Nomenclature of Carbohydrates (Recommendations 1996)
2-Carb-22
Continued from 2-Carb-21
Contents
2-Carb-22. Uronic acids
2-Carb-22.1. Naming and numbering
The names of the individual compounds of this type are formed by replacing (a) the '-ose' of the systematic or trivial name of the aldose by '-uronic acid', (b) the '-oside' of the name of the glycoside by '-osiduronic acid' or (c) the '-osyl' of the name of the glycosyl group by '-osyluronic acid'. The carbon atom of the (potential) aldehydic carbonyl group (not that of the carboxy group as in normal systematic nomenclature [13,14]) is numbered 1 (see 2-Carb-2.1, note 1).
2-Carb-22.2. Derivatives
Derivatives of these acids formed by change in the carboxy group (salts, esters, lactones, acyl halides, amides, nitriles, etc.) are named according to 2-Carb-20.2. The anion takes the ending '-uronate'. Esters are also named using the ending '-uronate'.
Examples:
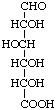
D-Glucuronic acid
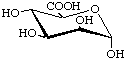
α-D-Mannopyranuronic acid
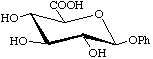
Phenyl β-D-glucopyranosiduronic acid
not phenyl β-D-glucuronoside or phenyl glucuronide
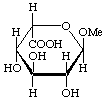
Methyl α-L-idopyranosiduronic acid 
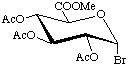
Methyl 2,3,4-tri-O-acetyl-α-D-glucopyranosyluronate bromide
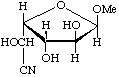
Methyl α-L-glucofuranosidurononitrile
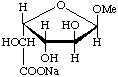
Sodium (methyl α-L-glucofuranosid)uronate
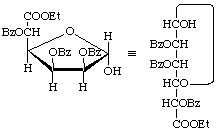
Ethyl 2,3,5-tri-O-benzoyl-α-D-mannofuranuronate
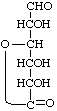
D-Glucurono-6,3-lactone
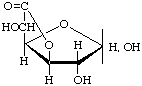
D-Glucofuranurono-6,3-lactone
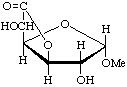
Methyl α-D-glucofuranosidurono-6,3-lactone
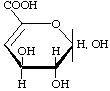
4-Deoxy-L-threo-hex-4-enopyranuronic acid
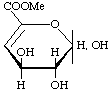
Methyl 4-deoxy-L-threo-hex-4-enopyranuronate
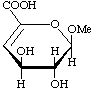
Methyl 4-deoxy-α-L-threo-hex-4-enopyranosiduronic acid
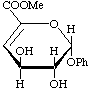
Methyl (phenyl 4-deoxy-β-L-threo-hex-4-enopyranosid)uronate
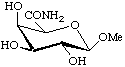
Methyl β-D-galactopyranosiduronamide
References
13. IUPAC Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 Edition, Pergamon Press, Oxford, U.K. Sections E and F are reprinted in ref. 2, pp. 1-18 and 19-26, respectively.
14. Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford (1993).
Continue to the next section with 2-Carb-23 of Nomenclature of Carbohydrates.
Return to Carbohydrates home page.