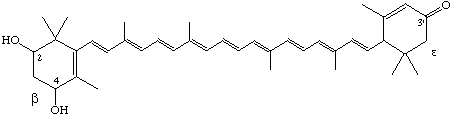
2,4-Dihydroxy-β,ε-caroten-3'-one
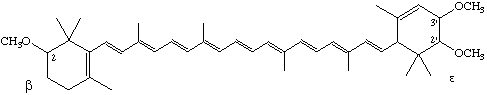
2,2',3'-Trimethoxy-β,ε-carotene
8.1. If the two C9 end groups of the parent carotenoid hydrocarbon are dissimilar, their oxygenated derivatives are numbered according to Rule Carotenoid 4, i.e. the end group designated by the Greek letter occurring earlier in the Greek alphabet receives unprimed locants.
Examples:

2,4-Dihydroxy-β,ε-caroten-3'-one

2,2',3'-Trimethoxy-β,ε-carotene
8.2.
(a) If the two C9 end groups of the parent carotenoid hydrocarbon are identical, then the lowest (see note 1) locant possible is assigned to the principal group, cited as suffix.
(b) If more than one of the group chosen to be cited as suffix is present, the numbering is determined by the principle of lowest locants (see note 2) applied to the suffixes.
(c) If no group qualifies to be cited as suffix, then the numbering is determined by the principle of lowest locants (see note 1) for all groups cited as prefixes.
Examples:
(a)
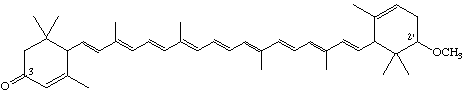
2'-Methoxy-ε,ε-caroten-3-one (not 2-Methoxy-ε,ε-caroten-3'-one)
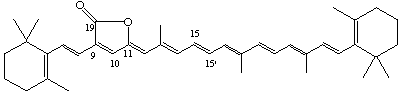
β,β-Caroten-19,11-olide
[The position of the lactone group is indicated following the principles of Rule C-472.2 (see ref 1)]
(b)
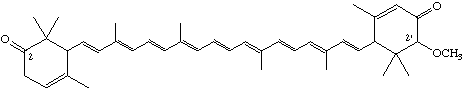
2'-Methoxy-ε,ε-carotene-2,3'-dione
[not 2-Methoxy-ε,ε-carotene-3,2'-dione; see para (b)]
(c)

3,2'-Dimethoxy-1,2,5',6'-tetrahydro-ψ,ψ-carotene
[not 2,3'-Dimethoxy-l',2',5.6-tetrahydro-ψ,ψ-carotene,
1,2,3,2',5',6' having priority over 2,5,6,1',2',3'; see para (c)]
Note 1 In the carotenoid series all unprimed numbers are cited before primed numbers and the former are therefore considered as 'lower than' primed numbers, e.g. 2,6,6,1',2',6', etc.
Note 2 When series of locants containing the same number of terms are compared term by term, that series is 'lowest' that contains the lowest number on the occasion of the first difference [IUPAC Rule C-13.11(e), footnote (see ref 1)].
Rule Carotenoid 9. retro Nomenclature
9.1. The prefix 'retro' (printed in italics) and a pair of locants are used to indicate a shift, by one position, of all single and double bonds of the conjugated polyene system delineated by the pair of locants.
9.2. The pair of locants precedes the prefix retro. The first locant is that of the carbon atom that has lost a proton, the second that of the carbon atom that has gained one.
9.3. The prefix and its accompanying locants are placed immediately before, and hyphenated to, the Greek-letter prefixes of the name defined according to Rule Carotenoid 3.
9.4. The prefix and its associated locants are not detachable from the names defined according to Rule Carotenoid 3.
Examples:
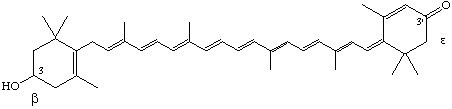
3-Hydroxy-6',7-retro-β,ε-caroten-3'-one
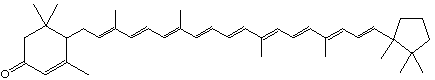
6',7-retro-ε,κ-Caroten-3-one
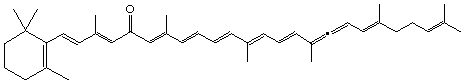
8,11-retro-β,ψ-Caroten-11-one
Rule Carotenoid 10. Apo nomenclature
It is often necessary to designate derivatives in which the carbon skeleton has been shortened by the formal removal of fragments from one or both ends of a carotenoid.
10.1. The unitalicized prefix 'apo', preceded by a locant, is used to indicate that all of the molecule beyond the carbon atom corresponding to that locant has been replaced by hydrogen atoms. A side-chain methyl group is not considered to be 'beyond' the carbon atom to which it is attached.
10.2. The prefix and its locant immediately precede the specific name (Rule Carotenoid 3) unless the locant associated with the prefix 'apo' is greater than 5, in which case there is no need to give a Greek-letter end-group designation for that end of the molecule.
10.3. For purposes of numbering, etc., an end that has been shortened by 5 or less skeletal carbon atoms is considered a ψ (acyclic) end group.
10.4. The prefix diapo, preceded by two locants, is used to indicate removal of fragments from both ends of the molecule.
10.5. If, in a diapo compound, the two ends of the carotenoid skeleton have been shortened unequally, the lower locant associated with the prefix 'diapo' is unprimed.
Examples:
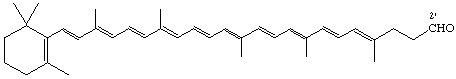
2'-Apo-β,ψ-caroten-2'-al
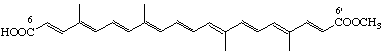
Methyl hydrogen 6,6'-diapocarotene-6,6'-dioate (trans-Bixin)
Rule Carotenoid 11. Higher carotenoids
The higher carotenoids are a class of hydrocarbons and their oxygenated derivatives consisting of more than eight isoprenoid units joined in a manner similar to that of the C40 carotenoids. They are named as mono- or disubstituted C40 carotenoids. The numbering of the normal carotenoid is retained.
Examples:
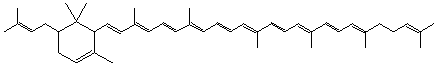
2-(3-Methyl-2-butenyl)-ε,ψ-carotene
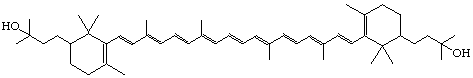
2,2'-Bis(3-hydroxy-3-methylbutyl)-β,β-carotene
or
2,2'-Bis(3-methylbutyl)-β,β-carotene-3",3"'-diol
Rule Carotenoid 12. Stereochemistry
12.1. Absolute Configuration at Chiral Centers.
The absolute configuration at chiral centers is designated by use of the RS convention (ref 5), the symbols being placed, with the corresponding locants, before the carotenoid name.
Example:
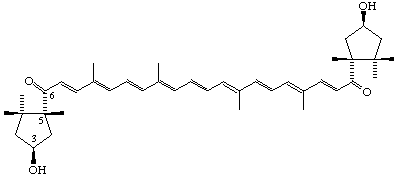
(3S,5R,3'S,5'R)-3,3'-Dihydroxy-κ,κ-carotene-6,6'-dione (Capsorubin)
12.2. Absolute Configuration of Allenic Compounds.
The absolute configuration around allene groups will be similarly designated, when known.
Example:
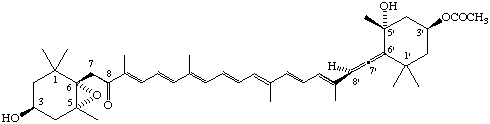
(3'S,5'R,6'R)-3'-Acetoxy-5,6-epoxy-3,5'-dihydroxy-6',7'-didehydro-5,6,7,8,5',6'-hexahydro-β,β-caroten-8-one (Fucoxanthin)
12.3. Geometrical Configuration around Double Bonds.
The stem name 'carotene' implies trans configuration about all double bonds unless the contrary is indicated. Following the designation of absolute configuration (if any), geometrical configuration is indicated by citing the double bond or bonds with a cis configuration.
Example:
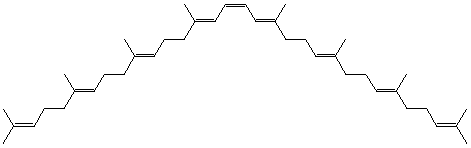
Natural phytoene = 15-cis-7,8,11,12,7',8',11',12'-octahydro-ψ,ψ-carotene
At trisubstituted double bonds, the term cis refers to the relative position of the two substituents forming parts of the main chain of carbon atoms.
Example: natural bixin = methyl hydrogen 9'-cis-6,6'-diapocarotene-6,6'-dioate.
In the absence of definite information on geometrical configuration, cis isomers may be distinguished by prefixes such as neo A, neo U, etc. (cf. Zechmeister, Cis-Trans Isomeric Carotenoids, Vitamins A and Aryl-polyenes, Springer-Verlag, Vienna, 1962).
The stereochemical prefixes E and Z (see footnote to Rule Carotenoid 12.1 and J. Amer. Chem. Soc. 90. 509 (1968)) may be used, especially when the prefixes cis and trans might lead to ambiguity.
12.4. Numbering of gem-Dimethyl Groups at C-1.
In an end group of β, γ, κ or ε type, the two methyl groups attached to C-1 are distinguished as follows: when the potential chirality is as shown in formula A, i.e. with the polyene chain (R) to the right of C-1, the methyl groups below and above the plane of the paper are numbered 16 and 17, respectively; if, with the polyene chain (R) to the left, the end group is as shown in B1, then these designations are unaltered; if the end group is as shown in B2, then they are reversed.
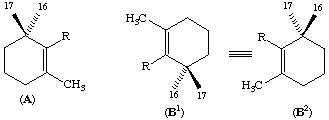
In an acyclic end group, the methyl group that is trans to the main skeletal chain is numbered 16 and the methyl group that is cis is numbered 17, as shown in C.
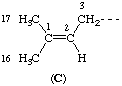
Rule Carotenoid 13. Trivial names
The preceding rules are designed to define precisely the structure of a given carotenoid by its name. Use of the semisystematic names derived from these rules will greatly assist communication between scientists and enable work to be more readily retrieved from the literature.
The appendix contains a list of some trivial names currently in use for naturally occurring carotenoids; it also gives their semisystematic names and structures. These trivial names will be of value in natural-product and biochemical work. but their use in systematic organic work should be restricted. If trivial names are used in a paper, the semisystematic names should always be clearly indicated.
While the need to coin a new trivial name must occasionally arise (e.g. because the structure of the compound is unknown) the list in the appendix should not be unnecessarily enlarged. Simple derivatives of known carotenoids should not be given new trivial names; they may, however, be named by modification of existing trivial names [cf. Steroid Rule 2S-A.2, Note (ref 2)], but the full semisystematic name according to these Rules should be given for each compound at the first mention in each paper.
1. IUPAC Nomenclature of Organic Chemistry (Pure Appl. Chem. 11, Nos. 1 and 2 (1965); Sections A, B and C published by Butterworths, London, 1971) indicates that, in ranking locants for priority, a primed numeral ranks immediately after the same numeral unprimed. In general organic nomenclature the common practice is therefore to cite locants in the order x,x',(x+n), (x+n)'. The established sequence in the carotenoid field is, however, to cite an unprimed numerals before any primed numerals, and this practice is followed in these rules. [Now included in the 1979 edition with sections D, E, F and H]
2. The contrast with steroid usage [IUPAC-IUB 1971 Definitive Rules for Steroid Nomenclature, Pure Appl. Chem. 31, Nos. 1-2 (1972)] Rule 2S-6, where the prefix 'nor' is associated with the highest permissible number, is to be noted. [Now as the 3rd edition, 1989.] The 1981 IUPAC-IUB Nomenclature of Retinoids recommended the use of the higher number to follow IUPAC Section F; Natural Product Nomenclature, and proposed that when the carotenoid rules are next revised this should be used there too.
3. IUPAC Nomenclature of Organic Chemistry (see ref 1), Rule C-16.1. allows the prefix 'hydro' to be detachable or nondetachable and the former has become the established usage in general organic chemistry. However, the common practice in carotenoid names is now to use this prefix as nondetachable, a practice that is followed in this set of rules.
4. IUPAC Rules, Nomenclature of Organic Chemistry (see ref 1), Subsections C-2 to C-4.
5. For a discussion on the RS convention and the use of thickened lines, broken fines, wavy lines, and wedges in displayed formulae, see IUPAC Tentative Rules for the Nomenclature of Organic Chemistry, Section E, Fundamental Stereochemistry, IUPAC Information Bulletin No. 35, p 68. [Now revised version included in the 1979 edition with sections A, B, C, D, F and H]