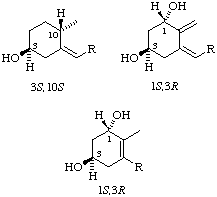
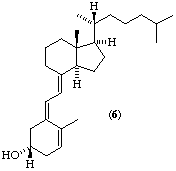
Fig. 1. Examples of stereochemistry in ring A. Note that the hydroxyl group in formulae (1) to (6) is 3S
IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN)
https://iupac.qmul.ac.uk/misc/D.html
World Wide Web version prepared by G. P. Moss
School of Physical and Chemical Sciences, Queen Mary University of London,
Mile End Road, London, E1 4NS, UK
e-mail g.p.moss@qmul.ac.uk
These recommendations are as close as possible to the printed version [see Arch. Biochem. Biophys., 1982, 218, 342-346; Endokrinol. Inform., 1982(2), 53-62; Eur. J. Biochem., 1982, 124, 223-227; Mol. Cell. Biochem., 1982, 49, 177-181; Pure Appl. Chem., 1982, 54, 1511-1516; Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pages 242-246; copyright IUPAC and IUBMB; reproduced with the permission of IUPAC and IUBMB]. If you need to cite these rules please quote these references as their source. In the web version footnotes have been converted into notes following the paragraph to which they apply. A PDF of the printed version is available.
Any comments should be sent to any member of the Committee
Contents
Introduction
1. Class Name
2. Semisystematic names
3. Stereoparent Compounds
4. Numbering
5. Modification of the Triene System
6. Side Chain Modification
7. Dihydro Derivatives
8. Other Modifications
9. Combination of Prefixes
10. Additional Hydroxyl Groups
a) designated by a suffix
b) designated by a prefix
11. Other Substituents
a) modification of the suffix
b) designated by a prefix
References
Appendix. Trivial names of vitamin D compounds
In the IUPAC Definitive Rules for the Nomenclature of Vitamins, published in 1960 [ref 1], and in the revised document of 1966 on Trivial Names of Miscellaneous Compounds of Importance in Biochemistry [ref 2], the trivial names ergocalciferol for Vitamin D2 and cholecalciferol for Vitamin D3 were recommended. The recent revival of interest in vitamin D analogues and their chemistry as a result of the developments in vitamin D metabolism and function has rendered cumbersome some of the older systems for naming these compounds, resulting in the use of undesirable abbreviations like 1α,25-(OH)2D3 in the literature. Therefore, the Commission on Biochemical Nomenclature asked H. F. DeLuca to develop, in consultation with other experts, a simplified and extended system of trivial names for vitamin D metabolites. This proposal was submitted to the International Union of Nutritional Sciences and the IUB-IUPAC Joint Commission on Biochemical Nomenclature. The present recommendations are based on H. F. DeLuca's proposal after further consultation with other workers active in this field. They extend the scope of Section M-2 of the 1966 Rules [ref 2] and recommend, new shorter trivial names. From the biochemical point of view, the most important ones are calciol, calcidiol and calcitriol for cholecalciferol, 25-hydroxycholecalciferol and 1α,25-dihydroxycholecalciferol, respectively. Calciol is not necessarily preferred to cholecalciferol for vitamin D3 itself, but the new names are recommended for hydroxylated derivativess A full list of recommended names is given in the appendix. These recommendations are intended to give convenient short trivial names to the common, biologically important derivatives of vitamin D. Synthetic derivatives that have a more complicated structure may be named more conveniently according to the steroid rules [ref 4], using 9,10-secocholestane and 9,10-secoergostane as parent compounds. Thus these recommendations do not supersede the steroid rules.
The term vitamin D should be used as a general term to describe all steroids that exhibit qualitatively the biological activity of calciol. This term should be used in derived terms such as vitamin D activity, vitamin D deficiency, vitamin D antagonist [ref 5].
The term vitamin D3 may be used as a synonym for calciol, but it should not be abbreviated to D3 and then modified to forms like 1,25-(OH)2D3. This type of representation of vitarnin D3 metabolites is strongly discouraged.
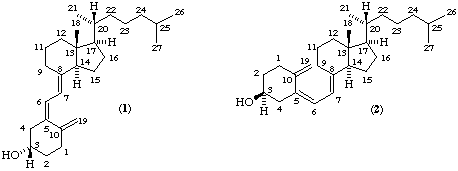
Although all compounds with vitamin D activity may be described using a semisystematic steroid name [ref 4], the names tend to be cumbersome for general use. A new and shorter, name for (5R,10R)-9,10-secocholestane was considered as a parent molecule, but it was decided that the shortening achieved was not worth the disruption involved in the change. The main confusion in the application of Steroid Rule 2S-8.1 [ref 4] to vitamin D derivatives is that the descriptors 'α' and 'β' only apply when ring A is orientated as in the parent steroid, although the vitamin is often represented in its alternative conformation [compare (1) with (2), (3) with (4), or (8) with (9)]. We recommend that these descriptors should never be applied to ring A or to C-6 or C-7 of vitamin D compounds; chiral centres should be designated R or S, and double bonds E or Z [ref 6]. Examples are given in Table 1.
Table 1. Nomenclature for vitamin D compounds
| Current trivial name | Recommended trivial name | Systematic steroid name a |
| Cholecalciferol | calciol or cholecalciferol | (5Z,7E)-(3S)-9,10-seco-5,7,10(19)-cholestatrien-3-ol |
| 25-Hydroxycholecalciferol | calcidiol | (5Z,7E)-(3S)-9,10-seco-5,7,10(19)-cholestatriene-3,25-diol |
| 1α,25-Dihydroxycholecalciferol | calcitriol | (5Z,7E)-(1S,3R)-9,10-seco-5,7,10(19)-cholestatriene-1,3,25-triol |
| Ergocalciferol | ercalciol or ergocalciferol | (5Z,7E,22E)-(3S)-9,10-seco-5,7,10(19),22-ergostatetraen-3-ol b |
| 1α,25-Dihydroxyergocalciferol | ercalcitriol | (5Z,7E,22E)-(1S,3R)-9,10-seco-5,7,10(19),22-ergostatetraen-1,3,25-triol c |
| 22,23-Dihydroergocalciferol | (24S)-methylcalciol or 22,23-dihydroercalciol | (5Z,7E)-(3S)-9,10-seco-5,7,10(19)-ergostatrien-3-ol c |
| 1α,24R,25-Trihydroxycholecalciferol | calcitetrol | (5Z,7E)-(1S,3R,24R)-9,10-seco-5,7,10(19)-cholestatriene-1,3,24,25-tetrol |
| Previtamin D3 | (6Z)-tacalciol | (6Z)-(3S)-9,10-seco-5(10),6,8-cholestatrien-3-ol |
| Tachysterol3 | tacalciol | (6E)-(3S)-9,10-seco-5(10),6,8-cholestatrien-3-ol |
| Isovitamin D3 | (5E)-isocalciol | (5E,7E)-(3S)-9,10-seco-1(10),5,7-cholestatrien-3-ol |
| Dihydrotachysterol3 | dihydroercalciol | (5E,7E)-(3S,10S)-9,10-seco-5,7-cholestadien-3-ol |
b 24R-configuration.
c 24S-configuration.
Because of the nature of the sequence rules it is not possible to transfer R or S from one compound to its derivatives. Examples of the effects of this are shown in Fig. 1.

Fig. 1. Examples of stereochemistry in ring A. Note that the hydroxyl group in formulae (1) to (6) is 3S
These recommendations do not apply to compounds in which ring B is unbroken. Thus lumisterol remains (22E)-9β,10α-ergosta-5,7,22-trien-3β-ol (Steroid Rule 2S-5.2 in [ref 4]).
Many investigators have used modifications of purely trivial names to show relationships between compounds. This aim can be combined with considerable shortening of names based on cholecalciferol and ergocalciferol if the name calciol is used for cholecalciferol [(1) which is the same as (2)]; cholecalciferol may still be used as an alternative trivial name for calciol but should not be used for naming metabolites. Although calciol is the parent name for the vitamin D3 series and is capable of further modification (see below), no new parent hydrocarbon is named. Hence the name should only be used for compounds containing a 3-hydroxyl group and a system of (or derived from) three conjugated double bonds. Unless otherwise specified the configuration of the 3-hydroxyl group remains unchanged from that of the 3β-hydroxyl of the parent tetracyclic steroid, i.e. in the absence of 2- and 4-substitution it is 3S if position 1 is unsubstituted and 3R if position 1 also carries a hydroxyl. The triene system is 5,7,10(19) with 5Z,7E stereochemistry unless otherwise specified.
Calciol
The numbering of the parent steroid is maintained as shown in formulae (1) and (2).
5. Modification of the Triene System
As mentioned in rule 3 the stem calci- includes the 5,7,10(19)-triene system with 5Z,7E stereochemistry unless otherwise specified. The prefix 'ta' is applied to calciol (Table 2) to change the location of the triene to 5(10),6,8 with 6E configuration implied, e.g. tacalciol [(3) which is the same as (4)]. The prefix 'iso' is applied to calciol (Table 2) to change the location of the triene to 1(10),5,7 with 7E configuration implied; this prefix requires designation of the stereochemistry at position 5, e.g. (5E)-isocalciol (5)
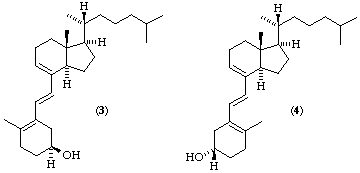
Tacalciol
(5E)-Isocalciol
(5Z)-Isocalciol
| Stem or prefix | Origin | Effect |
| calci | calciferol | indicates 9,10-seco-5,7,10(19)-cholestatriene with 5Z,7E-configuration |
| ta | tachysterol | changes the triene of calciol to 5(10),6,8 with 6E-configuration |
| iso | isovitamin D3 | changes the triene of calciol to 1(10),5,7 with 7E-configuration |
| er | ergosterol | introduces 22(23) double bond with 22E-configuration and 24-methyl group in the configuration that is 24R if no other changes are made |
The prefix 'er' is used (Table 2) to indicate the side chain (7) for the vitamin D2 series, e.g. ercalciol. This prefix implies the 22E,24R configuration shown in (7) unless otherwise specified. Ergocalciferol may still be used as an alternative trivial name for ercalciol but should not be used for naming metabolites.

(7)
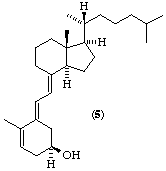
Note. Because of the nature of the sequence rules it is not possible to transfer R or S from one compound to its derivatives. Examples of the effect of this are shown in Fig. 2.
Dihydro tachy sterol is an important member of the vitamin D family. It should be called dihydrocalciol, although a more systematic name would be (5E)-(10S)-10,19-dihydrocalciol [(8) which is the same as (9)].
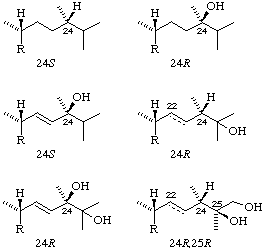
Fig. 2. Examples of stereochemistry at position 24 (and 25) in the vitamin D2 series. Note that the presence or absence of a 22(23) double bond in the bottom three examples does not change in this series the designation at position 24 (or 25).
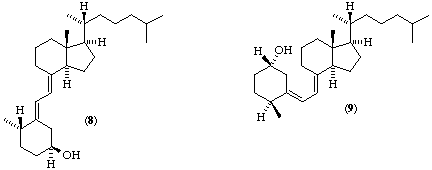
(5E)-(10S)-10,19-Dihydrocalciol
Note 1. Although this compound is derived from calciol by hydrogenation of the 10(19) double bond it may also be considered as a derivative of tacalciol formed by 1,6-addition of hydrogen to the 5(10),6,8-triene system, i.e. positions 9 and 10.Note 2. A new chiral centre is present at position 10. If a synthetic sample contains a mixture of both isomers, not necessarily in equimolar proportions, the affix ambo may be, used [ref 8] to indicate the presence of such a mixture, e.g. (5E)-10-ambo-10,19-dihydrocalciol. If only one isomer is present, but with unknown stereochemistry, then this may be indicated by the use of xi, e.g. (5E)-(10ξ)-10,19-dihydrocalciol. When the absolute stereochemistry at C-10 is know this is shown in the normal way, e.g. (5E)-(10S)-10,19-dihydrocalciol.
Other modifications of the parent compound may named by the appropriate prefix as outlined in the IUPAC Nomenclature of Organic Chemistry, Section F [ref 9]. Table 3 indicates possible modifications.
If there is a change of configuration from that implied by the stem and its suffix (see sections 3 and 10a), and the prefixes listed in Table 2, then this is stated by means of the appropriate locant and affix (R or S at positions 1, 3, 20 or 24; E or Z at positions 5, 6, 7 or 22; and α or β at 13, 14 or 17). Further details of these modifications are given in Rule F-6.3 [ref 9] and Steroid Rules 2S-3.2 and 2S-3.4 [ref 4]; the use of the prefixes 'ent-' and 'rac-' is also given in these references (F-6.4, F-6.5, F-6.6; 2S-5.1, 2S-5.3 and 2S-5.4). Examples: D-homocalciol, 24-azaercalciol, (3R)-calciol, and (22Z)-ercalciol.
Further details for the application of these prefixes may be found in the appropriate section of the F-rules [ref 9] as indicated in column 3
| Prefix | Effect | Rule |
| cyclo | an additional ring | F-4.1 |
| didehydro | an additional double bond | F-3.3 |
| homo | an additional methylene group | F-4.5 |
| dihydro | reduction of a double bond | F-3.1, see section 7 above |
| nor | loss of a methylene | F-4.2, F-4.4 |
| aza | group replacement of carbon by nitrogen | F-4.11 |
| oxa | replacement of carbon by oxygen | F-4.11 |
Modifying prefixes may be combined and are cited in the order (a) stereochemistry of double bonds requiring E or Z, (b) stereochemistry at chiral centres requiring R or S, (c) detachable prefixes (see sections 10a and 11b below), (d) modifying prefixes in the order given in Table 3, (e) changes in configuration at positions 13, 14 or 17, and (f) modifying prefixes given in Table 2 in alphabetical order.
Example: (24S)-24-hydroxy-22,23-didehydrotacalciol (cf. second example in Fig. 3).
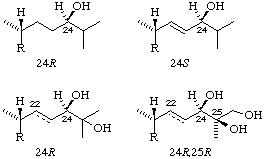
Fig. 3. Examples of stereochemistry at position 24 (and 25) in the vitamin D3 series.
Note that the presence or absence of a 22(23) double bond in the last two examples does not change the designation at position 24 (or 25)
10. Additional Hydroxyl Groups
a) Designated by a Suffix
The name calcidiol is reserved for the 3,25-diol, calcitriol for the 1,3,25-triol, and calcitetrol for the 1,3,24,25-tetrol. The configuration of the hydroxyl group(s) corresponds to the 3β-hydroxy or 1α,3β-dihydroxy tetracyclic steroid (see Fig. 1) or 24R-hydroxy group.
b) Designated by a Prefix
Any hydroxyl groups not included in the suffixes (see rules 3 and 10a) shall be designated by the prefix hydroxy, dihydroxy etc. together with the appropriate locant and where necessary indicating the stereochemistry of this substituent. Examples: (1S)-1-hydroxycalciol, 16β-hydroxycalciol, and 26-hydroxycalciol [or if the full stereochemistry is known, (25R)-26- or (25S)-26-hydroxycalciol].
Note. Because of the nature of the sequence rules it is not possible to transfer R or S from one compound to its derivatives. Examples of the effects of this are shown in Fig. 2 and Fig. 3.
a) Modification of the Suffix
Esters of the hydroxyl groups cited by a suffix (rules 3 and 10a) are given by the acyloxy group(s) in its anionic form, with locants when necessary (see Steroid rule 2S-4.1 [ref 4]). The ketone corresponding to calciol may be called calcione, but this name is restricted to the 3-ketone. Oxidation of calcidiol will give a hydroxy ketone, which should be named 25-hydroxycalcione. Examples: calciol acetate, and calcitriol 1-acetate 3-formate.
Note. If a methyl group of a vitamin D compound is oxidized to a carboxyl group (or derivative), the compound needs to be named using the appropriate suffix. The name calciol is not suitable for this, because its suffix indicates the oxygen function at C-3; we therefore recommend that carboxylic acids should be named as 9,10-secocholestane or 9,10-secoergostane derivatives. Example: (5Z,7E)-(3S,23R,25S)-3β,25-dihydroxy-9,10-seco-5,7,10(19)-cholestatrieno-26,23-lactone.
b) Designated by a Prefix
Substituents not cited by the parent compound and suffixes (sections 3, 10a and 11a) should be designated by a prefix together with the appropriate locant, indicating, where necessary, the stereochemistry with an affix. Vitamin D analogues where the hydroxyl group at C-3 is absent or is replaced by an amino group are named by the use of the prefix 3-deoxy (see Carbohydrate Rule 14 [ref 10]). Examples: 25-fluorocalciol, (3S)-3-amino-3-deoxycalciol, and 11α-acetoxycalciol.
1. International Union of Pure and Applied Chemistry (1960). Definitive rules for the nomenclature of vitamins, J. Am. Chem. Soc. 82, 5581-5583.
2. IUPAC-IUB Commission on Biochemical Nomenclature (CBN), Tentative rules, section on Trivial names of miscellaneous compounds of importance in biochemistry, 1966, Arch. Biochem. Biophys. 118, 505-507 (1967), Biochem. J. 102, 15-16 (1967), Biochim. Biophys. Acta, 107, 1-4 (1965), Bull. Soc. Chim. Biol. (in French) 49, 332-335 (1967), Eur. J. Biochem. 2, 1-2 (1967), Hoppe Seyler's Z. Physiol. Chem. (in German) 348, 266-268 (1967),. IUPAC Inf Bull. 25, 19-23 (1966), and J. Biol. Chem. 241, 2987-2988 (1966); also on pages 212-213 in [ref 3].
3. International Union of Biochemistry (1978) Biochemical Nomenclature and Related Documents, The Biochemical Society, London. [Now available as a 1992 second edition]
4. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC) and IUPAC-IUB Commission on Biochemical Nomenclature (CBN). The Nomenclature of Steroids, Revised tentative rules, 1967, Arch. Biochem. Biophys. 136, 13-35 (1970) amended 147, 4-7 (1971), Biochem. J. 113, 5-28 (1969) amended 127, 613-616 (1972), Biochemistry, 8, 2227-2242 (1969) amended 10, 4994-4995 (1971), Biochim. Biophys. Acta, 164, 453-486 (1968) amended 248, 387-390 (1971), Eur. J. Biochem. 10, 1-19 (1969) amended 25, 1-3 (1972) and - amendments incorporated - Pure Appl. Chem. 31, 285-322 (1972); also on pages 133-153 in [ref 3]. [Now available as a 1989 third edition.]
5. International Union of Nutritional Sciences, Committee 1/1, Nomenclature (1978) Nutr. Abstr. Rev. 48A, 831-835.
6. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC). Rules for the nomenclature of organic chemistry, Section E: Stereochemistry, Recommendations 1974, Pure Appl. Chem. 45, 11-30 (1976); also on pages 1-18 in [ref 3] and on pages 473-490 (section E) in [ref 7].
7. International Union of Pure and Applied Chemistry (1979). Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, Pergamon Press. Oxford.
8. IUPAC-IUB Commission on Biochemical Nomenclature (CBN). Nomenclature of tocopherols and related compounds, Recommendations 1973, Arch. Biochem. Biophys. 165, 6-8 (1974), Biochem. J. 147, 11-13 (1975), Eur. J. Biochem. 46, 217-219 (1974), IUPAC Inf. Bull., Append. Provisional Nomencl. Symb. Units Stand. No. 47 (1975); also on pages 171-173 in [ref 3]. An updated version of this document (Recommendations 1981) has appeared in Eur. J. Biochem. 123, 473-475 (1982).
9. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC). Nomenclature of organic chemistry, Section F: Natural products and related compounds, Recommendations 1976, Eur. J. Biochem. 86,1-8 (1978) and IUPAC Inf. Bull., Append. Provisional Nomencl. Symb. Terminol. Conv. No. 53 (1976); also on pages 19-26 in [ref 3] and on pages 497-511 (section F) in [ref 7]. [Now available as revised 1999 recommendations].
10. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC) and IUPAC-IUB Commission on Biochemical Nomenclature (CBN). Tentative rules for carbohydrate nomenclature, Part 1, 1969, Biochern. J. 125, 673-694 (1971), Biochemistry, 10, 3983-4004 and 4995 (1971), Biochim. Biophys. Acta, 244, 223-302 (1971), Eur. J. Biochem. 21, 455-476 (1971) and 25, 4 (1972), IUPAC Inf. Bull. Append. Tentative Nomencl. Symb. Units Stand. No. 7 (1970), and J. Biol. Chem. 247, 613-634 (1972); also on pages 174 - 195 in [ref 3]. [Now available as revised 1996 recommendations].
| Recommended name | Current trivial name | Other names |
| Calciol or cholecalciferol | cholecalciferol | vitamin D3, colecalciferol* |
| Ercalciol or ergocalciferol | ergocalciferol* | vitamin D2, calciferol |
| Calcidiol | 25-hydroxycholecalciferol | calcifediol* |
| (1S)-Hydroxycalciol | 1α-hydroxycholecalciferol | alfacaleidol* |
| (24R)-Hydroxycalcidiol | 24(R),25-dihydroxycholecalciferol | |
| Calcitriol* | 1,25-dihydroxycholecalciferol | |
| Calcitetrol | 1,24(R),25-trihydroxycholecalciferol | |
| 25-Fluorocalciol | 25-fluorocholecalciferol | |
| Ercalcidiol | 25-hydroxyergocalciferol | |
| Ercalcitriol | 1,25-dihydroxyergocalciferol | |
| Ertacalciol | tachysterol2 | |
| Tacalciol | tachysterol3 | |
| (5E)-Isocalciol | isovitamin D3 | |
| 22,23-Dihydroercalciol or (24S)-methylcalciol | vitamin D4 | |
| (5E)-(10S)-10,19-Dihydroercalciol | dihydrotachysterol2 | hytakerol, dihydrotachysterol* |
| (6Z)-Tacalciol | precalciferol | previtamin D |
| (24S)-Ethylcalciol | vitamin D5 | |
| (22E)-(24R)-Ethyl-22,23-didehydrocalciol | vitamin D6 |