Add after example 8
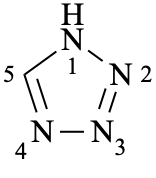
1H-tetrazole (PIN)
(not 1H-1,2,3,4-tetrazole)
Division VIII Chemical Nomenclature and Structure Representation Division
P-12.1, example 6. [corrected 26 January 2022]
Delete phenylethene and phenylethylene
P-14.3.3 [corrected 28 April 2023}.
Add at end
For omission of locants from a PIN see P-14.3.4.
P-14.3.4.4. [corrected 16 June 2021]
Add after example 8

1H-tetrazole (PIN)
(not 1H-1,2,3,4-tetrazole)
P-14.3.4.6, example 2. [modified 16 June 2021]
For (not 4-chorotrioxetane)
read (not 4-chloro-1,2,3-trioxetane)
P-14.6, example 3. [modified 28 April 2023]
For N-{2-[(acetyldimethylsilyl)methoxy]ethyl}-N′-[2-({2-[(2-aminoethyl)amino]ethyl}amino)ethyl]ethane-1,2-diamine
read N1-(2-{[acetyldi(methyl)silyl]methoxy}ethyl)-N2-[2-({2-[(2-aminoethyl)amino]ethyl}amino)ethyl]ethane-1,2-diamine
for N-[2-({2-[(acetyldimethylsilyl)methoxy]ethyl}amino)ethyl]-N′-{2-[(2-aminoethyl)amino]ethyl}ethane-1,2-diamine
read N1-{2-[(2-{[acetyldi(methyl)silyl]methoxy}ethyl)amino]ethyl}-N2-{2-[(2-aminoethyl)amino]ethyl}ethane-1,2-diamine
P-15.1.7.2.3, line 2. [corrected 26 January 2022]
For ...For example, in a cyclic unsaturated alcohol having one substituent group...
read ...For example, in an unsaturated alcohol having one substituent group...
P-15.1.8.2.1.2, example 2. [corrected 10 June 2021]
For (not N-nitrosoaniline)
read N-nitrosoaniline
P-15.3.1.2.2.2, example 2. [corrected 18 May 2023]
For ethane-1,2-diylidenebis(azanylylideneoxy)
read ethanediylidenebis(azanylylideneoxy)
P-16.5.1.3, lines 1-4. [modified 6 December 2023]
For P-16.5.1.3 Parentheses are placed around prefixes denoting simple substituent groups in front of parent hydrides when no locants are necessary in order to avoid any misunderstanding that the substituent group is modified by a preceding one. When the simple substituent groups are accompanied by multiplicative prefixes such as ‘di’ and ‘tri’, the multiplicative prefixes are not included in the parentheses. Parentheses are placed also around prefixes denoting simple substituent groups qualified by locants. A minimum of parentheses must be used. Enclosing marks are never used around the names of the first cited simple substituent group.
read P-16.5.1.3 Parentheses for substituents of parent structures.
P-16.5.1.3.1 For mononuclear parent hydrides with two or more substituents the first cited substituent never has enclosing marks unless it is a compound substituent group or includes a locant. The second and further substituents are each enclosed with parentheses even for simple substituents. When the simple substituent groups are accompanied by multiplicative prefixes such as ‘di’ and ‘tri’, the multiplicative prefixes are not included in the parentheses
P-16.5.1.3.1, example 9. [corrected 10 June 2021]
For ethyl(methyl)(propyl)silanecarboxylic acid
read ethyl(methyl)(propyl)silanecarboxylic acid (PIN)
P-16.5.1.3.1, example 10. [corrected 10 June 2021]
For methyl(phenyl)phosphinic acid
read methyl(phenyl)phosphinic acid (PIN)
P-16.5.1.3.1, example 11. [corrected 10 June 2021]
For cyclopropyl(phenyl)methanol
read cyclopropyl(phenyl)methanol (PIN)
P-16.5.1.3.2, example 1. [corrected 10 June 2021]
For bromo(nitro)(phenyl)acetic acid
read bromo(nitro)(phenyl)acetic acid (PIN)
P-16.5.1.3.2, example 2. [corrected 10 June 2021]
For diethyl ethyl(methyl)propanedioate
read diethyl ethyl(methyl)propanedioate (PIN)
P-16.5.1.3.2, example 3. [corrected 10 June 2021]
For bromo(chloro)acetic acid
read bromo(chloro)acetic acid (PIN)
P-16.5.1.3.2, example 4. [corrected 10 June 2021]
For cyclopropyl(fluoro)acetonitrile
read cyclopropyl(fluoro)acetonitrile (PIN)
P-16.5.4.1. [corrected 30 March 2022]
Replace section (retain box at end)
P-16.5.4.1 The presence of square brackets and/or parentheses that are an integral part of the name of a parent structure may affect the nesting order as described below.
P-16.5.4.1.1 In determining the nesting order in a name the parentheses of added indicated hydrogen are ignored.
Example:
Examples:
Examples:
Example:
Explanation: The order of insertion of stereochemistry for 2-{2-[(1-ethoxy-1-oxo-4-phenylbutan-2-yl)amino]propanoyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid is as shown below.
Stage 1 insert stereochemistry at the inner most position:2-{2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
The substituent (2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl requires square brackets to surround it hence subsequent enclosing marks need to be adjusted.
Stage 2 insert stereochemistry at the next position:
2-[(2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
The substituent (2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl has a parenthesis on the left and brace on the right so to make clear it requires square brackets around it.
Stage 3 insert stereochemistry at the third position:
(3S)-2-[(2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
P-16.5, contents list. [corrected 30 March 2022]
Add P-16.5.4 Multiple enclosing marks
P-16.5.4. [corrected 30 March 2022]
For P-16.5.4 When multiple types...
read P-16.5.4 Multiple enclosing marks
When multiple types...
P-16.9.4, line 2/3. [corrected 1 November 2023]
For ...(not 4a′,; for fusion locants, primes are added after the number, not after the letter)...
read ...(not 4a′; for locants of a fused ring component, primes are added after the number, not after the letter)...
After P-22.2.2.1.6 [corrected 16 June 2021]
Add:
P-22.2.2.1.7 Omission of locants
All locants are omitted for parent Hantzsch-Widman names if there is only one heteroatom or if there is no ambiguity if locants are omitted.
Examples:
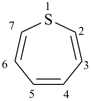
thiepine
(not 1-thiepine)
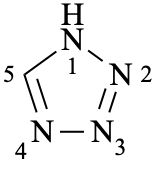
1H-tetrazole
(not 1H-1,2,3,4-tetrazole)
P-21.2.4.2, example 2. [corrected 28 April 2023]
For 2,5λ4,8,11-tetrathiadodecane
read 2,5λ6,8λ4,11-tetrathiadodecane
replace the structure with:
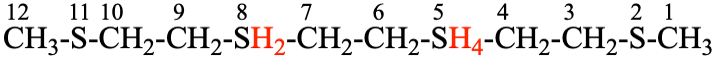
P-25.2.1, lines 7/8. [corrected 8.11.2023]
For Skeletal replacement (‘a’) nomenclature (see P-15.4), as described in Section P-25.5.4, is used...
read Skeletal replacement nomenclature, as described in Section P-15.5.2, is used...
P-25.2.2.4, example 1. [corrected 26 January 2022]
Delete 3-benzooxepine
P-25.2.2.4, example 2. [corrected 26 January 2022]
Delete 4H-3,1-benzooxazine
P-25.3.2.3.1. [1 December 2021]
For ...horizontal rows. Such rows...
read ...horizontal rows. If the compound requires distorted rings not shown above see P-25.3.2.2. Such rows...
P-25.3.2.3.1, before the example add. [corrected 23 February 2022)
Add 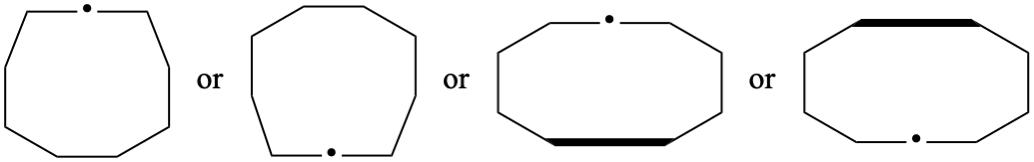
P-25.3.2.3.2 [corrected 1 December 2021]
For P-25.3.2.3.2
read P-25.3.2.3.3
add
P-25.3.2.3.2 Distorted ring shapes
If a compound cannot be drawn only using the shapes shown in P-25.3.2.3.1 a distorted ring shape will be required. The distorted ring should be as small as possible.
 | not |  |
| only preferred shapes used | distorted five-membered ring | |
 | not |  |
| only preferred shapes used (the separation to show the seven-membered ring is not fused to the left hand ring does not count as a distortion) | distorted seven-membered ring | |
 | not |  |
| distorted four-membered ring | distorted six-membered ring |
P-25.4.2.1.2, replace the structure of example 1. [corrected 26 January 2022]
P-25.4.2.1.2, replace structure of example 2. [corrected 26 January 2022]
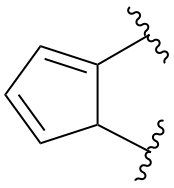
P-25.4.2.1.3, replace structure of example 4. [corrected 26 January 2022]
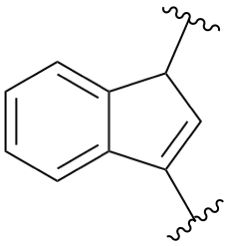
P-25.8.1, Note 3. [modified 11 August 2021]
For In the order of seniority used by CAS quinolizine precedes quinoline and the indacenes precede biphenylene.
read In the order of seniority used by CAS quinolizine precedes quinoline and isoquinoline; and indolizine preceeds indole and isoindole.
P-26.4.2.1, example. [corrected 1 November 2023]
For 1(3,10)-fluoranthenacyclononaphane (PIN)
read 1(3,10)-fluoranthenacyclononaphane
add 3,10-octanofluoranthene (PIN) [see P-52.2.5.1 (1)]
P-26.5.4.2, example 2. [corrected 30 March 2022]
For Step 2: 14,2,4,514,6,12-hexaoxa-114,54-dithia-3(2,5)-furana-1,5(1,5)dicyclotetradecanacyclododecaphane (I) (PIN)
read Step 2: 14,2,4,514,6,12-hexaoxa-114,54-dithia-3(2,5)-furana-1,5(1,5)-dicyclotetradecanacyclododecaphane (I) (PIN)
P-28.2.3. [corrected 1 December 2021]
For bivalent
read divalent
P-28.5, example 3. [corrected 30 March 2022]
For 11H,21H,31H,41H,51H,61H,71H-1,7(2),2,3,4,5,6(2,5)heptapyrrolaheptaphane (PIN)
read 11H,21H,31H,41H,51H,61H,71H-1,7(2),2,3,4,5,6(2,5)-heptapyrrolaheptaphane (PIN)
P-31.0, line 4. [corrected 26 January 2022]
For ...also called ‘mancude’ compounds (an acronym for MAximum Number of nonCUmulated DoublE bonds)....
read ...also called ‘mancude’ compounds (an acronym for MAximum number of NonCUmulated DoublE bonds)....
P-32.1.1, line before examples. [corrected 8.11.2023]
For ...formation of preferred IUPAC names, see P-57.1.4.1.
read ...formation of preferred IUPAC names, see P-57.1.1.1.
P-32.3, line 6. [corrected 18 May 2023]
For ...are the preferred IUPAC names.
read ...are the preferred prefixes.
P-32.3, line 8. [corrected 18 May 2023]
For ...preferred IUPAC name. It is...
read ... preferred prefix. It is...
P-32.3, line 9. [corrected 18 May 2023]
For ...The preferred IUPAC name is prop-1-en-2-yl.
read ... The preferred prefix is prop-1-en-2-yl.
P-32.3, line 11. [corrected 18 May 2023]
For ...The preferred IUPAC name is ..
read ... The preferred prefix is...
P-42.4, line 1 on this page. [corrected 6 December 2023]
For disulfurous acid
read disulfurous acid (ambiguous see P-67.3.2) p>
P-44.2.1.1, example 4, explanation line 3. [corrected 18 May 2023]
For ...criterion (f) in P-44.2.1...
read ...criterion (g) in P-44.2.1...
After P-44.4.1.12.2 add: [added 1 November 2023]
P-44.4.1.12.3 ‘cis/trans’ Isomerism (the ‘E/Z’ convention) and enantiomerism (the ‘R/S’ convention)
When there is a choice between parent structures that contain both ‘E/Z’ (or cis/trans) and ‘R/S’ descriptors the ‘E/Z’ (or cis/trans) descriptors are preferred to the ‘R/S’ descriptors.
Example
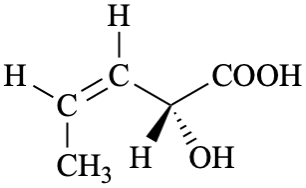 | > | 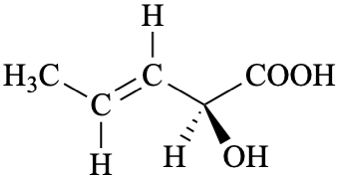 |
| (2R,3Z)-2-hydroxypent-3-enoic acid | (2S,3E)-2-hydroxypent-3-enoic acid | |
P-45.4.2 (formerly P-45.4.5), example. [corrected 30 March 2022]
For 2-[(13C)methyloxy]-N-{2-[methyl(18O)oxy]ethyl}ethan-1-amine (PIN)
read 2-(13C)methoxy-N-[2-(18O)methoxyethyl]ethan-1-amine (PIN)
for [not 2-[methyl(18O)oxy]-N-{2-[(13C)methyloxy]ethyl}ethan-1-amine]
read [not 2-(18O)methoxy-N-[2-(13C)methoxyethyl]ethan-1-amine]
P-45.4.3 (formerly P-45.4.6), [was a new example]. [corrected 30 March 2022]
For 4-([2-13C]ethyl)-2-[2-([2-14C]ethyl)pentyl]heptan-1-ol (PIN)
read 4-[2-13C]ethyl-2-(2-[2-14C]ethylpentyl)heptan-1-ol (PIN)
for [not 4-([2-14C]ethyl)-2-[2-([2-13C]ethyl)pentyl]heptan-1-ol
read [not 4-[2-14C]ethyl-2-(2-[2-13C]ethylpentyl)heptan-1-ol
P-51.2.1, example 3. [corrected 8.11.2023]
For acetyl chloride (PIN; P-65.5.1.1)
read acetyl chloride (PIN; P-65.5.1)
P-52.2.5.1 (1). [corrected 1 November 2023]
For (1) cyclophanes are cyclic phane structures containing one or more rings or ring systems, at least one ring or ring system of which must be a mancude system attached to adjacent atoms or chains at nonadjacent ring positions;
read
(1) cyclophanes are cyclic phane structures with at least six nodes including two or more rings or ring systems not ortho or ortho and peri fused to the cyclophane ring, and with at least one ring or ring system of which must be a mancude system;
P-52.2.5.2.1, example 2. [corrected 1 November 2023]
For 1(1,3)-benzenacyclopeptadecaphane (PIN; a phane name)
read 1(1,3)-benzenacyclopeptadecaphane (a phane name)
for bicyclo[16.3.1]docosa-1(22),18,20-triene (a von Baeyer name)
read bicyclo[16.3.1]docosa-1(22),18,20-triene (PIN; a von Baeyer name)
add Explanation: Phane name not permitted, only one ring system, see P-52.2.5.1 (1)
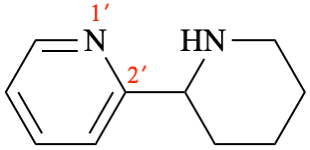
P-54.3, example 2. [corrected 6 December 2023]
Replace the structure with:
P-54.4.3.1 box, line 3. [corrected 8.11.2023]
For ...see also P-15.1.3.2, P-31.2, P-58.2), ...
read ...see also P-15.1.5.2, P-31.2, P-58.2), ...
P-57.4, example 4, explanation, line 1. [corrected 26 January 2022]
For ...involves a choice is between a ‘methyl’ group...
read ...involves a choice between a ‘methyl’ group...
P-58.2.1.2, example 4. [corrected 26 May 2021]
For 1H,2H-3a,7a-methano[2]benzofuran (PIN)
read 1H,2H-3a,7a-methano-2-benzofuran (PIN)
P-62.1 (a) (2), lines 1/2. [corrected 26 January 2022]
For ...compounds having the structure R-N=CR2 (R = H or hydrocarbyl),...
read ...compounds having the structure R2C=N-R (R = H or hydrocarbyl),....
P-62.2.1.2, Note line 5. [corrected 26 January 2022]
For ...functional class name were eliminated to form...
read ...functional class name is eliminated to form...
P-62.2.2.2, example 8. [corrected 26 January 2022]
For N-cyclopropylbutan-1-amine
read (not N-cyclopropylbutan-1-amine)
P-62.2.4.1.2, example 1. [corrected 23 February 2022)
Replace structure by
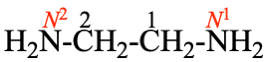
P-62.6.1 (1), line 2. [corrected 26 January 2022]
For ...the terminal letter, ‘e’ if present...
read ...the terminal letter ‘e’, if present...
P-63.1.5, line 9. [corrected 8.11.2023]
For ...are described in P-63.2.5.1. multiplicative names ...
read ...are described in P-63.2.5. multiplicative names ...
P-63.8.1, paragraph 3. [modified 1 November 2023]
For The traditional names methoxide, ethoxide, proproxide, butoxide, phenoxide, and aminoxide, , for CH3-O–, C2H5-O–, C3H7-O–, C4H9-O–, C6H5-O–, and H2N-O– are retained for use in general nomenclature and may be substituted in the same way as the corresponding alcohols. The traditional names isopropoxide and tert-butoxide for (CH3)CH-O– and (CH3)3C-O– also are retained but cannot be substituted.
read The traditional names methoxide, ethoxide, propoxide, butoxide and phenoxide, for CH3-O–, C2H5-O–, C3H7-O–, C4H9-O– and C6H5-O–, are retained as preferred IUPAC names and aminoxide, H2N-O–, as a preselected name, and they may be substituted in the same way as the corresponding alcohols. The traditional name tert-butoxide for (CH3)3C-O– is also retained as a preferred IUPAC name but cannot be substituted. The traditional name isopropoxide for (CH3)2CH-O– is retained for general nomenclature but cannot be substituted.
P-64.2.1.1, lines 2/3. [corrected 26 January 2022]
For ...lower than ‘ketone’. It is the stereospecific trans- or (E)- stereoisomer.
read ...lower than ‘ketone’. Chalcone refers only to the trans- or (E)- stereoisomer.
P-64.2.1.3, example 5. [modified 26 January 2022]
For benzal
read [not benzil]
P-65.1.1.1, line 1. [corrected 26 January 2022]
For Only the following five retained names are preferred IUPAC names....
read Only the following five carboxylic acids are retained names and are also preferred IUPAC names.....
P-65.5.4 (2) [corrected 1 December 2021]
For bivalent
read divalent
P-65.6.3.2.2, example 2 [corrected 11 December 2023]
For 1,4-phenylene bis(methyl propanedioate)
read 1,4-phenylene di(methyl propanedioate)
P-65.6.3.3.2.2.1, example 3. [modified 18 December 2023]
For (1) dimethyl butanedioylbis(oxy-2,1-phenylene) dibutanedioate (PIN)
read (1) dimethyl butanedioylbis(oxy-2,1-phenylene)dibutanedioate (PIN)
P-65.6.3.3.4.1, example. [corrected 18 December 2023]
For ethane-1,2-diyl bis(methyl butanedioate)
read ethane-1,2-diyl di(methyl butanedioate)
P-65.6.3.3.4.2, replace structure of example 9. [corrected 26 January 2022]
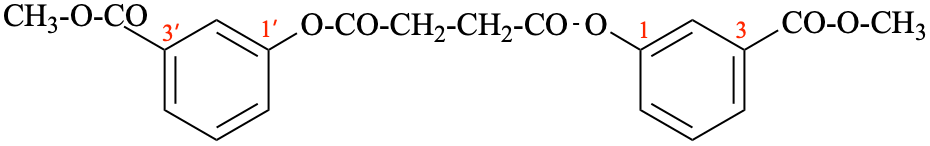
P-65.7.6.3, example 3. [corrected 16 June 2021]
For P,P′-diacetic P,P′-dipropanoic ethane-1,2-diylbis(phosphonic) tetraanhydride (PIN)
read P,P′-diacetic P,P′-dipropanoic (ethane-1,2-diyl)bis(phosphonic) tetraanhydride (PIN)
P-66 title. [corrected 26 January 2022]
For P-66 AMIDES, IMIDES, HYDRAZIDES, NITRILES, AND ALDEHYDES,
read P-66 AMIDES, IMIDES, HYDRAZIDES, NITRILES, AND ALDEHYDES
P-66.1.0, lines 3-5. [corrected 26 January 2022]
For ...three acyl groups on a single nitrogen atom are generically included and may be designated as primary, secondary, and tertiary amides, respectively.
read ...three acyl groups on a single nitrogen atom are generically included.
P-66.1.2.1. [corrected 6 December 2023]
For P-66.1.2.1 Amides having...
read Amides having...
P-66.1.1.3.1.1, example 1. [corrected 26 January 2022]
For N,N-dimethylformamide (PIN)
read dimethylformamide (PIN)
Delete dimethylformamide
P-66.1.6.1.1.1, line 1. [corrected 26 January 2022]
For ... H2N-CO-NH2 has the retained named ‘urea’, which...
read ... H2N-CO-NH2 has the retained name ‘urea’, which...
P-66.1.6.3, example 1. [corrected 23 February 2022)
For N-(propan-2-yl)dicarbonic diamide (PIN)
read N1-(propan-2-yl)dicarbonic diamide (PIN)
P-66.6.5.1.2, example 3. [corrected 30 March 2022]
For [2-(1,3-dioxolan-2-yl)ethyl]trimethylsilane (PIN)
read [2-(1,3-dioxolan-2-yl)ethyl]tri(methyl)silane (PIN)
P-67.1.2.3.2. [corrected 1 November 2023]
For P-67.1.2.3.2 Infixes denoting classes (in decreasing order of seniority except for halides that have the same rank but are cited in alphabetical order and pseudohalides that have the same rank but are cited in alphabetical order)
read P-67.1.2.3.2 Infixes denoting replacement of -OH group in decreasing order of seniority with all halides (class 1) and pseudohalides (class 2) having the same rank within the class.
P-67.1.2.3.4. [corrected 1 November 2023]
For P-67.1.2.3.4 Prefixes denoting classes (in decreasing order of seniority except for halides that have the same rank but are cited in alphabetical order and pseudohalides that have the same rank but are cited in alphabetical order)
read P-67.1.2.3.4 Prefixes denoting replacement of -OH group in decreasing order of seniority with all halides (class 1) and pseudohalides (class 2)
having the same rank within the class.
P-67.1.2.5.2, example 1. [corrected 30 March 2022]
For bromo(chloro)phenylborane (PIN)
read bromo(chloro)(phenyl)borane (PIN)
P-67.2.1, line 4. [corrected 6 December 2023]
For ...they are preferred IUPAC names if the...
read ...they are preselected names if the...
Page 959, P-67.2.5.2, example 4. [corrected 11 December 2023]
For (CH3)2CH-O-SO-O-SO-O-CH(CH3)2 di(propan-2-yl) disulfite (PIN)
read (CH3)2CH-O-SO-O-SO-O-CH(CH3)2 1,3-bis[(propan-2-yl)oxy]-1λ4,3λ4-dithioxane-1,3-dione (PIN)
[not di(propan-2-yl) disulfite, see P-67.3.2]
P-68.1.5.2.3, example 10. [corrected 30 Mach 2022]
For (difluoroboranyl)trimethylsilane (PIN)
read (difluoroboranyl)tri(methyl)silane (PIN)
P-68.1.6.3 (formerly P-68.1.6.1.2), example 2 on his page. [modified 23 February 2022)
Replace structure by
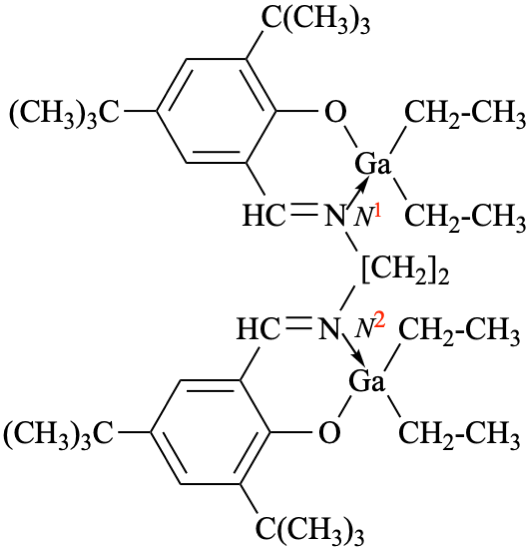
for N,N′-bis({3,5-di-tert-butyl-2-[(diethylgallanyl)oxy]phenyl}methylidene)-2(N—Ga)-ethane-1,2-diamine
read N1,N2-bis({3,5-di-tert-butyl-2-[(diethylgallanyl)oxy]phenyl}methylidene)-2(N—Ga)-ethane-1,2-diamine
for (μ-{2,2′-[ethane-1,2-diylbis(azanylylidene-1κN:2κN′-methanylylidene)bis(4,6-di-tert-butylphenolato-1κO:2κO′)di(ethanido-1κ2C1,2κ2C1)gallium
read (μ-{2,2′-[ethane-1,2-diylbis(azanylylidene-1κN1:2κN2-methanylylidene)bis(4,6-di-tert-butylphenolato-1κO:2κO′)bis[di(ethanido-1κ2C1,2κ2C1)gallium]
P-68.3.1.2.2, example 3. [modified 18 May 2023]
For ethane-1,2-diylidenebis(phenylhydrazine) (PIN)
read 1,1′-ethanediylidenebis(2-phenylhydrazine) (PIN)
P-68.3.1.3.2.3, example 1. [modified 30 March 2022]
For (1) (anthracen-2-yl)diazenyl]naphthalen-2-yl}phenyldiazene (PIN)
read (1) {7-[(anthracen-2-yl)diazenyl]naphthalen-2-yl}(phenyl)diazene (PIN)
for {not [7-(anthracen-2-yldiazenyl)naphthalen-2-yl]phenyldiazene
(numbering shown); alphanumerical order ‘anthracen-2-yl’ precedes ‘anthracen-2-yldiazenyl’}
read {not (anthracen-2-yl)[7-(phenyldiazenyl)naphthalen-2-yl]diazene;
each primary substituent has the same locant, i.e., none, so ‘anthracenyldiazenyl’ is alphabtically preferred to ‘anthracenylphenyl’}
P-73.2.3.3, paragraph above examples, lines 1/2. [corrected 1 November 2023]
For The names methoxylium, ethoxylium, propoxylium, butoxylium, phenoxylium, and aminoxylium are retained as preferred IUPAC names....
read The names methoxylium, ethoxylium, propoxylium, butoxylium and phenoxylium are retained as preferred IUPAC names, and aminoxylium as a preselected name....
P-73.4, example 2 of second set. [corrected 30 March 2022]
For 2-ethoxy-N-{2-[(2-ethoxyethyl)methylsulfaniumyl]ethyl}-N,N-dimethylethanaminium (PIN)
read 2-ethoxy-N-{2-[(2-ethoxyethyl)(methyl)sulfaniumyl]ethyl}-N,N-dimethylethanaminium (PIN)
P-82.2.5, example 1. [corrected 1 December 2021]
Delete acet(2H)amide
P-82.6.2, example 3. [corrected 1 December 2021]
Delete D-(O-2H)glycer(2H)aldehyde
P-82.6.3.3 was P-82.6.3.2 [corrected 1 December 2021]
For P-82.6.3.2
read P-82.6.3.3
Insert new P-82.6.3.2 after P-82.6.3.1 [corrected 1 December 2021]
P-82.6.3.2 When the nuclide is located at a position in a retained name that is not numbered a systematic name that identifies separately the relevant atom is used for the IUPAC preferred name. For general nomenclature an italicized prefixes or Greek letters may be used to denote its position.
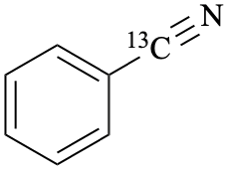
benzene(13C)carbonitrile (PIN)
(cyano-13C)benzonitrile
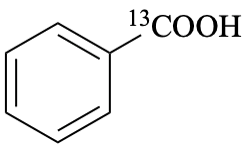
benzene(13C)carboxylic acid (PIN)
(carboxyl-13C)benzoic acid
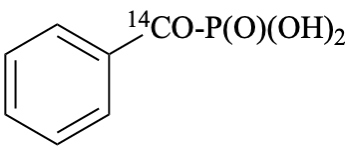
[benzene[14C]carbonyl]phosphonic acid (PIN)
[phenyl[14C]carbonyl]phosphonic acid
[carbonyl-14C]benzoylphosphonic acid
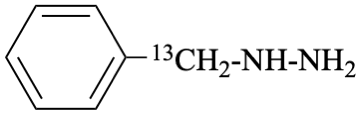
[phenyl(13C)methyl]hydrazine (PIN)
[(α-13C)benzyl]hydrazine
P-83.1.2.2, example 9. [modified 30 March 2022]
For O-ethyl 18O-methyl naphthalene-2-yl[18O]phosphonate (PIN)
read O-ethyl 18O-methyl (naphthalene-2-yl)[18O]phosphonate (PIN)
for ...(see P-82.6.3.2)
read ...(see P-82.6.3.3)
P-91.3, example 1. [corrected 28 April 2023]
For (R)-bromo(chloro)fluoromethane
read (R)-bromo(chloro)(fluoro)methane
P-92.2.1.1.1, example. [corrected 6 December 2023]
For (R)-bromo(chloro)fluoromethane (PIN)
read (R)-bromo(chloro)(fluoro)methane (PIN)
P-93.2.3, example 1. [corrected 10 June 2021]
Add [(S)-ethyl(methyl)(phenyl)azaniumyl]oxidanide
P-93.5.1.1.2 (b), end of paragraph. [corrected 31 May 2023]
Add The numbering for these examples is decided by application of criterion P-14.4 (j).
P-93.5.1.1.2 (b), example 3. [modified 31 May 2023]
For 3 (1R,2s,3S,4R,5s,6S)
read 3 (1R,2r,3S,4R,5s,6S)
Note R > r > S > s [see P-14.4 (j)]
replace the structure with:
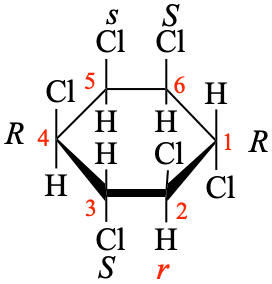
P-93.5.1.1.2 (b), example 4, add after the name. [corrected 31 May 2023]
Add Note R > r > S > s [see P-14.4 (j)]
P-102.3.4.2.2, example 3, left structure. [corrected 18 May 2023]
replace the structure with:
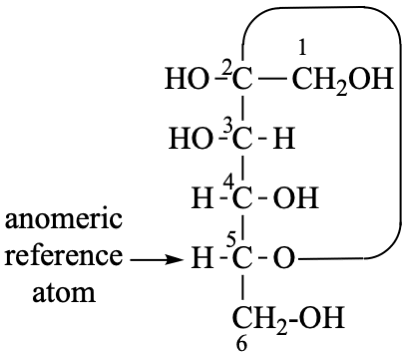
P-102.5.6.5.2, line 1. [corrected 18 May 2023]
For The prefix ‘meso’ must be included in the preferred name of erythritol....
read The prefix ‘meso’ may be included in the name of erythritol....
P-102.5.6.6.5.1, line 3/4. [corrected 18 May 2023]
For ...The descriptor ‘meso’ must be added for sake of clarity...
read ...The descriptor ‘meso’ may be added for the sake of clarity...
P-103.1.1.1, Table 10.4, entry 4, aspartic acid [corrected 28 April 2023]
For 2-aminobutanedioic acid
read aminobutanedioic acid
P-107.3.5 example [corrected 28 April 2023]
For (2R)-3-{[(2-aminoethoxy)hydroxyphosphoryl]oxy}propane-1,2-diyl dihexadecanoate
read (2R)-3-{[(2-aminoethoxy)hydroxyphosphoryl]oxy}propane-1,2-diyl di(hexadecanoate)
P-107.3.6 example. [modified 28 April 2023]
For (2R)-3-[({[(1s,2R,3S,4s,5R,6S)-hydroxy-2,3,4,5,6-pentahydroxycyclohexyl]oxy}hydroxyphosphoryl)oxy]propane-1,2-diyl dihexadecanoate
read (2R)-3-[(hydroxy{[(1s,2R,3R,4s,5S,6S)-2,3,4,5,6-pentahydroxycyclohexyl]oxy}phosphoryl)oxy]propane-1,2-diyl di(hexadecanoate)
P-107.4.2 example. [modifird 28 April 2023]
For (2S)-3-(O-β-D-galactopyranosyl)propane-1,2-diyl dioctadecanoate
read (2S)-3-(β-D-galactopyranosyloxy)propane-1,2-diyl di(octadecanoate)