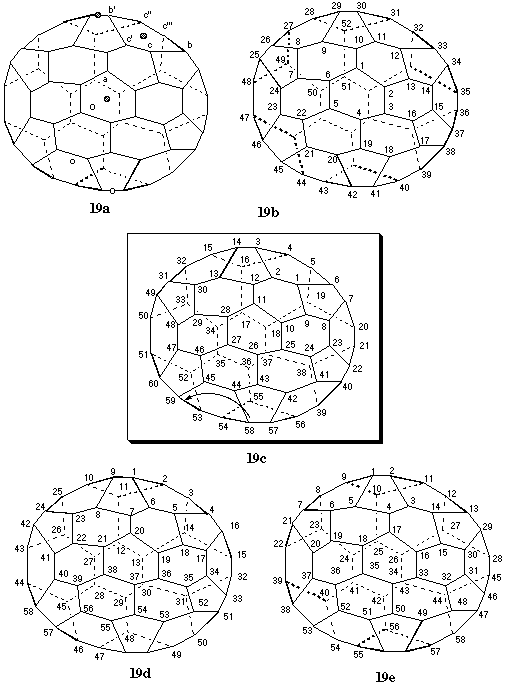
Fig. 19. Systematic numbering of (C60-D6h)[5,6]fullerene
This fullerene features one C6 axis, passing through the midpoints of opposite hexagons (centers of coronene substructures), three symmetry-equivalent C2 axes, also passing through the midpoints of opposite hexagons, and three symmetry-equivalent C2 axes, passing through the midpoint of opposite bonds (Fig. 19a). Starting from any of the six symmetry-equivalent atoms a of the six-membered ring intersected by the C6 axis does not lead to a contiguous helical pathway. The numbering built around the C6axis (Fig. 19b) becomes discontiguous at position 52. Three pathways must be considered for beginning the numbering based on one of the C2 axes passing through opposite hexagons: c to c' to b'; c' to b' to c"; and b' to c" to c'''. These pathways lead to numberings discontiguous at position 58 (Fig. 19c), 56 (not shown), and 58 (Fig. 19d), respectively. Finally, one pathway can also be constructed around one of the remaining C2 axes passing through the midpoints of opposite bonds. This pathway results in the numbering shown in Fig. 19e that is discontiguous at position 58. To select among the three numberings having the discontiguity at the same higher position and built around axes of the same order (Figs. 19c, 19d, and 19e), Fu-3.2.2 is first applied. The two numberings beginning in a ring (Figs. 19c and 19d) are preferred over the one beginning at a bond bisected by a C2 axis (Fig. 19e). Furthermore, according to Fu-3.2.3, the numbering beginning with a 6,6,5 atom (Fig. 19c) is preferred over the one beginning with a 6,5,5 atom (Fig. 19d). The completion of the numbering of Fig. 19c is straightforward on the basis of Fu-3.2.4.

Fig. 19. Systematic numbering of (C60-D6h)[5,6]fullerene