Nomenclature of Carbohydrates (Recommendations 1996)
2-Carb-18
Continued from 2-Carb-17
Contents
2-Carb-18. Branched-chain sugars
Note This is a modified form of the 1980 recommendations [4]. Priority is now given to naming cyclic forms, since in most cases branched-chain monosaccharides will form cyclic hemiacetals or hemiketals.
2-Carb-18.1. Trivial names
Several branched monosaccharides have trivial names, some established by long usage. Examples are given below, together with systematic names for the (cyclic or acyclic) forms illustrated. (See also the alphabetical listing of trivial names in the Appendix.) Enantiomers of the sugars listed should be named systematically.
Examples:
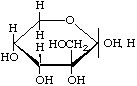
Hamamelose
2-C-(Hydroxymethyl)-D-ribopyranose
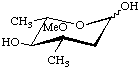
Cladinose
2,6-Dideoxy-3-C-methyl-3-O-methyl-L-ribo-hexopyranose
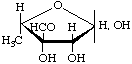
Streptose
5-Deoxy-3-C-formyl-L-lyxofuranose
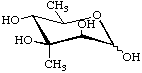
6-Deoxy-3-C-methyl-D-mannopyranose
(Evalose)
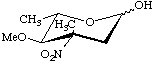
2,3,6-Trideoxy-3-C-methyl-4-O-methyl-3-nitro-L-arabino-hexopyranose
(Evernitrose)
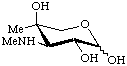
3-Deoxy-4-C-methyl-3-methylamino-L-arabinopyranose
(Garosamine)
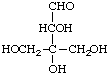
D-Apiose (Api)
3-C-(Hydroxymethyl)-D-glycero-tetrose
Note. For the cyclic forms of apiose, systematic names are preferred, e.g.
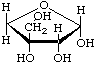
3-C-(Hydroxymethyl)-α-D-erythrofuranose
[The name α-D-erythro-apiofuranose is ambiguous; Chemical Abstracts Service (CAS) uses the trivial name D-apio-α-D-furanose; Beilstein gives (3R)-α-D-apiofuranose]
2-Carb-18.2. Systematic names
A branched-chain monosaccharide is named as a substituted parent unbranched monosaccharide, as outlined in 2-Carb-16.1 to 2-Carb-16.4.
Note. C-Locants are essential only where there is potential ambiguity, to make clear whether substitution is at carbon or at a heteroatom (cf. 2-Carb-16); however, they may also be used for emphasis.
2-Carb-18.3. Choice of parent
If the branched monosaccharide forms a cyclic hemiacetal or hemiketal, the chain which includes the ring atoms rather than any alternative open chain must be the basis of the name. Otherwise the parent is chosen according to the principles given in 2-Carb-2.1.
Examples (see also Chart V):
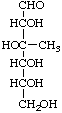
3-C-Methyl-D-glucose
(configuration determined by OH)
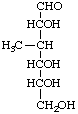
3-Deoxy-3-methyl-D-glucose
(configuration determined by CH3)
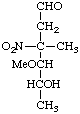
2,3,6-Trideoxy-3-C-methyl-4-O-methyl-3-nitro-D-lyxo-hexopyranose
(nitrogen has priority over carbon for determining configuration)
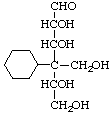
4-Cyclohexyl-4-deoxy-4-(hydroxymethyl)-D-allose
[oxygen (in CH2OH) has priority over carbon (in cyclohexyl) at C-4]
or (4R)-4-cyclohexyl-4-deoxy-4-(hydroxymethyl)-D-ribo-hexose
If the two substituents at the branch point are identical, so that this centre has become achiral, the stereochemistry is specified as described in 2-Carb-8.4.
Examples:
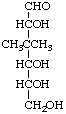
3-Deoxy-3,3-dimethyl-D-ribo-hexose
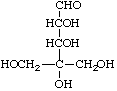
4-C-(Hydroxymethyl)-D-erythro-pentose
Note. Cyclization of the second example between C-1 and a CH2OH group would necessitate a three-centre configurational prefix for the ring form.
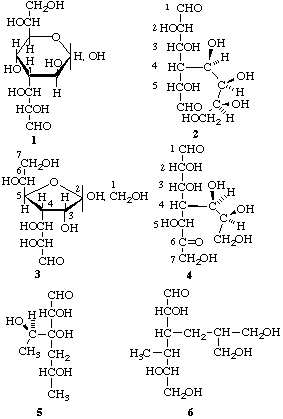
1 3-Deoxy-3-[(1R,2S)-1,2-dihydroxy-3-oxopropyl]-D-glycero-D-altro-heptopyranose
or 3-deoxy-3-(L-threo-1,2-dihydroxy-3-oxopropyl)-D-glycero-D-altro-heptopyranose
(not the alternative open-chain six-carbon dialdose or eight-carbon aldose, cf. 2)
2 4-Deoxy-4-(L-ribo-1,2,3,4-tetrahydroxybutyl)-D-altro-hexodialdose
3 4-Deoxy-4-[(1R,2R)-1,2-dihydroxy-3-oxopropyl]-D-allo-heptulo-2,5-furanose
or 4-deoxy-4-(L-erythro-1,2-dihydroxy-3-oxopropyl)-D-allo-heptulo-2,5-furanose
(not the alternative ketoaldose, cf. 4)
4 4-Deoxy-4-[(1R,2S)-1,2,3-trihydroxypropyl]-L-talo-heptos-6-ulose
or 4-deoxy-4-(L-erythro-1,2,3-trihydroxypropyl)-L-talo-heptos-6-ulose
5 4,6-Dideoxy-3-C-(L-glycero-1-hydroxyethyl)-D-ribo-hexose
(not the alternative pentose)
6 3,4-Dideoxy-3-[3-hydroxy-2-(hydroxymethyl)propyl]-4-C-methyl-L-mannose
(not the alternative threo-hexose)
Chart V. Choice of parent in branched-chain monosaccharides. In the first names given for examples 1, 3 and 4, side-chain configuration is specified by use of R and S. This approach is generally preferred in all but the simplest cases, as less open to misinterpretation.
Note. These recommendations may give rise to very different names for cyclic and acyclic forms of the same basic structures, resulting from different priorities. Thus, in Chart V, structures 1 and 2 are virtually identical, differing only by cyclization. The same holds for structures 3 and 4.
2-Carb-18.4. Naming the branches
Each branch will be named as an alkyl or substituted alkyl group replacing a hydrogen atom at the branch point of the parent chain. Within the branches, configurations around asymmetric centres can either be indicated using the R,S-system or, if they are carbohydrate-like and assignment is straightforward, by the use of the configurational prefix. For this purpose, and in the absence of a carbonyl group (or a terminal COOH or its equivalent) in the branch, the point of attachment of the branch (on the main chain) is regarded as equivalent to an aldehyde group.
Example:
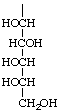
L-gluco-1,2,3,4,5-Pentahydroxypentyl
If there is a carbonyl group in the branch (or a terminal COOH or its equivalent), its position (assigned lowest number when stereochemistry is being considered) is used to define the configurational prefix (see examples 1 and 3 in Chart V). Use of the R,S system is generally preferred, as less open to misinterpretation.
For an alternative approach to naming carbohydrate residues as substituents see 2-Carb-31.2 [which would give the name (1R)- or (1S)-L-arabinitol-1-C-yl for the above example, depending on the ligands at the branch point].
2-Carb-18.5. Numbering
The carbon atoms of the parent chain are numbered according to 2-Carb-2.2.1. If a unique numbering is required for the branch(es) (e.g. for X-ray or NMR work), the carbon atoms may be given the locant of the appropriate branch point, with the internal branch locant as superscript, e.g. 42 for position 2 of the branch at position 4 of the main chain. This style of branch numbering is not to be used for naming purposes: e.g. the side-chain-O-methylated derivative of compound 5 is named 4,6-dideoxy-3-C-[(R)-1-methoxyethyl]-D-ribo-hexose, and not as a 31-O-methyl derivative. 
2-Carb-18.6. Terminal substitution
See 2-Carb-16.4.
Reference
4. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN), Nomenclature of branched-chain monosaccharides (Recommendations 1980), Eur. J. Biochem., 119, 5-8 (1981); corrections: Eur. J. Biochem., 125, 1 (1982); Pure Appl. Chem., 54, 211-215 (1982); ref.2, pp. 165-168.
Continue to the next section with 2-Carb-19 of Nomenclature of Carbohydrates.
Return to Carbohydrates home page.