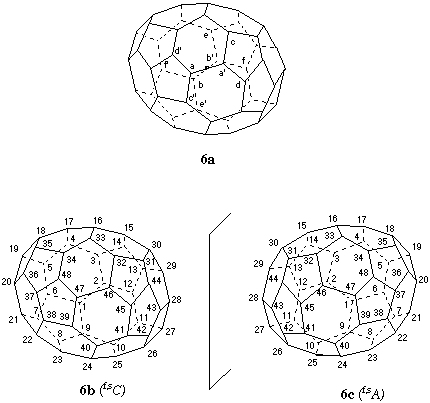
Fig. 6. Systematic numbering of (C48-C2)[5,6]fullerene
This is a chiral fullerene. The discussion will consider the enantiomer shown in Fig. 6a. The only symmetry element in this fullerene is a C2 axis which bisects midpoints of opposite bonds (Fig. 6a). Both bonds connect 6,6,5 atoms (a and a' at the near end of the axis; b and b' at the far end). Based on Fu-3.1.2.2 no selection between these bonds for beginning the numbering can be made. In Fig. 6a, the atoms indicated with the same letter (unprimed and primed) are identical for symmetry reasons. Also because of symmetry, only four pathways must be considered: a to a' to c; a to a' to d; b to b' to e; and b to b' to f. Of these, only the anticlockwise b to b' to e pathway results in a contiguous helical numbering that is shown in Fig. 6b. Because the pathway is clockwise for a viewer looking directly at the polygon where the numbering begins from the outside of the fullerene cage, the stereodescriptor for this inherently chiral fullerene is (f,sC) ("f" = fullerene; "s" = systematic numbering; "C" = clockwise) [ref 4]. The numbering of the (f,sA) enantiomer, shown in Fig. 6c, is the mirror-image of that shown in Fig. 6b.

Fig. 6. Systematic numbering of (C48-C2)[5,6]fullerene
4. International Union of Pure and Applied Chemistry, Division of Organic Chemistry, Commission on Nomenclature of Organic Chemistry. "Nomenclature for the (C60-Ih) and (C70-D5h(6))[5,6]fullerenes (IUPAC recommendations 2002)", Pure Appl. Chem. 2002, 74, 629-695.
5. P.W. Fowler and D.E. Manolopoulos, An Atlas of Fullerenes, Clarendon Press, Oxford, 1995