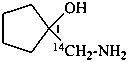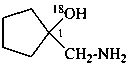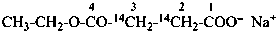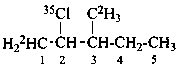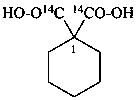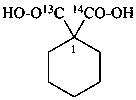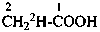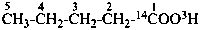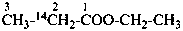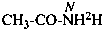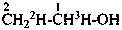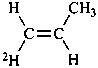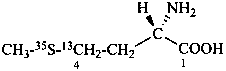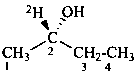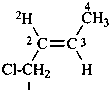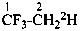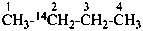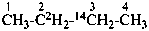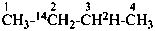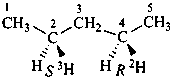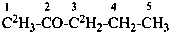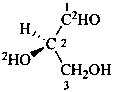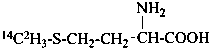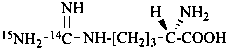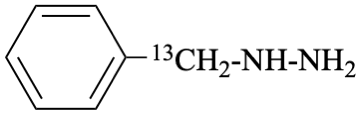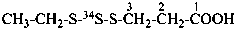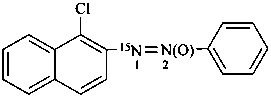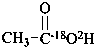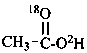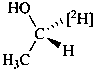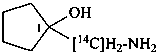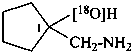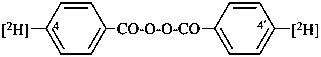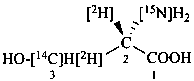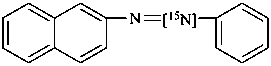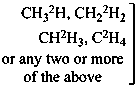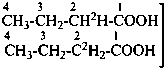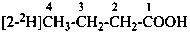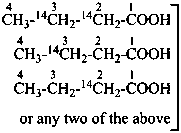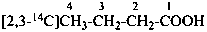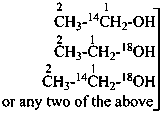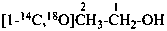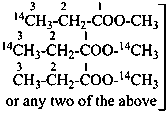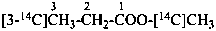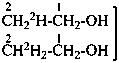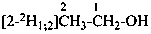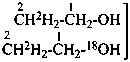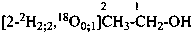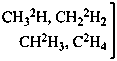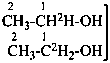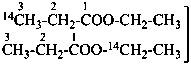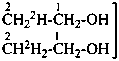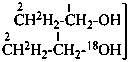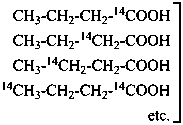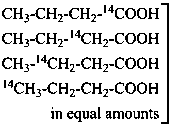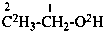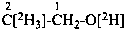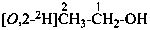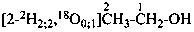International Union of Pure and Applied Chemistry
Division VIII Chemical Nomenclature and Structure Representation Division
Nomenclature of Organic Chemistry. IUPAC Recommendations and Preferred Names 2013.
Prepared for publication by Henri A. Favre and Warren H. Powell, Royal Society of Chemistry, ISBN 978-0-85404-182-4
Chapter P-8 ISOTOPICALLY MODIFIED COMPOUNDS
P-80 Introduction
P-81 Symbols and definitions
P-82 Isotopically substituted compounds
P-83 Isotopically labeled compounds
P-84 Comparative examples of formulas and names of isotopically modified compounds
P-80 INTRODUCTION
This Section describes a general system of nomenclature for organic compounds whose isotopic nuclide composition (refs. 12, 14 and 22) deviates from that occurring in nature (for a discussion of the meaning of ‘natural composition’ see ref. 29). Examples of isotopic nuclides for hydrogen are given in Table 8.1, below. This Chapter is derived from Section H of the 1979 Recommendations (ref. 1) and Chapter R-8 in the l993 Recommendations (ref. 2). The present Recommendations replace both those of 1979 and 1993. A brief discussion of common biochemical practices can be found in ref. 30.
There is one other system in use for describing isotopically modified compounds. It is based on an extension of the principles proposed by Boughton (see ref. 31) for designating compounds containing hydrogen isotopes and is used mainly in the Chemical Abstracts Service Index Nomenclature system (see ref. 32) for describing isotopic substitution and not isotopic labeling.
The system codified in these recommendations provides for recognition of various types of isotopic modification and thus was chosen in preference to the system based on the Boughton principles which deals only with isotopically substituted compounds.
P-81 SYMBOLS AND DEFINITIONS
P-81.1 NUCLIDE SYMBOLS
The symbol for denoting a nuclide in the formula and name of an isotopically modified compound consists of the atomic symbol for the element and an arabic numeral in the left superscript position indicating the mass number of the nuclide (see IR-3.2, ref.12).
P-81.2 ATOMIC SYMBOLS
The atomic symbols used in the nuclide symbol are those given in IUPAC Nomenclature of Inorganic Chemistry (see ref. 12). In the nuclide symbol, the atomic symbol is printed in roman type, italicized atomic symbols being reserved for letter locants, as is customary in the nomenclature of organic compounds and described in P-14.3.
For the hydrogen isotopes protium, deuterium, and tritium, the nuclides symbols 1H, 2H, and 3H, are used. The symbols D and T for 2H and 3H, respectively, are used, but not when other modifying nuclides are present, because this may cause difficulties in alphabetic ordering of the nuclide symbols in the isotopic descriptor.
Although the symbols d and t have been used and are still used in place of 2H and 3H in names formed according to the Boughton system (see ref 31), in no other cases are only lower-case letters used as atomic symbols. Therefore, the use of d and t in chemical nomenclature outside of the Boughton system is not recommended.
P-81.3 NAMES FOR HYDROGEN ATOMS AND IONS (see refs. 30, 33)
Table 8.1 Names of the hydrogen atoms and ions
|
| | 1H | 2H | 3H | natural
composition |
|
| atom | H | protium | deuterium | tritium | hydrogen |
| anion | H– | protide | deuteride | tritide | hydride |
| cation | H+ | proton | deuteron | triton | hydron |
|
P-81.4 ISOTOPICALLY UNMODIFIED COMPOUNDS
An isotopically unmodified compound has a macroscopic composition such that its constituent nuclides are present in the proportions occurring in nature. Its formula and name are written in the customary manner.
Examples:
CH4
methane
CH3-CH2-OH
ethanol
P-81.5 ISOTOPICALLY MODIFIED COMPOUNDS
An isotopically modified compound has a macroscopic composition such that the isotopic ratio of nuclides for at least one element deviates measurably from that occurring in nature. Isotopically modified compounds may be classified as:
(1) isotopically substituted compounds: or
(2) isotopically labeled compounds.
P-82 ISOTOPICALLY SUBSTITUTED COMPOUNDS
P-82.0 Introduction
P-82.1 Structures
P-82.2 Names
P-82.3 Order of nuclide symbols
P-82.4 Stereoisomeric isotopically substituted compounds
P-82.5 Numbering
P-82.6 Locants
P-82.0 INTRODUCTION
An isotopically substituted compound has a composition such that essentially all the molecules of the compound have only the indicated nuclide at each designated position. For all other positions, the absence of nuclide indication means that the nuclide composition is the natural one.
P-82.1 STRUCTURES
The structure of an isotopically substituted compound is written in the usual way except that appropriate nuclides symbols are used. When different isotopes of the same element are present in the same position, their symbols are written in order of increasing mass number.
Examples:
14CH4
12CHCl3
CH3-CH2H-OH
(not CH3-C2HH-OH)
P-82.2 NAMES
P-82.2.1 The name of an isotopically substituted compound is formed by adding or inserting the nuclide symbol(s) enclosed in parentheses, preceded by any necessary locant(s), letters, and/or numerals, before the part of the compound that is isotopically substituted. Immediately after the parentheses there is neither space nor hyphen, except that when the name, or a part of a name, includes a preceding locant, a hyphen is inserted. When two or more nuclide symbols appear at the same place in the name they are cited first in alphabetical order and then by their mass number, if necessary (see P-82.3)
When polysubstitution at a single position is possible, the number of atoms substituted is always specified as a right subscript to the atomic symbol(s), even in case of monosubstitution.
For polysubstitution with isotopically modified and unmodified atoms or groups, see P-82.2.2. For compounds modified by hydro prefixes, see P-82.2.3.
Examples:
14CH4
(14C)methane (PIN)
12CHCl3
trichloro(12C)methane (PIN)
(12C)chloroform
CH32H
(2H1)methane (PIN)
C2H2Cl2
dichloro(2H2)methane (PIN)
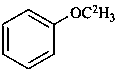
(2H3)methoxybenzene (PIN)
(α,α,α-2H3)anisole
C6H5-13CO-13CH3
1-phenyl(1,2-13C2)ethan-1-one (PIN)
(1,2-13C2)acetophenone
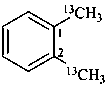
1,2-di[(13C)methyl]benzene (PIN)
(α,α′-13C2)-1,2-xylene
(α,α′-13C2)-o-xylene
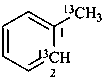
1-(13C)methyl(2-13C)benzene (PIN)
(α,2-13C2)-toluene
CH22H-CH2-OH
(2-2H1)ethan-1-ol (PIN)
13CH3-CH2-OH
(2-13C)ethan-1-ol (PIN)
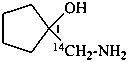
1-[amino(14C)methyl]cyclopentan-1-ol (PIN)
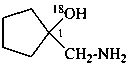
1-(aminomethyl)cyclopentan-1-(18O)ol (PIN)
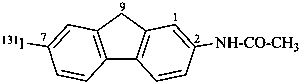
N-[7-(131I)iodo-9H-fluoren-2-yl]acetamide (PIN)
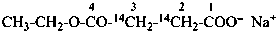
sodium 4-ethoxy-4-oxo(2,3-14C2)butanoate (PIN)
sodium ethyl (2,3-14C2)butanedioate
sodium ethyl (2,3-14C2)succinate
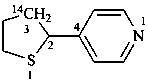
4-[(3-14C)thiolan-2-yl]pyridine (PIN)
4-[tetrahydro(3-14C)thiophen-2-yl]pyridine
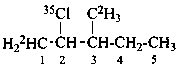
2-(35Cl)chloro-3-(2H3)methyl(1-2H1)pentane (PIN)
P-82.2.2 The name of an isotopically modified compound may differ from the name of the unmodified analogue when its structure contains identical units that are not identically modified in equivalent positions. Such different groups are expressed separately.
| Isotopically modified atoms or groups that are not identically modified in equivalent positions are expressed separately, which is a change from Section H of the 1979 Recommendations (ref. 1) and Chapter R-8 of the l993 Recommendations (ref. 2). |
|
P-82.2.2.1 Different isotopic modifications on otherwise identical substituents
When two substituent groups are isotopically modified in different ways so that they cannot be combined together using multiplicative terns such as ‘di-’, ‘bis-’, etc., they are cited separately. The isotopically modified substituent is preferred alphabetically to the unmodified substituent.
Examples:
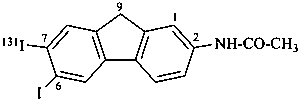
N-[7-(131I)iodo-6-iodo-9H-fluoren-2-yl]acetamide (PIN)
{not N-[6,7-(7-131I)diiodo-9H-fluoren-2-yl]acetamide}
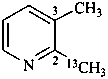
2-(13C)methyl-3-methylpyridine (PIN)
[not 2,3-(2-13C)dimethylpyridine]

2-(2,2-2H2)ethyl-3-ethylhexan-1-ol (PIN)
P-82.2.2.2 When two characteristic groups are isotopically modified in different ways so that they cannot be combined using multiplicative terms such as ‘di-’, ‘bis-’, etc., the isotopically modified characteristic group with the greater number of modifications is chosen as the principal characteristic group to be cited as a suffix; the other characteristic is then cited as a prefix. If a further choice is needed, the suffix with the nuclide of higher atomic number, then the nuclide of higher mass number, is chosen as the principal characteristic group to be cited as a suffix.
Examples:
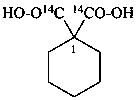
cyclohexane-1,1-di[(14C)carboxylic acid] (PIN)
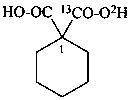
1-carboxycyclohexane-1-(13C,2H)carboxylic acid (PIN)
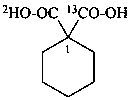
1-(2H)carboxycyclohexane-1-(13C)carboxylic acid (PIN)
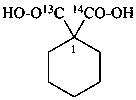
1-(13C)carboxycyclohexane-1-(14C)carboxylic acid (PIN)
P-82.2.3 Addition of hydro prefixes
Isotopically modified hydrogen atoms, when present, are always attached to the skeleton of the isotopically modified compound. According to P-82.2.2, the hydro prefixes must be
identical, whether unmodified or isotopically modified, and added in pairs. Isotopically modified or unmodified hydro prefixes are added as detachable substituent prefixes placed at the front of the parent hydride.
| In these recommendations hydrogenated mancude ring systems are treated as described in P-82.2.2 which is a change from previous recommendations. |
|
Examples:
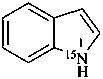
(15N)-1H-indole (PIN)
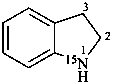
2,3-dihydro(15N)-1H-indole (PIN)
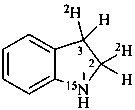
2,3-dihydro(2,3-2H2,15N)-1H-indole (PIN)
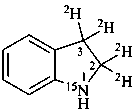
2,3-di[(2H)hydro](2,3-2H2,15N)-1H-indole (PIN)
[not (2,3-2H2)dihydro(2,3-2H2,1-15N)-1H-indole]
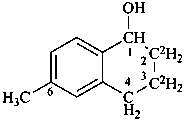
6-methyl-2,3-di[(2H)hydro]-1,4-dihydro(2,3-2H2)naphthalen-1-ol (PIN)
{not 6-methyl-1,4-dihydro-2,3-di[(2H)hydro](2,3-2H2)naphthalen-1-ol;
not 6-methyl[(2,3-2H2)-1,2,3,4-tetrahydro](2,3-2H2)naphthalen-1-ol;
these names do not conform to P-82.2.2}
P-82.2.4 In a name consisting of two or more words, the isotopic descriptor is placed before the appropriate word or part of the word that includes the nuclide(s), unless unambiguous locants are available or are unnecessary.
Examples:
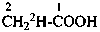
(2-2H1)acetic acid (PIN)
CH3-COO2H
(O-2H)acetic acid (PIN)
acetic (2H)acid
CH3-C18O-O2H
(O-2H,18O)acetic acid (PIN)
CH3-CO-18O2H
(18O-2H,18O)acetic acid (PIN)
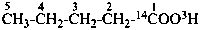
(1-14C)pentan(3H)oic acid (PIN)
H14COO– Na+
sodium (14C)formate (PIN)
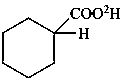
cyclohexane(2H)carboxylic acid (PIN)
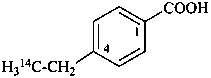
4-(2-14C)ethylbenzoic acid (PIN)

(1-14C)ethyl propanoate (PIN)
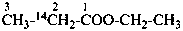
ethyl (2-14C)propanoate (PIN)
P-82.2.5 In a name consisting of one word, the isotopic descriptor is placed before the name, with an appropriate locant. This method is preferred to that of placing the descriptor before the implied name of the characteristic group.
Examples:
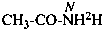
(N-2H1)acetamide (PIN) 
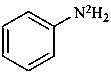
(N-2H2)aniline (PIN)
(N-2H2)benzenamine
P-82.3 ORDER OF NUCLIDE SYMBOLS
P-82.3.1 When isotopes of different elements are present as nuclides in an isotopically substituted compound, their symbols are arranged in alphabetical order if they are at the same place in the name.
Example:
CH318O2H
methan(2H,18O)ol (PIN)
P-82.3.2 When several isotopes of the same element are present as nuclides in an isotopically substituted compound, their symbols are arranged in the order of increasing mass number if they are inserted at the same place in the name.
Example:
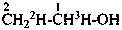
(2-2H1,1-3H1)ethan-1-ol (PIN)
P-82.4 STEREOISOMERIC ISOTOPICALLY SUBSTITUTED COMPOUNDS
Two types of stereoisomeric isotopically substituted compounds are possible:
(1) those in which the stereoisomerism results from isotopic modification;
(2) those whose analogous unmodified compounds are themselves stereoisomers.
The nomenclature for stereoisomers of isotopically substituted compounds follows the general methods described in Chapter P-9.
Stereodescriptors are cited at the specified place in the name according to the stereochemical rules. When they must be inserted into the name at the same place as isotopic descriptors, the stereodescriptors are cited first.
P-82.4.1 Examples in which stereoisomerism results from isotopic substitution
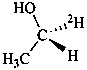
(1R)-(1-2H1)ethan-1-ol (PIN)
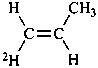
(1E)-(1-2H1)prop-1-ene (PIN)
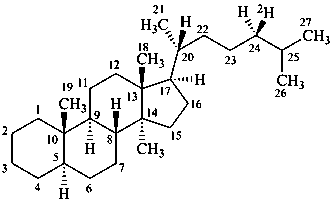
(24R)-5α-(24-2H1)cholestane
P-82.4.2 Examples of isotopically substituted stereoisomers
Stereochemical affixes (for example D and L) are added according to the rules of special classes, such as carbohydrates, amino acids, steroids, etc., described in Chapter P-10; they usually refer to the parent substance (or unmodified compound) according to the particular class of compounds. In these classes isotopic descriptors may follow the stereochemical descriptors according to biochemical usage (ref. 30).
Examples:

5α-(17-2H)pregnane
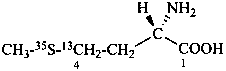
L-(4-13C,35S)methionine
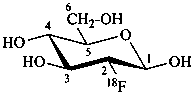
2-deoxy-2-(18F)fluoro-β-D-glucopyranose
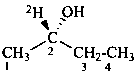
(2S)-(2-2H)butan-2-ol (PIN)
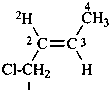
(2E)-1-chloro(2-2H)but-2-ene (PIN)
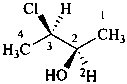
(2R,3R)-3-chloro(2-2H)butan-2-ol (PIN)
P-82.5 NUMBERING
P-82.5.1 Numbering in relation to the unmodified compound
Numbering of an isotopically substituted compound is not changed from that of an isotopically unmodified compound. Among the structural features of a compound to be considered successively for numbering as given by P-14.4, the presence of nuclides is considered last with the exception of chirality arising from isotopic modification.
Examples:
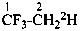
1,1,1-trifluoro(2-2H1)ethane (PIN)
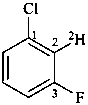
1-chloro-3-fluoro(2-2H)benzene (PIN)
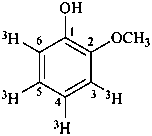
2-methoxy(3,4,5,6-3H4)phenol (PIN)
P-82.5.2 Priority between isotopically substituted and unmodified atoms or groups
When there is a choice between equivalent numberings in an isotopically unmodified compound, the starting point and the direction of numbering of the analogous isotopically substituted compound are chosen so as to give lowest locants to the modified atoms or groups considered together in one series in increasing numerical order. If a choice still remains, precedence for the lowest locants is given to the nuclide of higher atomic number. In the case of different nuclides of the same element, precedence is given to the nuclide of higher mass number.
Examples:
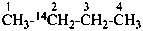
(2-14C)butane (PIN)
[not (3-14C)butane]
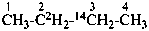
(3-14C,2,2-2H2)butane (PIN)
[not (2-14C,3,3-2H2)butane]
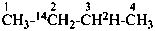
(2-14C,3-2H1)butane (PIN)
[not (3-14C,2-2H1)butane]
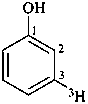
(3-3H)phenol (PIN)
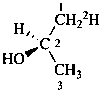
(2R)-(1-2H1)propan-2-ol (PIN)
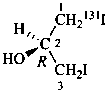
(2R)-(1-131I)iodo-3-iodopropan-2-ol (PIN)
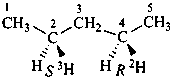
(2S,4R)-(4-2H1,2-3H1)pentane (PIN)
P-82.6 LOCANTS
P-82.6.1 Omission of locants (see also P-14.3.4)
P-82.6.1.1 In preferred IUPAC names, locants are omitted if no locants are necessary in unmodified names. However, if isotopic modification requires a locant to specify its position, then all locants must be specified and none are omitted.
Examples:
C2H3-CN
(2H3)acetonitrile (PIN)
(as in trichloroacetonitrile)
CH3-CH2-O2H
ethan(2H)ol (PIN)
(as in ethanol)
13CH3-CH2-OH
(2-13C)ethan-1-ol
[not (2-13C)ethanol]
CH22H-O-C2H2-S-CH2-OOH
{[(2H1)methoxy(2H2)methyl]sulfanyl}methaneperoxol (PIN)
P-82.6.1.2 Locants are omitted when there is only one atom of a given element.
Example:
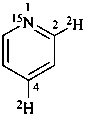
(2,4-2H2,15N)pyridine (PIN)
P-82.6.1.3 Locants are omitted in compounds or substituent groups in which all positions are completely isotopically substituted or modified in the same way.
Example:
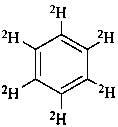
(2H6)benzene (PIN)
P-82.6.1.4 Locants are not omitted when there is a possibility of isomers
Example:
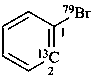
1-(79Br)bromo(2-13C)benzene (PIN)
P-82.6.2 Letter and/or numeral locants
When locants are needed for defining the structure of the parent structure or of a unit of structure as defined by its appropriate enclosing marks, then all locants must be cited for the parent structure or that structural unit. Specific positions of nuclides must be indicated in the isotopic descriptor by appropriate locants, letters, and/or numerals, preceding the nuclide symbol(s). In preferred names, all locants are placed before the nuclide that is multiplied.
Examples:
CH3-CH2H-O2H
(1-2H1)ethan-1-(2H)ol (PIN)
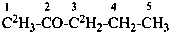
(1,1,1,3,3-2H5)pentan-2-one (PIN)
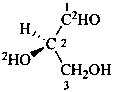
(2R)-2-(2H)hydroxy-3-hydroxy(1-2H)propanal (PIN)
D-(2-O,1-2H2)glyceraldehyde 
P-82.6.3 Location of nuclides on positions not normally denoted by locants
P-82.6.3.1 When a nuclide occupies a position that is not numbered, an italicized prefix or Greek letter may be used to denote its position.
Examples:
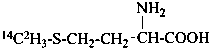
DL-[methyl-(14C,2H3)]methionine
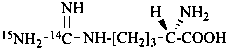
L-(carbamimidoyl-14C,N′-15N)arginine
L-(guanidino-14C,N′-15N)arginine (see Rule H-4.21, ref. 1)]
[not L-(amidino-14C,N′-15N)arginine]
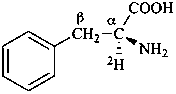
L-(α-2H)phenylalanine
P-82.6.3.2 When the nuclide is located at a position in a retained name that is not numbered a systematic name that identifies separately the relevant atom is used for the IUPAC preferred name. For general nomenclature an italicized prefixes or Greek letters may be used to denote its position.
When the nuclide is located at a position in a retained name that is not numbered a systematic name that identifies separately the relevant atom is used for the IUPAC preferred name. For general nomenclature an italicized prefixes or Greek letters may be used to denote its position.
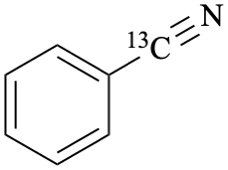
benzene(13C)carbonitrile (PIN)
(cyano-13C)benzonitrile
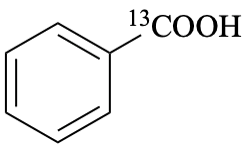
benzene(13C)carboxylic acid (PIN)
(carboxyl-13C)benzoic acid
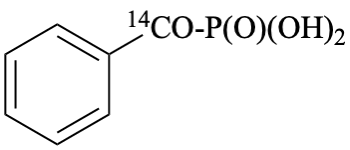
[benzene[14C]carbonyl]phosphonic acid (PIN)
[phenyl[14C]carbonyl]phosphonic acid
[carbonyl-14C]benzoylphosphonic acid
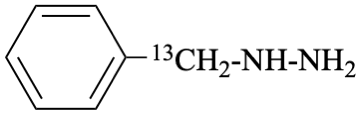
[phenyl(13C)methyl]hydrazine (PIN)
[(α-13C)benzyl]hydrazine
P-82.6.3.3 When a nuclide occupies a position that is not numbered or when its position cannot be easily defined according to rule P-82.6.3.1, the nuclide symbol is included in the entire symbol of the group through which it is linked to the main part of the structure. This rule is useful in general nomenclature in which many names are constructed without locants.
When a nuclide occupies a position that is not numbered or when its position cannot be easily defined according to rule P-82.6.3.1, the nuclide symbol is included in the entire symbol of the group through which it is linked to the main part of the structure. This rule is useful in general nomenclature in which many names are constructed without locants.
Examples:
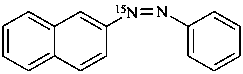
1-(naphthalen-2-yl)-2-phenyl(1-15N)diazene (PIN)
naphthalene-2-(15N=N)azobenzene
CH3-CH2-CH=15N-NH2
1-propylidene(1-15N)hydrazine (PIN)
propanal (15N-NH2)hydrazone
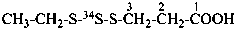
3-[ethyl(2-34S)trisulfanyl]propanoic acid (PIN)
3-[ethyl(S-34S-S)trithio]propionic acid (see Rule H-4.22. ref. 1)
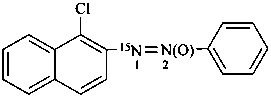
1-(1-chloronaphthalen-2-yl)-2-phenyl(1-15N)diazene 2-oxide (PIN)
1-chloro-2-(phenyl-ON15N-azoxy)naphthalene
P-82.6.4 Italicized nuclide symbols and/or italic capital letters are used to distinguish between different nuclides of the same element.
Examples:
CH3-CH2-CO-18O-CH2-CH3
18O-ethyl propan(18O1)oate (PIN)
CH3-CH2-C18O-O-CH2-CH3
O-ethyl propan(18O1)oate (PIN)
CH3-O-CO-18O-CH2-CH3
18O-ethyl O-methyl (18O1)carbonate (PIN)
CH3-CH2-O-C18O-18O-CH3
O-ethyl 18O-methyl (18O2)carbonate (PIN)
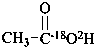
(18O-2H,18O)acetic acid (PIN)
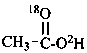
(O-2H,18O)acetic acid (PIN)
(the ‘18O’ is an isotopic descriptor and the ‘O’ is a locant)
P-83 ISOTOPICALLY LABELED COMPOUNDS
An isotopically labeled compound is a mixture of isotopically unmodified compound with one or more analogous isotopically substituted compound(s).
Although an isotopically labeled compound is really a mixture as far as chemical identity is concerned (in the same way as is an unmodified compound), for nomenclature purposes, such mixtures are called ‘isotopically labeled’ compounds.
P-83.1 Specifically labeled compounds
P-83.2 Selectively labeled compounds
P-83.3 Nonselectively labeled compounds
P-83.4 Isotopically deficient compounds
P-83.5 General and uniform labeling
P-83.1 SPECIFICALLY LABELED COMPOUNDS
An isotopically labeled compound is designated as ‘specifically labeled’ when a unique isotopically substituted compound is formally added to the analogous isotopically unmodified compound. In such a case, both position(s) and number of nuclides are defined.
Structures (see P-83.1.1) are identical to those given in Section P-82 for isotopically substituted compounds, except that brackets enclose the nuclide symbol.
Examples:
|
Isotopically
substituted
compound | when
added
to | Isotopically
unmodified
compound | gives
rise
to | Specifically
labeled
compound |
| 13CH4 | | CH4 | | [13C]H4 |
| CH22H2 | | CH4 | | CH2[2H2] |
|
P-83.1.1 Structures of specifically labeled compounds
The structural formula of a specifically labeled compound is written in the usual way, but with the appropriate nuclide symbol(s) and multiplying subscript enclosed in square brackets, [ ]. The structural formula is written in the same way as that of an isotopically substituted compound.
Although the formula for a specifically labeled compound does not represent the composition of the bulk material, which usually consists overwhelmingly of the isotopically unmodified compound, it does indicate the presence of the compound of chief interest, the isotopically substituted compound.
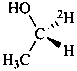
Examples:
Formulas of isotopically
substituted compounds | | Formulas of specifically
labeled compounds |
| |
|
| CH3-CO-NH2H | | CH3-CO-NH[2H] |
 | | 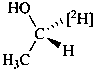 |
| C2H3-CO-C2H2-CH2-CH3 | | C[2H3]-CO-C[2H2]-CH2-CH3 |
A specifically labeled compound is:
(a) singly labeled when the isotopically substituted compound has only one isotopically modified atom;
Example:
CH3-CH[2H]-OH
(b) multiply labeled when the isotopically substituted compound has more than one modified atom of the same element at the same position or at different positions;
Examples:
CH3-C[2H2]-OH
CH2[2H]-CH[2H]-OH
(c) mixed labeled when the isotopically substituted compound has more than one kind of modified atom.
Example:
CH3-CH2-[18O][2H]
P-83.1.2 Names of specifically labeled compounds
P-83.1.2.1 The name of a specifically labeled compound is formed by adding or inserting in brackets, [ ], the nuclide symbol(s) preceded by any necessary locants before the denomination of that part of the compound that is isotopically modified. When polylabeling is possible, the number of atoms that have been labeled is always specified as a subscript to the atomic symbol(s) even in the case of monolabeling. This is necessary in order to distinguish between a specifically and a selectively or nonselectively labeled compound.
Examples:
[13C]H4
[13C]methane (PIN)
CH3[2H]
[2H1]methane (PIN)
C[2H2]Cl2
dichloro[2H2]methane (PIN)
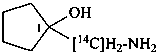
1-(amino[14C]methyl)cyclopentan-1-ol (PIN)
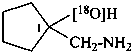
1-(aminomethyl)cyclopentan-1-[18O]ol (PIN)
P-83.1.2.2 All rules given in P-82 to construct names of isotopically substituted compounds are applicable to construct names of specifically labeled compounds, with the exception that the isotopic descriptor is placed in brackets and parentheses are used around complex prefixes. For examples of geminal dicarboxylic acids, see P-82.2.2.2.
Examples:
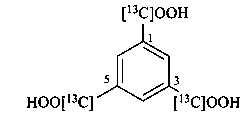
benzene-1,3,5-tri([13C]carboxylic acid) (PIN)
(not benzene-1,3,5-[1,3,5-13C3]tricarboxylic acid)
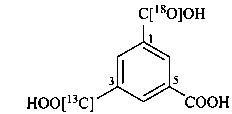
3-[13C]carboxy-5-carboxybenzene-1-[18O]carboxylic acid (PIN)
(not benzene-1,3,5-[3-13C,1-18O]tricarboxylic acid)
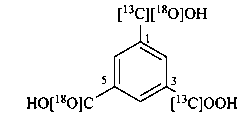
3-[13C]carboxy-5-[18O]carboxybenzene-1-[13C,18O]carboxylic acid (PIN)
(not benzene-1,3,5-[1,3-13C2,1,5-18O2]tricarboxylic acid)
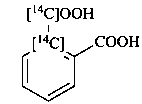
2-carboxy[1-14C]benzene-1-[14C]carboxylic acid (PIN)
(not [1-14C]benzene-1,2-[1-14C]dicarboxylic acid)
[1-14C]phthalic [1-14C]acid
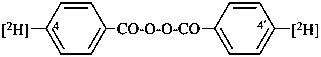
di([4-2H]benzoyl) peroxide
[4-2H]benzoic peroxyanhydride (PIN)
CH3-[14C]OOH
[1-14C]acetic acid (PIN; see P-82.2.4)
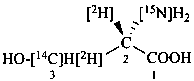
L-[3-14C,2,3-2H2,15N]serine (see P-82.4.2)
(2S)-2-[15N]amino-3-hydroxy[2,3-2H2,3-14C]propanoic acid
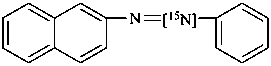
1-(naphthalen-2-yl)-2-phenyl[2-15N]diazene (PIN)
naphthalene-2-[N=15N]azobenzene (see P-82.6.3.3)
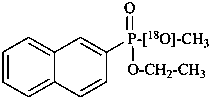
O-ethyl 18O-methyl (naphthalen-2-yl)[18O]phosphonate (PIN)  (see P-82.6.4)
(see P-82.6.4)
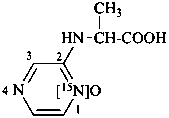
2-[(1-carboxyethyl)amino][1-15N]pyrazine 1-oxide
N-([1-15N]pyrazin-2-yl)alanine 15N-oxide (see P-82.6.4)
P-83.2 SELECTIVELY LABELED COMPOUNDS
An isotopically labeled compound is designated as selectively labeled when a mixture of isotopically substituted compounds is formally added to the analogous isotopically unmodified compound in such a way that the position(s) but not necessarily the number of each labeling nuclide is defined. A selectively labeled compound may be considered as a mixture of specifically labeled compounds. A selectively labeled compound may be:
(a) multiply labeled when in the unmodified compound there is more than one atom of the same element at the position where the isotopic modification occurs, for example H, in CH4; or there are several atoms of the same element at different positions where the isotopic modification occurs, for example C, in C4H8O;
(b) mixed labeled when there is more than one labeling nuclide in the compound, for example, C and O in CH3-CH2-OH.
When there is only one atom of an element that can be modified in a compound, only specific labeling can result.
P-83.2.1 Structures of selectively labeled compounds
P-83.2.1.1 A selectively labeled compound cannot be described by a unique structural formula; therefore it is represented by inserting the nuclide symbol(s) preceded by any necessary locant(s) (letters and/or numbers) but without multiplying subscripts, enclosed in square brackets, [ ], directly before the usual formula or, if necessary, before parts of the formula that have an independent numbering. Identical locants are not repeated. When different nuclides are present, the nuclide symbols are written in alphabetical order according to their symbols, or when the atomic symbols are identical, in order of increasing mass number.
Examples:
Mixture of
isotopically
substituted
compounds | when
added
to | Isotopically
unmodified
compound | gives
rise
to | Selectively
labeled
compound |
|
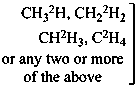 | | CH4 | | [2H]CH4 |
| |
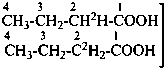 | | CH3-CH2-CH2-COOH | | 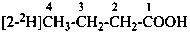 |
| |
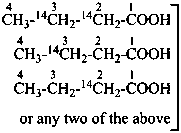 | | CH3-CH2-CH2-COOH | | 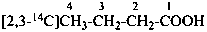 |
| |
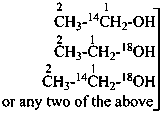 | | CH3-CH2-OH | | 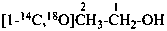 |
| |
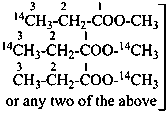 | | CH3-CH2-COO-CH3 | | 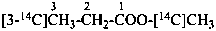 |
|
P-83.2.1.2 In a selectively labeled compound formally arising from mixing several known isotopically substituted compounds with the analogous isotopically unmodified compound, the number or the possible number of labeling nuclide(s) for each position may be indicated by subscripts to the atomic symbol(s). Two or more subscripts referring to the same nuclide symbol are separated by a semicolon. For a multiply labeled or mixed labeled compound, the subscripts are written successively in the same order as the various isotopically substituted compounds are considered. The subscript zero is used to indicate that one of the isotopically substituted compounds is not modified at the indicated position.
Examples:
Mixture of
isotopically
substituted
compound | when
added
to | Isotopically
unmodified
compound | gives
rise
to | Selectively
labeled
compound |
|
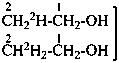 | | CH3-CH2-OH | | 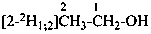 |
| |
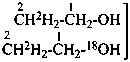 | | CH3-CH2-OH | | 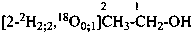 |
|
P-83.2.2 Names of selectively labeled compounds
The name of a selectively labeled compound is formed in the same way as the name of a specifically labeled compound, except that the multiplying subscripts following the atomic symbols are generally omitted. Identical locants corresponding to the same element are not repeated. The name of a selectively labeled compound differs from the name of the corresponding isotopically substituted compound in the use of square brackets, [ ], surrounding the nuclide descriptor rather than parentheses and in the omission of repeated identical locants and multiplying subscripts.
Examples:
Mixture of isotopically
substituted compounds | when added to | is named |
|
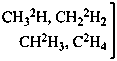 | CH4 | [2H]methane (PIN)
(not [2H4]methane) |
| |
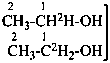 | CH3-CH2-OH | [1-2H]ethan-1-ol (PIN)
(not [1,1-2H2]ethan-1-ol) |
| |
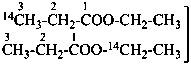 | CH3-CH2-COO-CH2-CH3 | [1-14C]ethyl [3-14C]propanoate (PIN) |
| |
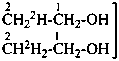 | CH3-CH2-OH | [2-2H1;2]ethan-1-ol (PIN) |
| |
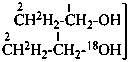 | CH3-CH2-OH | [2-2H2;2]ethan-1-[18O0;1]ol (PIN) |
|
P-83.3 NONSELECTIVELY LABELED COMPOUNDS
P-83.3.1 An isotopically labeled compound is designated as nonselectively labeled when the position(s) and the number of the labeling nuclide(s) are both undefined.
When only atoms of an element to be modified are present at the same position in a compound, only specific or selective labeling can result. Nonselective labeling requires that the element to be modified be at different positions in the structure. For example, CH4 and CCl3-CH2-CCl3 can only be specifically or selectively labeled with a hydrogen isotope.
P-83.3.2 Structures
Nonselective labeling is indicated in a formula by inserting the nuclide symbol, enclosed in brackets, directly before the line formula without locants or subscripts.
Example:
[13C]CH3-CH2-CH2-COOH
P-83.3.3 Names
The name of a nonselectively labeled compound is formed in the same way as the name of a selectively labeled compound but contains neither locants nor subscripts in the nuclide descriptor.
Examples:
chloro[2H]benzene (PIN)
[13C]propane-1,2,3-triol (PIN)
[13C]glycerol
P-83.4 ISOTOPICALLY DEFICIENT COMPOUNDS
P-83.4.1 An isotopically labeled compound may be designated as isotopically deficient when the isotopic content of one or more elements has (have) been depleted, i.e. when one or more nuclide(s) is(are) present in less than the natural ratio.
P-83.4.2 Structures
Isotope deficiency is denoted in the formula by adding the italicized symbol ‘def’ immediately preceding, and without a hyphen, the appropriate nuclide symbol.
Example:
[def13C]CHCl3
P-83.4.3 Names
The name of an isotopically deficient compound is formed by adding the italicized symbol def immediately preceding, and without a hyphen, the appropriate nuclide symbol, both enclosed in brackets and cited before the name or the part of the name that is isotopically modified.
Example:
trichloro[def13C]methane (PIN)
[def13C]chloroform
P-83.5 GENERAL AND UNIFORM LABELING
P-83.5.1 In the name of a selectively labeled compound in which all positions of the designated element are labeled, but not necessarily in the same isotopic ratio, the symbol ‘G’ is used in place of locants to indicate a ‘general’ labeling.
Examples:
Isotopically
substituted
compounds | when added to | is designated as |
|
mixture of substituted
compounds (selective labeling)
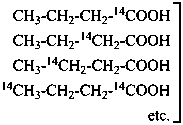 | CH3-CH2-CH2-COOH | [G-14C]butanoic acid (PIN) |
| |
D-Glucose in which all six positions are labeled with 14C, but not necessarily uniformly, is designated as
D-[G-14C]glucose.
P-83.5.2 In the name of a selectively labeled compound in which all positions of the designated element are labeled in the same isotopic ratio, the symbol ‘U’ is used in place of locants to denote ‘uniform’ labeling.
Examples:
Isotopically
substituted
compounds | when added to | is designated as |
|
mixture of substituted
compounds (uniform labeling)
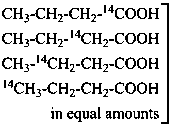 | CH3-CH2-CH2-COOH | [U-14C]butanoic acid (PIN) |
| |
D-Glucose in which 14C is equally distributed among the six positions is designated as
D-[U-14C]glucose.
Note: In the case of radioactive nuclides, ‘same isotopic ratio’ means ‘same specific radioactivity’.
P-83.5.3 In the name of a selectively labeled compound, the symbol ‘U’ (see P-83.5.2) followed by appropriate locants is similarly used to indicate labeling in the same isotopic ratio at the specified positions.
Example:
D-glucose in which 14C is equally distributed among positions 1, 3, and 5 is designated as
D-[U-1,3,5-14C]glucose.
P-84 COMPARATIVE EXAMPLES OF FORMULAS AND NAMES OF ISOTOPICALLY MODIFIED COMPOUNDS
| Types of compounds | Formula | Name |
|
| Unmodified | CH3-CH2-OH | ethanol (PIN) |
| Isotopically substituted | 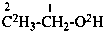 | (2,2,2-2H3)ethan-1-(2H)ol (PIN)
(O,2,2,2-2H4)ethan-1-ol |
| Specifically labeled | 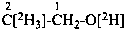 | [2,2,2-2H3]ethan-1-[2H]ol (PIN)
[O,2,2,2-2H4]ethan-1-ol |
| Selectively labeled | 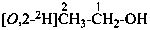 | [2-2H]ethan-1-[2H]ol (PIN) |
| 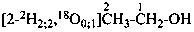 | [2-2H2;2]ethan-1-[18O0;1]ol (PIN) |
| Nonselectively labeled | [2H]CH3-CH2-OH | [2H]ethanol (PIN) |
| Isotopically deficient | [def13C]CH3-CH2-OH | [def13C]ethanol (PIN) |
Continued with P-9 Specification of Configuration and Conformation.
Return to:
IUPAC Chemical Nomenclature home page.
Blue Book home page.