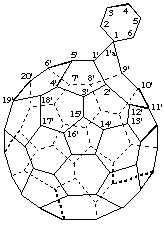
Spiro[cyclohexane-1,1'a-[1(9)a]homo(C60-Ih)[5,6]fullerene]
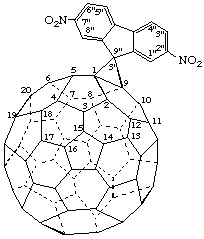
2",7"-Dinitrospiro[cyclopropa[1,9](C60-Ih)[5,6]fullerene-3',9"-fluorene]
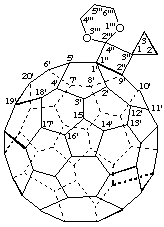
Dispiro[cyclopropane-1,3"-cyclobuta[1,9](C60-Ih)[5,6]fullerene-4",2'''-[1,3]dioxane]
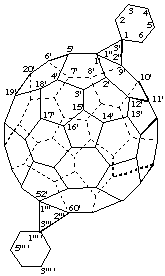
Dispiro[cyclohexane-1,3"-dicyclopropa[1,9:52,60](C60-Ih)[5,6]fullerene-3''',1''' '-cyclohexane]