose by sulfur is indicated in the same way, the number of the non-anomeric adjacent carbon atom of the ring being used as locant.
Selenium and tellurium compounds are named likewise, by use of the prefix 'seleno' or 'telluro'.
Sulfoxides (or selenoxides or telluroxides) and sulfones (or selenones or tellurones) may be named by functional class nomenclature [13].
Note. The appropriate prefix is thio, not thia; the latter is used in systematic organic chemical nomenclature to indicate replacement of CH2 by S.
Examples:
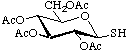
2,3,4,6-Tetra-O-acetyl-1-thio-β-D-glucopyranose

5-Thio-β-D-glucopyranose
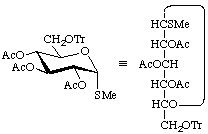
Methyl 2,3,4-tri-O-acetyl-1-thio-6-O-trityl-α-D-glucopyranoside
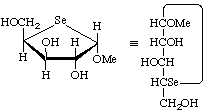
Methyl 4-seleno-α-D-xylofuranoside
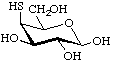
4-Thio-β-D-galactopyranose
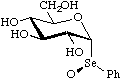
α-D-Glucopyranosyl phenyl (R)-selenoxide
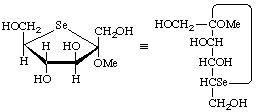
Methyl 5-seleno-α-D-fructofuranoside
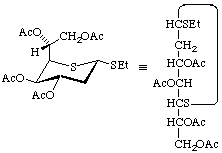
Ethyl 3,4,6,7-tetra-O-acetyl-2-deoxy-1,5-dithio-α-D-gluco-heptopyranoside
Note. It is common practice in carbohydrate names to regard 'thio' as detachable, and therefore alphabetized with any other prefixes.
Reference
13. IUPAC Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 Edition, Pergamon Press, Oxford, U.K. Sections E and F are reprinted in ref. 2, pp. 1-18 and 19-26, respectively.
Continue to the next section with 2-Carb-16 of Nomenclature of Carbohydrates.
Return to Carbohydrates home page.