Organic Chemistry Division
Commission on Nomenclature of Organic Chemistry
a) 2031 Leclaire, Montréal, Québec H1V 3A1, Canada; Chairman, CNOC
b) Stoltzestrasse 61, D-63073 Offenbach, Germany
c) Queen Mary and Westfield College, London, U.K.
d) 1436 Havencrest Ct, Columbus, Ohio 43220, USA
e) 122 Highland Trace Drive, Baton Rouge, Louisiana 70810-5061, USA; Secretary, CNOC
These Corrections are as close as possible to the published version [see Pure Appl. Chem., 1999, 71, 1327-1330. Copyright IUPAC; reproduced with the permission of IUPAC]. If you need to cite these rules please quote this reference as their source. To assist readers for some corrections the corrected drawing has been added.
Among the names and structures used as examples in the 1993 Guide to IUPAC Nomenclature of Organic Compounds (Guide) [1] some errors [2] appear, several of which are likely to be quickly obvious and untroublesome. To reduce the possibility of confusion among users of the Guide, however, we publish a list of corrections here rather than waiting until a new edition of the Guide has been prepared. The on-line edition of the Guide has been corrected to correspond with this list. The formulae in this list that is intended to specify stereochemical information is drawn in the style recommended in the 1996 publication "Basic Terminology of Stereochemistry" [3].
Page 2, R-0.1.3.1, second example: Close up a,j within brackets.
Page 8, footnote 5: Insert space after second 'i'-.
Page 9, line 13 [R-0.1.7.1 (c)]: Change first 1,3,5,7-tetraoxocane to 1,3,5,7-tetroxocane.
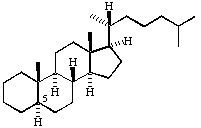
Page 18: Change formula for 5a-cholestane to

Page 19, Table 1, name for structure 5: Close up space after ] in 1-Azabicyclo[2.2.2]octane-2-carboxylic acid.
Page 24, bottom right formula: Add H with broken wedge bond at position 9.
Page 30, R-1.2.6.1, line 5: Replace g (gamma) by x (xi).
Page 31, R-1.2.7.1, line 3: Change prefix to read x(yz)abeo.
Page 31, bottom left formula: Move broken wedge bond and H from position 5 to position 6;
change prefix in name to read 10(56)abeo (i.e., no spaces).
Page 33, third example: Capitalize first c in name.
Page 39, R-2.3.1.2, line 2: Change second formula to CnHn+1
Page 42, Table 4, footnote b, line 2: Change Table 1 to Table 3.
Page 43, explanation under top right formula: Change 1,5,4 to 1,4,5 in next-to-last line.
Page 45, footnote 26: Change name to 7H-benzocyclononene and change A-21.5 to A-21.4.
Page 46, name for top right example: Insert comma between locants 1' and 2' within last square brackets.
Page 46, R-2.4.1.2, first example: Insert additional bond line between positions 4a and 9a:
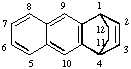
Page 49, R-2.4.1.4.1, line 4: Change R-2.3.3.3 to R-2.3.3.1.
Page 52, name for third formula (middle of page): Replace comma within square brackets by period.
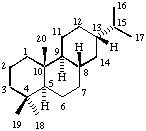
Page 56, top left formula (Abietane): Replace H at the end of wedge bond line from position 10 by locant number 20:
Page 58, line 4: Delete furfuryl, and thenyl.
Page 61, formulas for Cholesterol and 7,8-Didehydrocholesterol: Delete H and broken wedge line at position 5.
Page 61, footnote 42, line 1: Change locants in name to 3,4,5,6 and 2H.
Page 62, R-3.1.4, formula for hepta-1,6-diene-3,5-diyl: Delete subscript 2 at position 3.
Page 63, Table 5, Alcohols and Phenols entry: For suffix read -ol.
Page 66, Table 8, Isocyano- entry: Change Replacing atom(s) to -NC.
Page 67, last name: Capitalize p of phenyl.
Page 71, line 4 following heading Examples: Change three to two.
Page 75, line 2 of text: Replace -CH2-COOH by -[CH2]2-COOH.
Page 78, R-5.1.3.1, second name under heterocyclic formula: Change -dihyro- to -dihydro-; change (R-2.3.3.3) to (R-2.3.3.1).
Page 80, line 4: Change R-2.3.3.2 to R-2.3.3.3.
Page 84, name of last example: Capitalize first letter (c).
Page 85, second formula: Reverse numbering in benzene ring (i.e. -SO2-OH has locant 1):
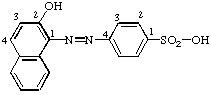
Page 85, R-5.3.3.1, first name for last formula: Change to (2-Anthryl)[7-(phenyldiazenyl)-2-naphthyl]diazene.
Page 88, R-5.4. 1, names for fourth example: Move second square bracket in (a) and (c). (a) [1,1'-Binaphthalene]-3,3',4,4'-tetrayltetrakis(azane); (c) [1,1'-Binaphthalene]-3,3',4,4'-tetrayltetraamine.
Page 90, R-5.4.3, second example, second name: Change dash to hyphen.
Page 90, R-5.4.4, line 2: Change dashes (after N and O) to hyphens.
Page 94, R-5.5.3, example on right: Add reference (R-5.5.4.2) after second name.
Page 98, R-5.5.7, last two examples, second names: Insert 1H- before 1l4 and 5H- before 5l6, respectively.
Page 105, R-5.6.6.3, name for second example: Delete () and close up.
Page 106, R-5.6.6.4, fourth example, first name: Insert () around Propan-2-ylidene.
Page 111, Table 13, third column: Change -dithoic to -dithioic.
Page 116, Table 15, second column, fifth name: Change dash to hyphen.
Page 120, R-5.7.5.1, second example, first name: Change locants 4,6 to 2,10.
Page 120, R-5.7.5.1, last example, second name: Change R-2.4.4.1 to R-2.4.1.1.
Page 121, R-5.7.5.3, second example, first name: Final character is letter l, not numeral 1.
Page 129, line 1 after first two examples: Change hydrazides to hydrazine.
Page 133, R-5.8.1.2, top right example, second formula: Delete one CH2 group.
Page 141, R-5.8.4, first example: Delete first bond line and close up to leave (CH3)3N+-N--CH3.
Page 154, top, formula for rotenone: Change locant 5 to 5'.
Page 166, Table 21, formula for cubane: Interchange locants 4 and 8:
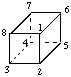
Page 166, Table 23, formula for acridarsine: Change numbering to 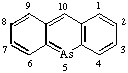 and delete An exception to.
and delete An exception to.
Page 170, second column: Change Phenothiarsine to Phenothiarsinine, Phenoxantimonine to Phenoxastibinine, Phenoxarsine to Phenoxarsinine, and Phenoxaphosphine to Phenoxaphosphinine.
Page 175, Table 28, formula for terephthalic acid: Change numbering to assign locants 1 and 4 to the substituted positions:
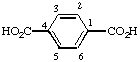
Page 177, Table 29, formula for rhodanine: Replace S= at position 4 by 0=.
Page 179, Table 31, formula for formazan: Reverse the order of locants (i.e., NH2 is 5):
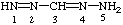
Page 180, bottom left example: Change locant 3 in formula to 2, delete [1,3] from name, and add after name, ([1,2] is implied).
Index:
Amides--Change 175 to 63, 125-127, 175, 176.ReferencesAmines--Change 125-127 to 63, 87-89.
Amino--Add 74 and 88.
Aminocarbonyl--Delete this entry entirely.
Amminium--Change to Ammonium.
Cyclo--Change 38 to 39.
Methylene--Add 163.
After Nitriles--Add Nitrilo 33
Phosphorus--Change tetravalent to trivalent
Prefixes, detachable--Change 19 to 10.
Radicofunctional name--Add 14 and 25.
Replacement nomenclature--Change 51 to 15, 23, 43.
Selanyl--Add 102.
Under Seniority--Add of classes of compounds 70.
Thio--Delete 91.
-thiol--Change 91 to 92, 93.
1. International Union of Pure and Applied Chemistry. Division of Organic Chemistry. Commission on Nomenclature of Organic Chemistry, A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, R. Panico, W. H. Powell, and J-C. Richer, eds., Blackwell Scientific Publications, Oxford, U.K., 1993, 190 pp.
2. A substantial number of these errors were discerned during the preparation of a German translation of the Guide: Nomenklatur der Organischen Chemie - Eine Einführung, G. Kruse, ed., VCH, Weinheim, Germany, 1997. We are grateful to Gerlinde Kruse for alerting us to these errors.
3. International Union of Pure and Applied Chemistry. Division of Organic Chemistry. Commission on Nomenclature of Organic Chemistry, "Basic Terminology of Stereochemistry", Pure Appl. Chem. 1996, 68, 2193-2222.