Fig. 1
https://iupac.qmul.ac.uk/misc/quinone.html
World Wide Web version Prepared by G. P. Moss
School of Physical and Chemical Sciences, Queen Mary University of London,
Mile End Road, London,
E1 4NS, UK
g.p.moss@qmul.ac.uk
These Rules are as close as possible to the published version [see Arch. Biochem. Biophys., 1974, 165, 1-5; Biochem. J., 1975, 147, 15-21; Biochim. Biophys. Acta 1975, 387, 397-401; Eur. J. Biochem., 1975, 53, 15-18; Pure Appl. Chem., 1974, 38, 439-447; Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pages 222-225. Copyright IUPAC and IUBMB; reproduced with the permission of IUPAC and IUBMB]. If you need to cite these rules please quote these references as their source. Footnotes in the published version are presented here as notes under the paragraph where they apply. A PDF of the printed version is available.
At its meeting in April 1961, the IUPAC Commission for.the Nomenclature of Biological Chemistry appointed a Subcommittee, consisting of K. Folkers, D. E. Green, O. Isler, C. Martius, R. A. Morton and E. C. Slater, to report on the standardization of the nomenclature of the quinones with an isoprenoid side-chain. The Subcommittee met once in April 1963, and otherwise carried out its activities by correspondence. In June 1964, it reported to the IUPAC-IUB Commission on Biochemical Nomenclature, which adopted the report, with minor modifications, at its meeting in September 1964 and published it as Tentative Rules in 1965. (See IUPAC Information Bulletin No. 25, February 1966, page 24, also in Arch. Biochem. Biophys., 1967, 118, 507-511; Biochem. J., 1967, 102, 17-19; Biochim. Biophys. Acta, 1965, 107, 5-10; Eur. J. Biochem., 1967, 2, 3-5; J. Biol. Chem., 1966, 241, 2989-2991.)
The present Recommendations differ from the 1964 Tentative Rules principally in the deletion of the alternative Proposal II (see 3.3.1) from the latter.
Contents
1. Designation.of Length of Side-Chains
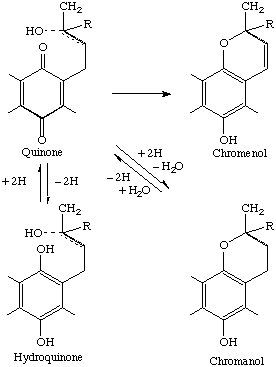
An inspection of the chemical structures involved (Table 3) indicates that there are three types of quinone nuclei (1,4-naphthoquinone, methyl-substituted 1,4-benzoquinone, methyl- and methoxy-substituted 1,4-benzoquinone) and two types of side-chains (phytyl or derived phytyl, multi-isoprenyl). On reduction, the quinones yield the corresponding hydroquinones, and each of these has an isomer formed by ring closure; these are known as chromenols and chromanols, respectively. The interrelationships are shown in Fig. 1.

Fig. 1
These Recommendations are concerned solely with the naturally occurring compounds of these types and with those compounds formed (e.g. by cyclization) from them, with respect to (a) designation of the length of the isoprenoid side-chains, (b) trivial names for each group of quinones and (c) designation of individual members of each group.
Recommendations
1. Designation.of Length of Side-Chains
1.1. The number of isoprene units, not the number of carbon atoms, is chosen as the basis of side-chain designation.
1.2. An isoprene unit is designated 'prenyl'. The hexahydrotetraprenyl side-chain (cf. Table 3) is termed 'phytyl'.
Note 1 'Prenyl' is 3-methyl-2-butenyl (IUPAC Rule A-3.5).1.3. The oxygen-containing ring of a chromenol or chromanol incorporates three of the five carbon atoms of the first prenyl of the corresponding (non-cyclized) quinone or hydroquinone. The number of intact prenyls to he designated is therefore reduced by one to reflect the actual number of prenyls remaining.Note 2 'Phytyl' is (E)-(7R,11R)-3,7,11,15-tetramethyl-2-hexadecenyl (IUPAC Rule A-75.1).
2. Trivial Name for Groups of Quinones
2.1. Because it is against IUPAC practice to designate chemical compounds by terms such as vitamin X or coenzyme X, and because the cyclized forms of the quinones and hydroquinones cannot easily be designated in such terms, the trivial names vitamin E-2, vitamins K-1 and K-2 and coenzyme Q are replaced by appropriate chemical names.
2.2. Because it is desirable to retain links with the older names, the chemical names or abbreviations recommended have been selected with that in mind.
2.3. The group names shown in Table 1 are recommended.
| Quinoid nucleus | Side-chain | Class name | Abbreviation |
| 2,3,5-Trimethylbenzoquinone | Multiprenyl | Tocoquinone | |
| 2,3-Dimethylbenzoquinone | Multiprenyl | Plastoquinone | PQ |
| 2,3-Dimethoxy-5-methylbenzoquinone | Multiprenyl | Ubiquinone | Q |
| 2-Methylnaphthoquinone | Multiprenyl | Menaquinone a | MK |
| 2-Methylnaphthoquinone | Phytyl | Phylloquinone | K |
a It is realized that menaquinone has sometimes been used to designate the parent quinone, 2-methyl-1,4-naphthoquinone (menadione).2.4. The corresponding hydroquinones, chromenols and chromanols are named by replacing the suffix 'quinone' with 'quinol', 'chromenol' and 'chromanol', respectively; e.g. ubiquinone becomes ubiquinol, ubichromenol and ubichromanol.Note Although by IUPAC Rules A-54 and C-71, governing assemblies of identical cyclic units, the prefixes ter-, quater-, . . . novi- and deci- might seem more appropriate, the traditional terms tri-, tetra-, . . . nona- and deca- are used for the multiprenyl side-chains. Because of the single locant preceding the side-chain term, this designation is unlikely to be mistaken for multiple substitution by independent prenyl groups. See also notes to 1.2
2.5. It is realized that some confusion may arise from the fact that 6-(3-hydroxy-3,7,11,15-tetramethylhexadecanyl)-2,3,5-trimethyl-1,4-benzoquinone, the quinone form of α-tocopherol, has been referred to as α-tocoquinone in the literature. Usually, however, the name of α-tocopherylquinone has been used. It is recommended that this compound be named α-tocopherolquinone. Similarly, the analogous compounds formed by oxidation of β-, γ- and δ-tocopherol should be named β-, γ- and δ-tocopherolquinone, respectively.
2.6. A reduced and cyclized tocopherolquinone, a chromanol, is a tocopherol. The tocopherols may also be regarded as methylated tocols (tocol is 2-methylbenzopyran-6-ol or 2-methyl-6-chromanol). When the side-chain is triprenyl rather than substituted phytyl, such chromanols are termed tocotrienols, the triene referring to the three double bonds in the side-chain (see 3.1). The oxidation of these to the 1,4-benzoquinone form yields tocotrienolquinones.
2.7. The abbreviations for tocopherol, tocopherolquinone, tocotrienol and tocotrienolquinone are, respectively, T, TQ, T-3 and TQ-3. Each is prefixed with the appropriate Greek letter or numerals; thus, for example:
| α-Tocopherol | α-Τ |
| β-Tocopherolquinone | β-TQ |
| 7,8-Dimethyltocotrienol | 7,8-T-3 (or PQ-3-al; cf. 3.2 and Table 3) |
| 2,3,5-Trimethyltocotrienolquinone | 2,3,5-TQ-3 |
Note The Greek-letter prefixes for the tocopherols are defined in the CBN 1965 reference given above and in a 1973 revision of that document [Arch. Biochem. Biophys., 1974, 165, 6-8; Biochem. J., 1975, 147, 11-14; Eur. J. Biochem., 1974, 46, 217-219; IUPAC Information Bulletin No. 47, 1975]. The latter includes the stereochemical description and designation of the tocopherols; it also recommends against the use of Greek letters in the names and abbreviations of the tocotrienols and their quinones. [See also revised version of Nomenclature of Tocopherols and Related Compounds published 1981]2.8. The hydroquinones may be abbreviated by the addition of H2 to the abbreviation of the quinone. If an abbreviation of a chromanol or chromenol is required, it is suggested that the suffixes al and el, respectively, be added to the abbreviation of the quinone (e.g. PQ-3-al for plastochromanol-3).
3. Designation of Individual Members of Each Group
3.1. In its earlier deliberations, the Commission recognized that there were two schools of thought concerning the designation of the individual members of each group of quinones. One school considered that the links with vitamin E, coenzyme Q and vitamin K are retained sufficiently by the name tocoquinone and by the abbreviations Q and K (or MK), and proposed that the different members should be designated -quinone-n (abbreviation X-n), where n is the number of intact isoprene units in the side-chain. The other school considered it necessary to retain E, Q and K as a part of the name and to designate the number of isoprene units by the lower subscript n, as was the practice in the coenzyme Q series.
The Commission favours the first proposal, summarized in Table 2. In many cases n may be omitted from the abbreviation, especially in chemical equations.
| Name | Abbreviation |
| Tocoquinone-n | |
| Plastoquinone-n | PQ-n |
| Ubiquinone-n | Q-n |
| Menaquinone-n | Mk-n |
| Phylloquinone | K |
3.2. According to these recommendations, 5,7,8-trimethyltocotrienol, also known as α-tocotrienol and ξ1-tocopherol, may be designated tocochromanol-3. It is recommended that 5,7,8-trimethyltocotrienol be used when the relationship with tocols and tocoenols (with vitamin E activity) is relevant, and tocochromanol-3 when the relationship with the quinones with isoprenoid side-chains (K and Q series) is more important. Similarly, 7,8-dimethyltocotrienol, also known as γ-tocotrienol and η-tocopherol, may be designated plastochromanol-3.
3.3. Saturation of one or more (but not all) of the double bonds in a multiprenyl side-chain should be indicated in the following way:
a) The isoprene units are designated by Roman numerals (I, II, III, etc.) starting from the quinone or chroman nucleus.Summary and Examplesb) Additional hydrogen atoms are indicated by the prefixes dihydro, tetrahydro, hexahydro, etc., with the Roman numerals of the units that are reduced, e.g. II-dihydro ... ; I, II; III-hexahydro ....
c) These abbreviations may be abbreviated if necessary (e.g. in tables) to II-H2; I, II, III-H6, etc.
Note. Arabic numerals should not be employed for the units, because such numerals are generally employed for individual atoms.
The recommended trivial names (in boldface) and abbreviations for some naturally occurring quinones of the vitamins E and K and coenzyme Q series and related compounds are displayed in Table 3. Older and superseded names, marked by a dagger, are listed for convenience; their use is not recommended.
Table 3. Summary of chemical relationships and nomenclature of some biologically active quinones with isoprenoid side-chains, including vitamins E and K and coenzyme Q. Recommended names are in boldface type. In the published version this table was in two columns with details of the cyclised form opposite the quinone it is derived from. Here, to assist viewing, the cyclised form is placed after the corresponding quinone.
| Aromatic nucleus | Side-chain | Trivial name(s) | Abbrevs. |
| 1,4-Naphthoquinone 2-methyl *Menaquinone (Menadione) | 3-(prenyl)n | Menaquinone-n *Prenylmenaquinone-n | MK-n *MQ-n |
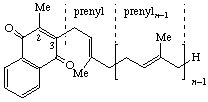 | |||
| 2H-Naphtho[1,2-b]pyran-6-ol, (2,5-substituted) | Menachromenol-(n-1) | MK-n-el | |
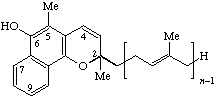 | |||
| 1,4-Naphthoquinone 2-methyl | 3-Phytyl | Phylloquinone *Phytylmenaquinone | K *PMQ |
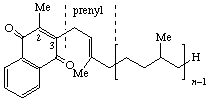 | |||
| 2H-Naphtho[1,2-b]pyran-6-ol, (2,5-substituted) | Phyllochromenol | K-el | |
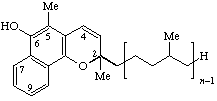 | |||
| 1,4-Benzoquinone, 2,3-dimethoxy-5-methyl- | 6-(prenyl)n | Ubiquinone-n n =10: coenzyme Q10 # | Q-n Q-10 Q-6 |
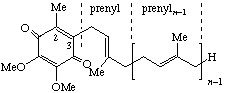 | |||
| 7,8-dimethoxy-2H-chromen-6-ol (2,5-substituted) | Ubichromenol-(n-1) n = 10: ubichromenol.9 | Q-n-el | |
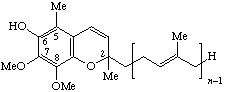 | |||
| 1,4-Benzoquinone, (2,3,5-trimethyl-) | 6-(prenyl)n | Tocoquinone-n n = 10: vitamin E2(50) # | |
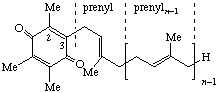 | |||
| 2H-chromen-6-ol, 2,5,7,8-tetramethyl | Tocochromanol-(n-1) | ||
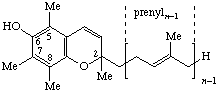 | |||
| 1,4-Benzoquinone, 2,3-dimethyl- | 6-(prenyl)n | Plastoquinone-n n = 9: plastoquinone # | PQ-n PQ-9 PQ-8-el |
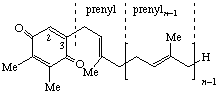 | |||
| 2H-chromen-6-ol, 2,7,8-trimethyl | Plastochromanol-(n-1) Plastochromanol-3 | PQ-n-al PQ-3-al | |
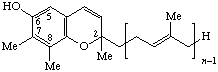 | |||
| 1,4-Benzoquinone, (2,3,5-substituted) | 6-'phytyl' | x-Tocopherolquinone(s) x Ra Rb Rc α Me Me Me (oxidized vit. E) | x-TQ |
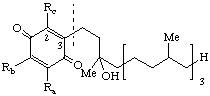 | |||
| Chroman-6-ol (Benzopyran-6-ol) (2,5,7,8-substituted) | x-Tocopherol(s) Tocol: Ra = Rb = Rc = H α (Ra = Rb = Rc = Me) | x-T | |
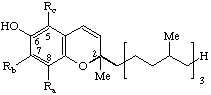 | |||
| 1,4-Benzoquinone, (2,3,5-substituted) | 6-'tetraprenyl' | x-Tocotrienolquinone(s) x = 2,3,5-trimethyl- | x-T-3 2,3,5-TQ-3 2,5-TQ-3 2,3-TQ-3 |
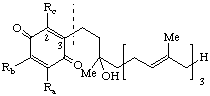 | |||
| Chroman-6-ol (Benzopyran-6-ol) (2,5,7,8-substituted) | x-Tocotrienol(s) x = 5,7,8-trimethyl | x-T-3 5,7,8-T-3
5,8-T-3 7,8-T-3 | |
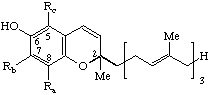 | |||
# Names previously used (see para. 3 of Introduction on changes from the provisional version.), not recommended.