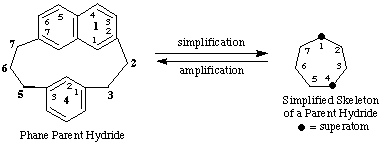
Phane nomenclature is a new method for building names for organic structures by assembling names that describe component parts of a complex structure (ref. 1). It is based on the idea that a relatively simple skeleton for a parent hydride structure can be modified by an operation called 'amplification', a process that replaces one or more special atoms (superatoms) of the simplified skeleton by multiatomic structure(s).
Examples:

Simplified skeletal name: cycloheptaphane
Phane parent hydride name: 1(2,7)-naphthalena-4(1,3)-benzenacycloheptaphane
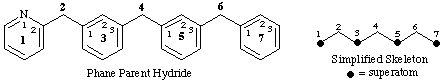
Simplified skeletal name: heptaphaneThe multiatomic structure is a fully saturated or mancude ring or ring system. A mancude ring or ring systems contains the maximum number of noncumulative double bonds (ref. 2). In the amplification operation each superatom is replaced by an amplificant denoted by an 'amplification prefix' attached to a stem called a 'simplified skeletal name'. The latter ends with the term 'phane' and is formed according to the principles for deriving names of saturated hydrocarbons. Accordingly, all of the atoms implied by the skeletal name, except for those replaced by amplification prefixes are, by convention, saturated carbon atoms. An amplification prefix is derived from the name of the corresponding cyclic parent hydride by the addition of the terminal letter 'a' with elision of a terminal vowel of the parent hydride name, if present (ref. 1a). Phane prefixes thus resemble the prefixes, such as 'oxa', 'aza', etc., that indicate replacement of a single atom, usually a carbon atom, by a different atom.Phane parent hydride name: 1(2)-pyridina-3,5(1,3),7(1)-tribenzenaheptaphane
The locants in front of the parentheses in the phane parent hydride name identify the positions of the superatoms in the simplified skeleton that are replaced by the ring structure specified by the amplification prefix immediately following. By the same token, they also identify the positions of the rings and ring systems in the phane parent hydride. These locants are determined by the inherent numbering of the simplified skeleton and the seniority of the rings and ring systems in the phane parent hydride. The locants within the parentheses specify the atoms of the ring structure specified by the amplification prefixes that are linked to the adjacent normal atoms of the simplified parent skeleton.
In addition to the basic principles, rules and conventions of Phane Nomenclature, Part I (ref. 1) contains the fundamental methodology for numbering phane parent hydrides and the application of skeletal replacement ('a') nomenclature for naming heterophane parent hydrides.
Part II of Phane Nomenclature describes derivatives of phane systems formed by substitutive nomenclature (ref. 3a). As parent hydrides, phane systems are totally integrated in substitutive nomenclature and therefore follow the general rules of this type of nomenclature. When required, other types of nomenclature are used to name derivatives, for example, functional class nomenclature for naming esters (ref. 3b), anhydrides (ref. 3c) and acid halides (ref. 3d).
PhII-1.1. Starting point and direction of numbering.
Insofar as the general rules of substitutive nomenclature leave a choice, the starting point and direction of numbering of a compound to be named by phane nomenclature are chosen so that lowest locants are given to the following structural features, if present, considered successively in the order listed until a decision is reached (ref. 3e).
1. numbering of phane parent hydride
2. heteroatoms introduced by skeletal replacement ('a') nomenclature
3. indicated hydrogen
4. nondetachable 'hydro-'/'dehydro-' prefixes
5. principal characteristic group (named as suffix)
6. unsaturation ('-ene'/'-yne' endings and 'hydro-'/'dehydro-' prefixes)
7. substituents named as prefixes (alphabetized substituents)
PhII-1.2. 'Hydro-' and 'dehydro-' prefixes.
In these recommendations, 'hydro-' and 'dehydro-' prefixes are classified as nondetachable (ref. 3f). Since nondetachable hydro prefixes define specific parent hydrides, they take precedence for low locants over substituent groups, but not over indicated hydrogen. 'Hydro-' and 'dehydro-' prefixes are cited in names immediately in front of the name of the phane parent hydride, or in front of skeletal replacement ('a') prefixes, when present.
When there is a choice, the established procedure for lowest locants is applied. The lowest locant set (ref. 3g) is the one that has the lowest numerical value at the first point of difference when the sets are compared term by term in order of increasing alphanumerical value.
PhII-1.4. Termination of acyclic phane parent hydrides.
Acyclic phane parent hydrides are, by definition, terminated by an amplificant at each end of the acyclic phane system. Alkyl substituents on these terminal amplificants do not extend the phane system beyond the terminal amplificants. Names are constructed in conformity with Section PhI-3.3 (ref. 1b).
Examples:
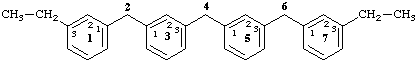
13,73-diethyl-1,7(1),3,5(1,3)-tetrabenzenaheptaphane
and not
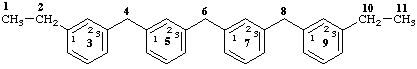
3,5,7,9(1,3)-tetrabenzenaundecaphane
PhII-2. Specification of indicated hydrogen
Indicated hydrogen, when present in an amplificant, is placed before the phane parent hydride name and preceded by appropriate locants (ref. 3h).
Examples:
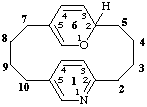
62H-1(2,5)-pyridina-6(2,5)-pyranacyclodecaphane
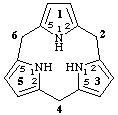
11H,31H,51H-1,3,5(2,5)tripyrrolacyclohexaphane
PhII-3. Substituent groups derived from phane parent hydrides
Names of substituent groups derived from phane parent hydrides are formed in accordance with the general methods (ref. 3i). The suffixes '-yl', and '-ylidene' are added to the name of the phane parent hydride preceded by appropriate locants. Low locants are assigned to these suffixes in accordance with the fixed numbering of the phane parent hydride or phane parent hydrides modified by skeletal replacement ('a') nomenclature. If a choice is possible, low locants are assigned to the suffix '-yl'. In names, the suffix '-yl' precedes '-ylidene'.
Examples:
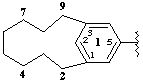
1(1,3)-benzenacyclononaphan-15-yl
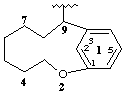
2-oxa-1(1,3)-benzenacyclononaphan-9-yl
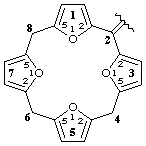
1,3,5,7(2,5)-tetrafuranacyclooctaphan-2-ylidene
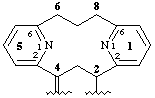
1,5(2,6)-dipyridinacyclooctaphan-2-yl-4-ylidene
PhII-3.2. The 'added hydrogen' method
The 'added hydrogen' method is applied when '-ylidene' free valences are attached to a mancude ring or ring system (ref. 3h). The method called 'added hydrogen' describes hydrogen atoms added to a specific structure as a consequence of the addition of a suffix describing a structural modification. 'Added hydrogen' is cited in parentheses after the locant describing the suffix. This type of substituent group may also be named by using nondetachable 'hydro-' prefixes (ref. 3f).
Example:
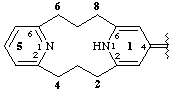
1,5(2,6)-dipyridinacyclooctaphan-14(11H)-ylidene
('added hydrogen' method)
or
11,14-dihydro-1,5(2,6)-dipyridinacyclooctaphan-14-ylidene
(nondetachable 'hydro'- prefix method)
1. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry."Phane Nomenclature, Part I: Phane Parent Names (IUPAC Recommendations 1998)" Pure Appl. Chem. 70, 1513-1545 (1998). (a) PhI-2.2, pp. 1522-1523; (b) PhI-3.3, pp. 1534-1536. (See also IUPAC chemical nomenclature database https://iupac.qmul.ac.uk/phane/).
2. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry/Commission on Physical Organic Chemistry. "Glossary of Class Names of Organic Compounds and Reactive Intermediates Based on Structure" Pure Appl. Chem. 67, 1307-1375 (1995) (See also IUPAC chemical nomenclature database https://iupac.qmul.ac.uk/class/).
3. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry. A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, R. Panico, W. H. Powell and J.-C. Richer, eds. Blackwell Scientific Publications, Oxford, 1993. (a) R-4.1, pp. 68-72; (b) R-5.7.4.2, pp. 117-119; (c) R-5.7.7, pp. 123-125; (d) R-5.7.6, pp. 122-123; (e) R-4.1, p. 72; (f) R-0.1.8, pp. 10-12; (g) R-0.2.4.2, p. 17; (h) R-1.3, pp. 34-35; (i) R-2.5, pp. 56-58. (See also http://www.acdlabs.com/iupac/nomenclature/).
Return to IUPAC nomenclature home page
Return to Phane nomenclature part II home page