IUPAC-IUB Commission on Biochemical Nomenclature (CBN)
https://iupac.qmul.ac.uk/misc/phospho.html
World Wide Web version prepared by G. P. Moss
School of Physical and Chemical Sciences, Queen Mary University of London,
Mile End Road, London, E1 4NS, UK
e-mail g.p.moss@qmul.ac.uk
These recommendations are as close as possible to the published version [see Biochem. J., 1978, 171, 1-19; Eur. J. Biochem., 1977, 79, 1-9; Hoppe-Seyler's Z. Physiol. Chem., 1977, 358, 599-616; Proc. Natl Acad. Sci. USA, 1977, 74, 2222-2230; Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pages 256-264.; Copyright IUPAC and IUBMB; reproduced with the permission of IUPAC and IUBMB]. If you need to cite these rules please quote these references as their source. Some errors have been noted and appropriate corrections have been made. The changes have been marked by which is a link to details of the change and where it applies.
Any comments should be sent to any member of the Committee
Contents
Introduction
1. Phosphoric esters, RO-PO(OH)2
2. Phosphate
3. Phosphoric anhydrides
4. Phosphodiesters
5. Nucleoside Triphosphate Analogs
6. Phosphoric amides
7. Fluorophosphoric acids
References.
Table 1. Phosphoric esters (phosphates)
Table 2. Representative oligophosphoric esters (oligophosphates)
Table 3. Representative bisnucleoside phosphates and cyclic phosphates
Table 4. Representative phospholipids (involving diesterified phosphoric acid)
Table 5. Phosphoric amides (phosphoramidic acids or amidophosphoric acids)
Table 6. Representative phosphoric anhydrides and fluorophosphates
Table 7. Representative C-phosphonates
Table 8. Adenosine 5'-triphosphate analogs
The IUPAC Commissions on the Nomenclature of Inorganic and of Organic Chemistry (CNIC and CNOC) have recently provided, in the "D Rules" (ref 1), recommendations for naming a large number of organic compounds containing phosphorus. Many such compounds are extremely important in biochemistry and hence in nearly all branches of biology and medicine. Most of the biochemically important ones are esters and/or anhydrides of various phosphorus containing acids with complex organic alcohols and organic acids. Strict application of the "D Rules" (ref 1) to such compounds would result, in many cases, in rather complicated names, and these would be inconvenient for most biochemists and biologists to use.
However, other systems of nomenclature, in use in the biochemical literature, are available (ref 2-5). It is the purpose of this document to define and recommend certain of these for naming organic phosphorus-containing compounds in biochemical, biological, and medical publications.
A general summary and explanation of the principles involved in the nomenclature of biochemically important organic phosphorus compounds is given below. Representative compounds and their recommended names, together with those derived from more systematic nomenclature (ref 4, 6, 7), including names formed according to the "D Rules" (ref 1) where appropriate, are listed in the tables.
1. Phosphoric esters, RO-PO(OH)2, are named as O-substituted phosphoric acids or as substituted alcohols (Table 1). Thus, choline O-(dihydrogen phosphate) and O-phosphonocholine are both appropriate names. The latter may be contracted to phosphocholine, but not changed to phosphorylcholine; "phosphoryl" is defined (ref 1, Rule 5.66) as 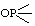 and requires, if used, the naming of all three groups attached to the phosphorus atom. However, "phosphoryl" is retained in derived terms such as the names of enzymes (e.g., phosphorylase) or of processes (e.g., phosphorylation).
and requires, if used, the naming of all three groups attached to the phosphorus atom. However, "phosphoryl" is retained in derived terms such as the names of enzymes (e.g., phosphorylase) or of processes (e.g., phosphorylation).
Comment. The form O-phosphono-R stems from two considerations, (i) the definition (ref 1, Rule 5.51) of phosphonic acid as HPO(OH)2, and (ii) the principle in organic nomenclature of substitution of another atom or group for a hydrogen atom of a parent molecule, which, in this case, involves the replacement of the H of an OH group by P(O)(OH)2, the phosphono group (ref 1, Rule 5.52).
2. Phosphate may be used for "(dihydrogen phosphate), "(disodium phosphate)," etc., (a) if the nature of the counterions is not known or is of no importance in the context, or (b) if a mixture of ionic forms (free acid,and/or monoanion and/or dianion) is in question. Thus, in most biochemical or biological systems, where the pH is around 7, "glucose phosphate" may be used in place of "glucose dihydrogen phosphate," the proper names for the protonated form.
Comments. (i) Although glucose phosphate is an ester, the term "phosphate ester" should not be used: "phosphoric ester" is the appropriate generic term. (ii) When "phosphoric" is followed by a generic term (e.g., ester, amide, group), the word "acid" need not intervene. Hence, "phosphoric ester" is complete and sufficient, and the residue transferred to glucose to form O-phosphonoglucose (see Section 1 above) is a "phosphoric residue." (iii) To distinguish choline phosphate (ester) from choline phosphate (salt), the former could he written "choline O-phosphate." However, "phosphocholine" is unambiguous. (For N-phosphono compounds, see Section 6.)
3. Phosphoric anhydrides are of two types, (a) those in which two or more phosphoric residues are linked by oxygen atoms to yield diphosphates, triphosphates, etc. (e.g., ADP, ATP, etc.; Table 2) and (b) those in which phosphoric acid forms a "mixed anhydride" with a different type of acid (generally a carboxylic acid, e.g., acetic acid) (Table 6). The latter are named (ref 1, Rule 5.64) as "R-ic phosphoric anhydrides" or as "R-yl phosphates" (e.g., acetic phosphoric anhydride or acetyl phosphate).
Comments. (i). "Pyro" should not be used for the substituted phosphoric anhydrides (ref 6, Rule 4.12) (Table 2), but may be retained in such terms as inorganic pyrophosphate (ref 6, Rule 5.213), pyrophosphatase, pyrophosphateglycerol transferase, and pyrophosphorolysis (ref 2). (Compare Section 1 above re "phosphoryl".)
(ii) The prefixes di, tri, tetra, etc. should not be used to indicate two or more independent phosphoric residues substituted on different oxygen (or other) atoms in a single compound; the appropriate multiplying prefixes for such compounds are bis, tris, tetrakis, etc. (ref 1, Rule 5.51; ref 6, Rules 2.251 and 4.12). For example, "fructose 1,6-diphosphate" could indicate a diphospboric residue bridging positions 1 and 6 of fructose; the common biochemical substance is correctly named fructose 1,6-bis(phosphate) (ref 3, Rule 4.4).
4. Phosphodiesters (Tables 3 and 4), which involve the bridging group -PO(OH)-, could be named in terms of phosphinic acid, H2PO(OH), for which the prefix form is phosphinico (ref 1). However, the use of this prefix, as in ref 2, presents complications in placing the locants for unsymmetrical diesters. Hence, phosphinico is contracted to phospho, which is used as an infix between the names of the two alcohols. Thus, glycerophosphocholine is recommended (ref 5) for the well-known substance [previously (ref 8), but incorrectly, called glycerophosphorylcholine; compare Section 1 above]. This recommendation also illustrates the convention by which glycerol phosphate is contracted to glycerophosphate (ref 8), but this should not be done in a context where "glycero" may he confused with the residue of glyceric acid, as in glycerolactone, or with the prefix glycero used in carbohydrate nomenclature (ref 4, Rule 9). (For the placement of locants, see examples in Tables 1 and 4.)
Comments. (i) The use of "phosphoryl" in this situation requires an indication in the name that there is one hydroxyl group remaining on the phosphoric residue, and would thus further lengthen the name (see Section 1 above).
(ii) The diacyl derivatives of glycerophosphoeholine are commonly expressed as derivatives of phosphatidic acid (Table 4), i.e., diacylglycerophosphocholine phosphatidylcholine (ref 8),
(iii) The trivial names for the acid radicals of nucleotides (Table 3) include the phosphoric residue, hence the latter is not specified in oligo- or polynucleotide names, e.g., adenylylcytidine suffices for Ado-P-Cyd (locants omitted for clarity; cf. ref 9).
(iv) The so-called cyclic phosphates (Table 3), of which adenosine 3',5'-phosphate (cyclic AMP or cAMP) is the best known example, are named in this form rather than in an inverted form, which would yield 3',5'-phosphoadenosine. The word "cyclic," often added before "phosphate," is unnecessary if the locants are given.
(v) The infix "phospho" gives precedent for "diphospho," "triphospho," "tetraphospho," etc. for the doubly esterified oligophosphoric acids (Table 2), e.g., uridinediphosphoglucose, adenosinediphosphoribose.
5. Nucleoside Triphosphate Analogs, in which a methylene group (-CH2-), an imido group (-NH-), or a sulfur atom replaces an oxygen atom bridging two phosphorus atoms, could be named by an extension of the convention of inorganic nomenclature (ref 6, Rule 4.15) that employs μ to indicate a bridging group. Thus the compound symbolized as Ado(5')P[CH2]PP could he named adenosine 5'-(1,2-μ-methylene)triphosphate, and Ado(5')PP[CH2]P might be named adenosine 5'-(2,3-μ-methylene)triphosphate. However, for the "methylene" part of these names, [α,β-methylene] and [β,γ-methylene] are unambiguous, are consistent with the use of Greek letters as locants in other situations (ref 7), and are therefore recommended (see Table 8). (The latter compound can be termed 5'-adenylyl methylenediphosphonate, but this name does not contain the significant term "triphosphate".)
Comments. (i) The use of square brackets here is similar to their use in amino-acid replacement (ref 11), indicating a replacement of the normal constituent.
(ii) Although the bridging methylene group in the Ado(5')PP[CH2]P example should receive priority for numbering, i.e., should be 1,2-μ-methylene to accord with inorganic nomenclature (ref 6, Rule 4.15), this would require an additional term (as in Table 8, column 2); it is therefore not suitable in this context, in which it is desirable to give adenosine first consideration (i.e., it is always considered to be linked to the α phosphorus atom).
(iii) A terminal substitution (e.g., sulfur replacing oxygen on P3) might he named adenosine 5'-[3-thio]triphosphate, but adenosine 5'-[γ-thio]triphosphate is recommended (see ii above).
(iv) The rules of inorganic nomenclature (ref 6, Table 11) specify "imido" as the ligand name for -NH-; it is, in this case, an imide of phosphoric acid, hence "imido" is recommended for biochemical use with "triphosphate" or "diphosphate" (see Table 8).
(v) The symbol for the nucleoside does not include the 5'-oxygen atom when the rest of the formula is written out in extenso. Thus, Ado(5')P[CH2]P Ado(5')-O-PO(OH)-CH2-PO3H2. Such extended representation may be useful for analogs such as Ado(5')-CH2-PO3H2, a methylene analog of AMP, and Ado(5')-O-PO(OH)-CH2-AsO3H2.
6. Phosphoric amides (Table 5) are named by changing "acid" in the original acid name to "amide" (ref 1, Rule 5.62). However, when the nitrogenous group supplying the amide moiety is known by a trivial name and that name is to be retained, the phosphoric amide may be named in the same manner as the esters (see Section 1 above), but not in the form in which "phosphate" is used as a suffix (see Section 2 above); e.g., phosphocreatine (for N-phosphonocreatine), but not "creatine phosphate," because the term "phosphate" means that all atoms attached to the phosphorus atom are oxygen atoms.
Comment. The contraction "phosphoamide" for phosphoric amide is often seen, but becomes unwieldy when either the amide or the phosphoric residue is substituted. Such compounds should be named as derivatives of phosphoramidic acid (or of a phosphoramidate), or of amidophosphoric acid (amidophosphate) [ref 1, Rules 5.0(e), 5.53(a, b), and 5.61(a, b)].
7. Fluorophosphoric acids, when doubly esterified, become fluorophosphates or phosphorofluoridates [ref 1, Rules 5.53(a, b), 5.61(a, b), and 5.0(e)]. Thus, the well-known compound (PriO)2PO-F or iPr2P-F (ref 4) may be called diisopropyl fluorophosphate or diisopropyl phosphorofluoridate (see Table 6).
1. Nomenclature of Organic Chomistry, Section D, IUPAC Information Bulletin, Appendix 31, August 1973, pp. 60-86. [Incorporated into 1979 recommendations.]
2. Enzyme Nomenclature (1972), (Elsevier Scientific Publishing Company, Amsterdam and New York), [Supplement 1: Biochem. Biophys. Acta 429, 1-45 (1976)]. [Now as 1992 edition.]
3. Abbreviations and Symbols for Chemical Names of Special Interest in Biological Chemistry (1965 Tentative Rules); Biochem. J. 101, 1-7 (1966); Biochemistry 5, 1445-1453 (1966); and elsewhere.
4. Nomenclature of Carbohydrates (1969), Biochemistry 10, 3983-4004 (1971); Eur. J. Biochem. 21, 455-477 (1971); and elsewhere. [Now as 1996 recommendations.]
5. Nomenclature of Lipids (1976), Hoppe-Seyler's Z. Physiol. Chem., in press; Lipids 12, 455-468 (1977).
6. IUPAC Nomenclature of Inorganic Chemistry, (1970) (Butterworths, London) [Now as 1990 recommendations.]
7. IUPAC Nomenclature of Organic Chemistry, Sections A, B, C (1971) (Butterworths, London). [Incorporated into 1979 recommendations.]
8. The Nomenclature of Lipids (1967), J. Biol. Chem. 242, 4845-4849 (1967); and 245, 1511 (1970); and elsewhere. Revision (1976), in press (ref 5).
9. Abbreviations and Symbols for Nucleic Acids, Polynucleotides, and their Constituents (1970), Arch. Biochem. Biophys. 145, 425-436 (1971); Eur. J. Biochem. 15, 203-208 (1970); and elsewhere.
10. Symbols for Amino-Acid Derivatives and Peptides (Recommendations 1971), Biochim. Biophys. Acta 263, 205-212 (1972); J. Biol. Chem. 247, 977-983 (1972); and elsewhere. [Now incorporated into 1983 recommendations.]
11. Rules for Naming Synthetic Modifications of Natural Peptides (Tentative Rules 1966, amended 1972), Biochemistry 6, 362-364 (1975); and elsewhere. [Now incorporated into 1983 recommendations.]
12. Nomenclature of α-Amino Acids (Recommendations 1974), Biochemistry 14, 449-462 (1975), Biochem. J. 149, 1-16 (1975), and elsewhere. [Now incorporated into 1983 recommendations.]
| Names recommended for biochemical usagea | |||||
| Phosphate names | O-Phosphono/phospho names | Systematic names | Abbreviations a,b | Structure | |
| 1. | D-Ribose 5-phosphate | 5-O-Phosphono-D-ribose; 5-phospho-D-ribose | D-Ribofuranose 5-(dihydrogen phosphate) | ribose-5-P; Rib5P | 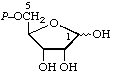 |
| 2. | α-D-Ribose 1-phosphate; α-D-ribosyl phosphatec | 1-O-Phosphono-α-D-ribose; 1-phospho-α-D-ribose | α-D-Ribofuranose 1-(dihydrogen phosphate); α-D-ribofuranosyl dihydrogen phosphatec | ribose-1-P; Rib1P |  |
| 3. | Adenosine 5'-phosphate; 5'-adenylic acidd | 5'-O-Phosphonoadenosine; 5'-phosphoadenosine | Adenosine 5'-(dihydrogen phosphate) | denosine-5'P; Ado5'P; PAdo; 5'AMP | 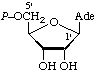 |
| 4. | 2-Aminoethyl phosphate; 2-aminoethanol O-phosphate; 2-aminoethanol phosphate (ester) | 2-Amino-O-phosphonoethanol; phosphoethanolaminee | 2-Aminoethyl dihydrogen phosphate; 2-aminoethanol (dihydrogen phosphate) (ester); 2-aminoethanol O-(dihydrogen phosphate) | P-ethanolamine | 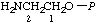 |
| 5. | 2-Hydroxy-2-propenoate phosphate (ester) | O-Phosphono-enol-pyruvate; phosphoenolpyruvatee | 2-(Phosphonooxy)-2- -propenoate; 2-hydroxy-2-propenoate (dihydrogen phosphate) (ester) | P-enolpyruvatef | 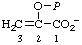 |
| 6. | D-Fructose 1,6-bis(phosphate) | 1,6-Di-O-phosphono-D-fructose; 1,6-bis(phospho)-D-fructose | D-Fructofuranose 1,6-bis(dihydrogen phosphate) | fructose-1,6-P2; Fru(1,6)P2 | 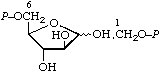 |
| 7. | myo-Inositol hexakis(phosphate)g | Hexa-O-phosphono-myo-inositol; hexakis(phospho)-myo-inositol | myo-Inositol hexakis(dihydrogen phosphate) | P6-inositol | 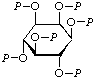 |
| 8. | sn-Glycerol 1-phosphateh; sn-glycero-1-phosphate | 1-O-Phosphono-sn-glycerol; 1-phospho-sn-glycerol | (S)-[2,3-Dihydroxypropyl dihydrogen phosphate]; (S)-[glycerol 1-(dihydrogen phosphate)] | sn-glycerol-1-P | 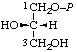 |
| 9. | sn-Glycerol 3-phosphatei; sn-glycero-3-phosphate | 3-O-Phosphono-sn-glycerol; 3-phospho-sn-glycerol | (R)-[2,3-Dihydroxypropyl dihydrogen phosphate]; (R)-[glycerol 1-(dihydrogen phosphate)] | sn-glycerol-3-P | 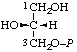 |
| 10. | sn-Glycerol 1,2-bis(phosphate)j; sn-glycero-1,2- -bis(phosphate) | 1,2-Di-O-phosphono-sn-glycerol; 1,2-bis(phospho)-sn-glycerol | (S)-[(Hydroxymethyl)ethylene bis(dihydrogen phosphate)]; (S)-[glycerol 1,2-bis(dihydrogen phosphate)]; (S)-[2,3-bis(phosphonooxy)-1- -propanol] | sn-glycerol-1,2-P2 | 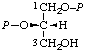 |
| 11. | sn-Glycerol 2,3-bis(phosphate)k; sn-glycero-2,3- -bis(phosphate) | 2,3-Di-O-phosphono-sn-glycerol; 2,3-bis(phospho)-sn-glycerol | (R)-[(Hydroxymethyl)ethylene bis(dihydrogen phosphate)]; (R)-[glycerol 1,2-bis(dihydrogen phosphate)]; (R)-[2,3-bis(phosphonooxy)-1- -propanol] | sn-glycerol-2,3-P2 | 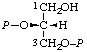 |
| 12. | D-Glyceraldehyde 3-phosphate | 3-O-Phosphono-D-glyceraldehyde; 3-phospho-D-glyceraldehyde | 2-Hydroxy-3-oxopropyl dihydrogen phosphate; 2-formyl-2-hydroxyethyl dihydrogen phosphate; D-glyceraldehyde 3-(dihydrogen phosphate) | D-glyceraldehyde-3-P | 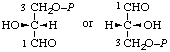 |
| 13. | Glycerone phosphatel; 1,3-dihydroxyacetone phosphate | 1-O-Phosphonoglycerone; 1-phosphoglyceronel | 3-Hydroxy-2-oxopropyl dihydrogen phosphate; 1-hydroxy-3-(phosphonooxy)-2- -propanone | glycerone-P1; dihydroxyacetone-P | 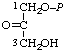 |
| 14. | D-Glycerate 3-phosphate | 3-O-Phosphono-D-glycerate; 3-phospho-D-gIycerate | (R)-[2-Hydroxy-3- -(phosphonooxy)- propanoate]; (R)-[2,3-dihydroxypropanoate 3-(dihydrogen phosphate)] | D-glycerate-3-P | 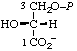 |
| 15. | D-Glycerate 2,3-bis(phosphate)m | 2,3-Di-O-phosphono-D-glycerate; 2,3-bis(phospho)-D-glyceratem | (R)-[2,3-Bis(phosphonooxy)- propanoate]; (R)-[2,3-dihydroxypropanoate 2,3-bis(dihydrogen phosphate)] | D-glycerate-2,3-P2 | 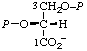 |
| 16. | (D-Glyceroyl phosphate) 3-phosphaten | 3-O-Phosphono-D-glyceroyl phosphate; 3-phospho-D-glyceroyl phosphate; 3-O-phosphono-D-glyceric phosphoric monoanhydride; 3-phospho-D-glyceric phosphoric monoanhydride | 2-Hydroxy-3-(phosphonooxy)- propionyl dihydrogen phosphate; 2,3-dihydroxypropionyl dihydrogen phosphate 3-(dihydrogen phosphate); 2-hydroxy-3-(phosphonooxy)- propionic phosphoric monoanhydride | D-glyceric-1,3-P2 | 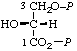 |
a See Section 2 in the text. Stereospecific numbering denoted by sn is defined in ref 8. If fully defined in a paper, sn and D may be omitted from biochemical names and abbreviations.
b The symbol P represents a phosphoric residue and may precede or follow the organic residue. Approved symbols, such as Rib, Ado, Fru, etc. (ref 3, 9, 10) are used to represent the organic residues. The symbols Ins, for inositol, and Gro, Gra, Grn, Gri, for glycerol, glyceraldehyde, glycerone (see footnote 1, below), and glyceric acid, respectively, are defined in ref 5.
c Locants are not needed because the glycosyl radical, by definition, is formed at the hemiacetal position (ref 4).
d Nucleotide trivial name.
e Most commonly used name.
f "Pyruvenol" with the symbol ePrv has been suggested for "enolpyruvate."
g Phytic acid.
h Previously named either L-glycerol 1-phosphate or D-glycerol 3-phosphate (ref 5, 8). Originally named L-glycerol 1-phosphate or D-α-glycerophosphoric acid (ref 3).
i Previously named L-glycerol 3-phosphate or D-glycerol 1-phosphate (ref 5, 8). Originally named D-glycerol 1-phosphate or L-α-glycerophosphoric acid (ref 3).
j Previously named L-glycerol 1,2-bis(phosphate) or D-glycerol 2,3-bis(phosphate).
k Previously named D-glycerol 1,2-bis(phosphate) or L-glycerol 2,3-bis(phosphate).
l The name "glycerone" has been proposed for 1,3-dihydroxyacetone (ref 5).
m Often called "diphosphoglycerate"; "bis" is correct (see Section 3).
n One phosphoric residue is an ester; the other is an anhydride. Glyceroyl is the acyl radical derived from glyceric acid (ref 7, Rule C-411.1).