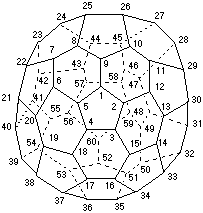
Fig. 1. Systematic numbering of (C60-Ih)[5,6]fullerene
Contents
2.1. (C60-Ih)[5,6]fullerene and (C70-D5h(6))[5,6]fullerene
Rule Fu-3.1.1
Rule Fu-3.1.2
Rule Fu-3.1.3
2.1.1. Systematic numbering for (C60-Ih)[5,6]fullerene
2.1.2. Systematic numbering for (C70-D5h(6))[5,6]fullerene
The following rules were adopted for numbering (C60-Ih)[5,6]fullerene and (C70-D5h(6))[5,6]fullerene [ref 4].
Rule Fu-3.1.1: Proper rotation axes (Cn) are examined in sequence from the highest-order to the lowest-order axis, until at least one contiguous helical pathway is found that begins in a ring through which a proper rotation axis passes, or at one end of a bond bisected by a proper rotation axis, or at an atom through which a proper rotation axis passes. Numbering begins at the end of such a contiguous helical pathway, and the corresponding axis is called the "reference axis".
Rule Fu-3.1.2: If there is a choice for the selection of a reference axis or for the end of the reference axis to begin the numbering, a ring is preferred to a bond which, in turn, is preferred to an atom.
Rule Fu-3.1.3: When there is a choice among helical numbering pathways, the preferred pathway terminates as close as possible, in terms of the number of bonds, to the reference axis.
The numberings of (C60-Ih)[5,6]fullerene and (C70-D5h(6))[5,6]fullerene illustrate the application of these rules.
2.1.1. Systematic numbering for (C60-Ih)[5,6]fullerene (Fig. 1)
(Atlas [ref 5] Ref. No. 60:1; CAS Reg. No. 99685-96-8).
This fullerene has six symmetry-equivalent C5 axes passing through opposite pentagons, each of which gives identical contiguous helical pathways in either direction from any atom of any pentagon (all atoms are symmetry-equivalent in (C60-Ih)[5,6]fullerene). Any of these C5 axes can be the reference axis. According to Fu-3.1.1, it is not necessary to consider any of the lower order C3 and C2 axes. The systematic numbering is given in Fig. 1.

Fig. 1. Systematic numbering of (C60-Ih)[5,6]fullerene
2.1.2. Systematic numbering for (C70-D5h(6))[5,6]fullerene (Fig. 2)
(Atlas [ref 5] Ref. No. 70:1; CAS Reg. No. 115383-22-7).
The principal axis for this fullerene is the C5 axis passing through opposite pentagons. There are no contiguous helical pathways from any atom in either pentagon (all symmetry-equivalent) and therefore the C5 axis cannot be the reference axis. Following rule Fu-3.1.1, one of the five equivalent C2 axes that pass through the center of a six-membered ring at one end and bisect the bond between two six-membered rings at the other end (see Fig. 2a) must be evaluated. Since, according to Fu-3.1.2, a ring is preferred to a bond for the beginning of numbering, the search for a helical pathway must start in the six-membered ring. A priori there are twelve pathways, one in each direction, clockwise and anticlockwise, from each atom of the six-membered ring to be examined. However, because of symmetry, atoms a and a' in Fig. 2a are equivalent, as are atoms b, b', b'', and b''' (throughout this document symmetry-related atoms are indicated by unprimed and primed identical letters; for sake of clarity, however, atoms located at the end of a pathway are identified by letters such as z/y/x... even if symmetry-related to a/b/c...). Therefore, there are only three different pathways to explore: clockwise b' to a' to b'', clockwise a to b to b', and clockwise b to b' to a'. The pathway b' to a' to b'' terminates at the atom marked z at the end of the bond bisected by the reference axis. The pathway a to b to b' terminates at the atom marked y, one bond removed from the bond at the end of the reference axis. The pathway b to b' to a' terminates at the atom marked x, two bonds removed from the bond bisected by the reference axis. According to Fu-3.1.3, the preferred contiguous pathway for numbering is b' to a' to b''; the resulting systematic numbering is shown in Fig. 2b.
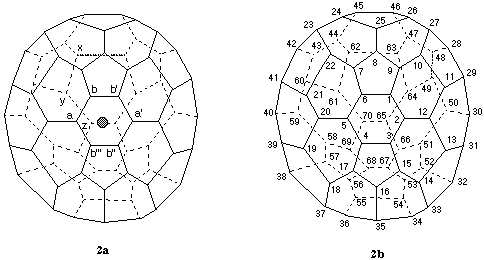
Fig. 2. Systematic numbering of (C70-D5h(6))[5,6]fullerene
4. International Union of Pure and Applied Chemistry, Division of Organic Chemistry, Commission on Nomenclature of Organic Chemistry. "Nomenclature for the (C60-Ih) and (C70-D5h(6))[5,6]fullerenes (IUPAC recommendations 2002)", Pure Appl. Chem. 2002, 74, 629-695.
5. P.W. Fowler and D.E. Manolopoulos, An Atlas of Fullerenes, Clarendon Press, Oxford, 1995