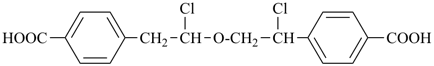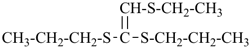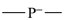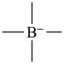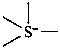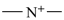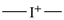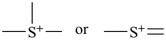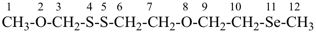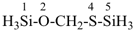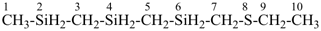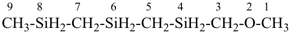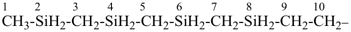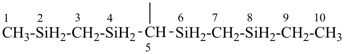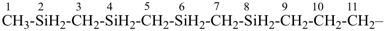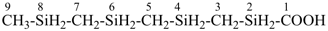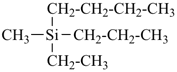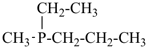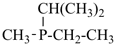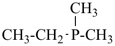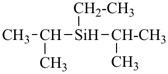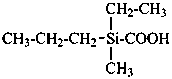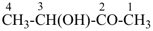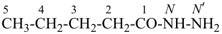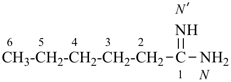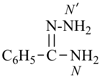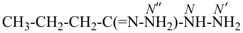International Union of Pure and Applied Chemistry
Division VIII Chemical Nomenclature and Structure Representation Division
Nomenclature of Organic Chemistry. IUPAC Recommendations and Preferred Names 2013.
Prepared for publication by Henri A. Favre and Warren H. Powell, Royal Society of Chemistry, ISBN 978-0-85404-182-4
Chapter P-1 GENERAL PRINCIPLES, RULES, AND CONVENTIONS.
| P-10 | Introduction |
| P-11 | Scope of nomenclature for organic compounds |
| P-12 | Preferred, Preselected, and Retained IUPAC Names |
| P-13 | Operations in Nomenclature of Organic Compounds |
| P-14 | General Rules |
| P-15 | Types of Nomenclature |
| P-16 | Name Writing |
P-10 INTRODUCTION
For nomenclature purposes, a structure containing at least one carbon atom is considered to be an organic compound and can be named using the principles of organic nomenclature, such as substitutive or replacement nomenclature, as described in this book.
The formation of a systematic name for an organic compound requires first selection and then naming of a parent structure. This basic name may then be modified by prefixes, infixes, and, in the case of a parent hydride, suffixes, which convey precisely the structural changes required to generate the compound in question from the parent structure. In contrast to such systematic names, there are traditional names which are widely used both in industry and academic circles. Examples are acetic acid, benzene, and pyridine. Therefore, when they meet the requirements of utility and when they fit into the general pattern of systematic nomenclature, these traditional names are retained.
A major new principle is elaborated in these Recommendations; the concept of ‘preferred IUPAC names’ (PINs) is developed and systematically applied. Up to now, the nomenclature developed and recommended by IUPAC has emphasized the generation of unambiguous names in accord with the historical development of the subject. In 1993, due to the explosion in the circulation of information and the globalization of human activities, it was deemed necessary to have a common language that would prove important in legal situations, with manifestations in patents, export-import regulations, environmental and health and safety information, etc. However, rather than recommend only a single ‘unique name’ for each structure, we have developed rules for assigning ‘preferred IUPAC names’, while continuing to allow alternative names in order to preserve the diversity and adaptability of the nomenclature to daily activities in chemistry and in science in general.
Thus, the existence of preferred IUPAC names does not prevent the use of other names to take into account a specific context or to emphasize structural features common to a series of compounds. Preferred IUPAC names (PINs) belong to a ‘preferred IUPAC nomenclature’. Any name other than a preferred IUPAC name, as long as it is unambiguous and follows the principles of the IUPAC recommendations herein, is acceptable as a ‘general IUPAC name’, in the context of a ‘general IUPAC nomenclature’.
The concept of preferred IUPAC names is developed as a contribution to the continuing evolution of the IUPAC nomenclature of organic compounds. This book (Recommendations 2013) covers and extends the principles, rules, and conventions described in two former publications: Nomenclature of Organic Chemistry, 1979 Edition (ref. 1) and A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993 (ref. 2). In a few instances, the 1979 rules and the 1993 recommendations have been modified to achieve consistency within the entire system. In case of divergence among various sets of recommendations, Recommendations 2013 prevail.
P-11 SCOPE OF NOMENCLATURE FOR ORGANIC COMPOUNDS
For nomenclature purposes we consider all compounds containing carbon as the principal element to be organic compounds as qualified above (see P-10). Oxygen, hydrogen, and nitrogen are the three elements usually associated with carbon to form the system of functional or characteristic groups. Other elements, among them the halogens and sulfur, complete the basic core of elements found in organic compounds. Substitutive nomenclature was first applied to compounds containing this set of atoms. The success of this type of nomenclature was such that it was extended to all elements of Groups 14, 15, 16, 17, and in Group 13, to boron; it could be extended to all elements of Group 13.
Table 1.1 Elements included in these recommendations
| Groups | 13 | 14 | 15 | 16 | 17 |
| B
boron | C
carbon | N
nitrogen | O
oxygen | F
fluorine |
| Al
aluminium | Si
silicon | P
phosphorus | S
sulfur | Cl
chlorine |
| Ga
gallium | Ge
germanium | As
arsenic | Se
selenium | Br
bromine |
| In
indium | Sn
tin | Sb
antimony | Te
tellurium | I
iodine |
| Tl
thallium | Pb
lead | Bi
bismuth | Po
polonium | At
astatine |
|
The elements Al, Ga, In, Tl have been added to the elements recommended in the 1979 edition (ref. 1) and the 1993 Guide (ref. 2) |
The ending ‘ane’, characteristic of alkanes, was borrowed from methane, ethane, etc., and attached to terms forming the roots of the names of the various elements, for example sulfane, H2S; phosphane, PH3; silane, SiH4; alumane, AlH3. The resulting names constitute the basis of substitutive nomenclature; this treatment of parent hydrides is called generalized ‘ane’ nomenclature because all the rules applicable to alkanes are applicable to all hydrides of the elements of Groups 13, 14, 15, 16, and 17. The nomenclature of the carbon hydrides may be conveniently termed ‘carbane nomenclature’; whereas the term ‘heterane nomenclature’ covers the hydrides of elements other than carbon. Names of mononuclear parent hydrides are listed in Table 2.1 in Chapter P-2.
Organometallic compounds, i.e., compounds in which one or more carbon atom(s) is (are) directly attached to a metal atom, have been regarded as organic compounds for nomenclature purposes. This association is maintained in these recommendations (see P-69), for the metals, semimetals, and nonmetals included in Groups 13, 14, 15, 16, and 17. However, the nomenclature for other organic derivatives of the elements in Groups 1 through 12 is considered as part of the nomenclature of inorganic compounds.
Likewise, IUPAC preferred names for polymers and IUPAC preferred names for Natural Products and related compounds are outside the scope of this book. The former is to be developed in conjunction with the Polymer Committee on Polymer Terminology and the latter in conjunction with the IUPAC-IUBMB Joint Commission on Biochemical Nomenclature
The construction of systematic names is based on general nomenclature operations and rules, and on operations and rules specific to different types of nomenclature. These aspects are discussed in the following sections.
P-12 PREFERRED, PRESELECTED, AND RETAINED IUPAC NAMES
P-12.1 Preferred IUPAC names
P-12.2 Preselected names
P-12.3 Retained names
P-12.4 Methodology
P-12.1 PREFERRED IUPAC NAMES
Preferred IUPAC names are names for structures or structural components that are preferred among two or more names for the same structure generated from two or more recommended IUPAC rules for organic compounds or the many synonyms that have been coined and used over the years.
Preferred IUPAC names, or PINs for short, are names selected according to the set of principles, conventions, and rules given herein. They originate from the strict application of the rules; in this sense, they can be referred to as ‘single names’. All preferred IUPAC names for organic compounds are identified by the parenthetical abbreviation ‘(PIN)’ following the name. Names used in the past, but now discarded or no longer recommended, are placed in parentheses and preceded by the word ‘not’. Names of organic compounds based on aluminium, gallium, indium, and thallium are not followed by the parenthetical abbreviation (PIN), because the decision to choose between a name based on organic or inorganic principles has not yet been reached.
It is necessary to select a preferred alternative in many instances in the construction of the names of organic compounds. Preferred IUPAC names are given to parent structures; characteristic groups denoted by prefixes and suffixes used in PINs are designated as preferred prefixes or suffixes. They also result from the choice to be made among the different types of nomenclature, for example, substitutive nomenclature, functional class nomenclature, and multiplicative nomenclature; and among the different types of operations, for example substitutive, additive, and subtractive.
Most commonly, a parent structure is a parent hydride, i.e., a structure containing, in addition to one or more hydrogen atoms, a single atom of an element, for example, methane; or a number of atoms (alike or different) linked together to form an unbranched chain, for example, pentane; or a monocyclic or polycyclic ring system, for example, cyclohexane and quinoline. Methane is a retained name (see P-12.3) that is preferred to the systematic name ‘carbane’, a name never recommended to replace methane, but used to derive the names ‘carbene’ and ‘carbyne’ for the radicals H2C2• and HC3•, respectively. Similarly, the retained names ‘ethane’, ‘propane’, and ‘butane’ were never replaced by systematic names ‘dicarbane’, tricarbane’, and ‘tetracarbane’ as recommended for analogues of silane, ‘disilane’; phosphane, ‘triphosphane’; and sulfane, ‘tetrasulfane’. The name ‘pentane’ is formed by application of P-21.2.1 and is marked as the preferred IUPAC name, or PIN, even though no rule has been cited giving an alternative name. The same reasoning applies to cyclohexane, an IUPAC name resulting from the application of P-22.1.1. The name ‘quinoline’ is a retained name that is preferred to the alternative systematic fusion names ‘1-benzopyridine’ or ‘benzo[b]pyridine’.
Examples:
CH4
methane (preferred IUPAC name or PIN, a retained name)
carbane
CH3-CH2-CH2-CH2-CH3
pentane (preferred IUPAC name or PIN)
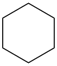
cyclohexane (PIN)
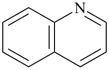
quinoline (PIN, a retained name, P-25.2.1)
1-benzopyridine (P-25.2.2.4)
benzo[b]pyridine (P-25.3.1.3)
(not 1-benzazine, see P-22.2.2.1.1)
It is sometimes convenient to employ parent hydrides of more complex structure, such as ring or ring-chain assemblies, for example biphenyl and styrene. The name ‘1,1′-biphenyl’ results from the application of Rule P-28.2.1; it is the preferred IUPAC name and the locants ‘1,1′’ are compulsory; the name ‘biphenyl’, without locants, can be used in general IUPAC nomenclature. The name ‘styrene’ is a retained name acceptable in general IUPAC nomenclature as being clear and unambiguous along with the substitutive name ‘vinylbenzene’. The name ‘ethenylbenzene’ is the preferred IUPAC name (PIN). 
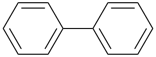
1,1′-biphenyl (PIN)
biphenyl
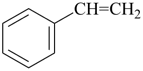
styrene (a retained name; P-31.1.3.4)
vinylbenzene
ethenylbenzene (PIN; P-31.1.3.4) 
A special class of parent structures having retained names (see P-12.3) is called functional parent compounds, for example, phenol and acetic acid. These two names are preferred IUPAC names; the corresponding systematic alternatives, benzenol and ethanoic acid, may be used in general IUPAC nomenclature. On the other hand, although acetone is a retained name recommended for general nomenclature, the preferred IUPAC name is the substitutive name propan-2-one.
Examples:
C6H5-OH
phenol (PIN)
benzenol
CH3-COOH
acetic acid (PIN)
ethanoic acid
CH3-CO-CH3
acetone
propan-2-one (PIN)
In order to generate the parent structure from a compound to be named, various formal operations must be carried out. For example, in naming the structure below, the parent hydride ‘pentane’ is formally derived by replacing the oxygen and chlorine atoms by the appropriate number of hydrogen atoms. When constructing the name, the formal operation is reversed; the suffix ‘one’ and the prefix ‘chloro’, indicating substitution of
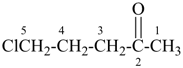
the hydrogen atoms of pentane, are attached to the name of the parent hydride to give the name ‘5-chloropentan-2-one’. Suffixes and prefixes can represent a number of different types of formal operations on the parent structure. Frequently, the suffix or prefix denotes the attachment of a characteristic group (functional group), for example, ‘one’ or ‘oxo’ for =O. A prefix may also describe a group derived from a parent hydride, for example ‘pentyl’, CH3-CH2-CH2-CH2-CH2–, from pentane.
The substitutive operation, described in P-13.1, is the operation used most extensively in organic nomenclature. Indeed, the comprehensive nomenclature system based largely on the application of this operation to parent structures is, for convenience, termed substitutive nomenclature, although this nomenclature also involves many of the other types of operations described in P-13. Substitutive nomenclature is the set of substitutive names, principles, conventions, and rules used for name construction. Examples of substitutive and other nomenclature operations are shown in Table 1.2
Table 1.2 Nomenclature operations
CH3-CH2-O-CH2-CH2-CH3
1 | CH3-CHCl-CH2-CO-CH3
2 | P(OCH3)3
3 |
4 | 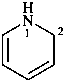
5 | CH3-O-CH2-CH2-O-CH2-CH2-O-CH2-CH2-O-CH2-CH3
6 |
7 | 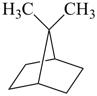
8 | 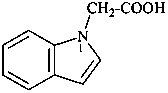
9 |
| Formula | Parent Structure
(class name) | Operation | Name | Reference |
| 1 | propane (PIN) (ether) | substitutive functional class | 1-ethoxypropane (PIN) ethyl propyl ether | P-13.1 P-13.3.3.2 |
| 2 | pentane (PIN) (ketone) | substitutive functional class | 4-chloropentan-2-one (PIN) 2-chloropropyl methyl ketone | P-13.1 P-13.3.3.2 |
| 3 | phosphane (preselected name) (phosphite) | substitutive functional class | trimethoxyphosphane trimethyl phosphite (PIN) | P-13.1 P-13.3.3.2 |
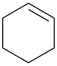
| 4 | cyclohexane (PIN) | substractive | cyclohexene (PIN) | P-31.1.3.1 |
| 5 | pyridine (PIN) | additive | 1,2-dihydropyridine (PIN) | P-31.2.3.1 |
| 6 | ethane (PIN) tridecane (PIN) | substitutive skeletal (‘a’) replacement | 1-ethoxy-2-[2-(2-methoxyethoxy)ethoxy]ethane 2,5,8,11-tetraoxatridecane (PIN) | P-13.1 P-13.2.1.1 |
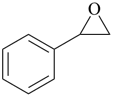
| 7 | oxirane (PIN) styrene + oxide | substitutive additive | phenyloxirane (PIN) styrene oxide | P-13.1 P-13.3.3.1 |
| 8 | bornane bicyclo[2.2.1]heptane (PIN) | subtractive substitutive | 10-norbornane 7,7-dimethylbicyclo[2.2.1]heptane (PIN) | P-13.4.3.2 P-13.1 |
| 9 | acetic acid acetic acid + indole | substitutive conjunctive | (1H-indol-1-yl)acetic acid (PIN) 1H-indole-1-acetic acid | P-13.1 P-13.5.2 |
Another type of nomenclature expresses the principal characteristic group not as a suffix but as a term denoting its functional class cited in a name as a separate word; in Table 1.2, the name ‘ethyl propyl ether’ is a typical functional class name based on the functional class name ‘ether’. The corresponding substitutive name ‘1-ethoxypropane’ is constructed by using the prefix ‘ethoxy’ and the parent hydride name ‘propane’.
Substitutive and functional class names are written differently. Generally, substitutive names are unitary names that combine prefixes, names of parent hydrides, endings, and suffixes in one word. In contrast, a functional class name is written as separate words [in English], even though the part describing the parent hydride or the modified parent hydride is the result of the same operations used to construct substitutive names.
The great majority, if not all, of organic compounds can be named in accordance with the principles of substitutive and functional class operations. However, in these recommendations, where there is a choice, names obtained by the substitutive operation are preferred IUPAC names. In Table 1.2, examples 1, 2, and 3 illustrate this preference. The substitutive names 1-ethoxypropane and 4-chloropentan-2-one are preferred to the functional class names based on the names of the corresponding class, ether and ketone, ethyl propyl ether and 2-chloropropyl methyl ketone, respectively. In contrast, a functional class name is preferred for the ester ‘trimethyl phosphite’ to the substitutive name trimethoxyphosphane. Esters, along with acid halides, anhydrides, and amine oxides, linked to a nitrogen atom are preferably named by using functional class nomenclature; substitutive nomenclature is less preferred for naming these compound classes.
Other types of operations are widely used, alone or along with substitutive nomenclature. There are two major types of replacement operations, the skeletal replacement operation (often referred to as skeletal replacement nomenclature or simply ‘a’ nomenclature) and functional replacement nomenclature. The former is used as a necessary complement in order to introduce heteroatoms into cyclic hydrocarbons and to avoid highly complex prefixes in names for acyclic systems. For example, the name ‘2,5,8,11-tetraoxatridecane’ formed by skeletal replacement is preferred to the substitutive name ‘1-ethoxy-2-[2-(2-methoxyethoxy)ethoxy]ethane’ (see Table 1.2, example 6). The latter is used to derive a very large number of suffixes and prefixes from basic oxygen names. Additive and subtractive operations have been extended for naming radicals and ions. They are the sole method for modification of the degree of hydrogenation, by adding or subtracting pairs of hydrogen atoms. Examples 4 and 5 illustrate this methodology. The conjunctive operation eliminates hydrogen atoms from two different parent structures and then combines them; this method is used to name parent hydrides composed of repeated identical units or to link rings and chains under specific conditions. Example 9 in Table 1.2 illustrates such an operation; in IUPAC nomenclature, however, a substitutive name is always preferred to a conjunctive name, for example ‘(1H-indol-1-yl)acetic acid’ is preferred to ‘1H-indole-1-acetic acid’ (see P-51.1.2).
A nomenclature embraces the major operations along with the principles, conventions, and rules necessary to construct names of a particular type. Substitutive nomenclature and functional class nomenclature have been discussed above. Replacement nomenclature and conjunctive nomenclature also require specific principles, conventions, and rules. In contrast, additive and subtractive operations do not correspond to nomenclatures in their own right, but are necessary complements to other nomenclatures.
It is very important to recognize that, in general, the rules of the nomenclature of organic compounds are written in terms of classical valence bonding. The principles and general rules for the nomenclature of organic compounds are described in this Chapter. Substitutive nomenclature is then elaborated in Chapter P-2 (parent hydride names), in Chapter P-3 (endings, suffixes, and prefixes), and in Chapter P-4 (selection rules for parent structures and unique names). Chapter P-5 describes selection rules for construction of preferred IUPAC names. In Chapter P-6 the naming of compounds arranged in classes and groups related to the Periodic Table (Groups 13-17) is described. In Chapter P-7, nomenclature for radicals, ions, and related species is discussed. Chapter P-8 describes isotopic modifications of organic compounds. Chapter P-9 deals with configuration and conformation specification and Chapter P-10 deals with natural products. Preferred IUPAC names (PINs) for the natural products in Chapter P-10 are not identified. Although most of the names are in fact generally accepted, there is a nebulous grey area where a distinction between a natural product name and a systematic name based solely on principles of organic nomenclature has not been defined. This likely will be the task of a future project consisting of organic and biochemical nomenclaturists.
Several topics discussed in these recommendations have been published since 1993 as fully comprehensive documents: radicals and ions (ref. 3), fused and bridged fused ring systems (ref. 4), phane nomenclature (refs. 5, 6), the von Baeyer system for polycyclic compounds (ref. 7), spiro compounds (ref. 8), natural products (ref. 9), and fullerenes (refs. 10, 11). They are not reproduced in extenso in these recommendations. Rather, the principles, conventions, and rules are discussed in a less extensive manner. Readers should use the full publications to deal with more complex cases; these publications are not superseded in these recommendations unless specifically noted in boxed comments. Again, all modifications to previously published recommendations made to achieve consistency are clearly signalled in these recommendations and prevail over any former rules or interpretations.
In this book, the label ‘PIN’ is added to the names of compounds whose parent hydride contains at least one of the following elements: B, Si, Ge, Sn, Pb, N, P, As, Sb, Bi, O, S, Se, Te, Po, F, Cl, Br, I, At, and that also contain at least one carbon atom in their structure and that can be named by substitutive nomenclature or one of its related nomenclatures according to the principles described in these recommendations. (see P-11)
Rules for the selection of preferred IUPAC names (PINs) for compounds containing Al, Ga, In, Tl, as well as for compounds containing B, Si, Ge, Sn, Pb, N, P, As, Sb, Bi, O, S, Se, Te, Po, F, Cl, Br, I, At, and that do not contain carbon, or that cannot be named on the basis of the principles of organic nomenclature as described in this book will be discussed in a further publication. Examples are discussed in these recommendations to illustrate the scope and limitations of the substitutive nomenclature extended from carbon to all elements of Groups 13 through 17; the label ‘preselected name’ is added to appropriate names.
P-12.2 PRESELECTED NAMES
Preselected names are names for structures or structural components chosen among two or more names for noncarbon-containing (inorganic) parents to be used as the basis for preferred IUPAC names for organic derivatives in the nomenclature of organic compounds. Although systematic names alumane, gallane, indigane and thallane are preselected names, the names based on these
parent hydrides currently do not have PIN status. However such names can be used in general nomenclature.
In the context of substitutive organic nomenclature, we need to select names for parent hydrides or other parent structures that do not contain carbon, in order to name derivatives that do contain carbon. The names chosen here for this purpose are termed 'preselected'. Each noncarbon-containing parent structure capable of substitution or functionalization by carbon-containing groups is assigned a unique 'preselected name’ to be used as the basis for deriving a preferred IUPAC name; noncarbon-containing characteristic groups, prefixes, and suffixes used in PINs are designated as preselected prefixes or suffixes.
Names of parent structures, prefixes, and suffixes identified herein as 'preselected' may not necessarily emerge as preferred IUPAC names in the context of inorganic chemical nomenclature.
All names listed in Table 2.1, with the exception of methane (carbane), are preselected names, and the concept is illustrated by the following examples.
Examples:
SnH3-[SnH2]11-SnH3
tridecastannane (preselected name)
CH3-SnH2-[SnH2]11-SnH3
1-methyltridecastannane (PIN)
(HO)3PO
phosphoric acid (preselected name)
(CH3-O)3PO
trimethyl phosphate (PIN)
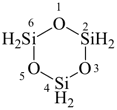
1,3,5,2,4,6-trioxatrisilinane (preselected name see P-22.2.2.1.6)
cyclotrisiloxane (P-22.2.6)
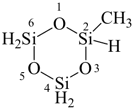
2-methyl-1,3,5,2,4,6-trioxatrisilinane (PIN)
P-12.3 RETAINED NAMES
Retained names are traditional or common, well-established names that may be either preferred IUPAC names, such as naphthalene, pyridine, and acetic acid; or preselected names, such as hydrazine and hydroxylamine; or as alternative names allowed in general nomenclature, for example, allene.
P-12.4 METHODOLOGY
In this book, names of parent structures, characteristic groups and their prefixes, and organic compounds are systematically identified as preferred IUPAC names, prefixes, and suffixes; or as preselected names, prefixes, or suffixes. Preferred IUPAC stereodescriptors are described and used in Chapter P-9. To facilitate the construction of the names of organic compounds, preferred and preselected prefixes for use in generating preferred IUPAC names are listed in Appendix 2 along with other prefixes that are acceptable in general nomenclature.
P-13 OPERATIONS IN NOMENCLATURE OF ORGANIC COMPOUNDS
The operations described in this section all involve structural modifications, and are classified first according to the type of modification, for example ‘replacement’; and then according to the way in which the modification is expressed, for example ‘by use of replacement infixes’. The structures to which the various modifications are applied can be regarded as parent structures, and the modifications are expressed by suffixes, affixes, infixes, and prefixes, or by a change of endings.
P-13.1 The substitutive operation
P-13.2 The replacement operation
P-13.3 The additive operation
P-13.4 The subtractive operation
P-13.5 The conjunctive operation
P-13.6 The multiplicative operation
P-13.7 The fusion operation
P-13.8 Operations used only in the nomenclature of natural products
P-13.1 THE SUBSTITUTIVE OPERATION
The substitutive operation involves the exchange of one or more hydrogen atoms for another atom or group of atoms. This process is expressed by a suffix or a prefix denoting the atom or group being introduced.
Examples:
CH3-CH3
ethane (PIN) |  | CH3-CH2-SH
ethanethiol (PIN)
(substitutive suffix = ‘thiol’) |
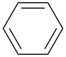
benzene (PIN) |  | 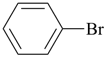
bromobenzene (PIN)
(substitutive prefix = ‘bromo’)
|
P-13.2 THE REPLACEMENT OPERATION
P-13.2.1 The replacement operation involves the exchange of one group of atoms or a single nonhydrogen atom for another. This can be expressed in several ways, as shown in the following subsections.
P-13.2.1.1 By replacement (‘a’) prefixes representing the element being introduced. This type of replacement is called ‘skeletal replacement’. The most common type of replacement in the nomenclature of organic compounds is replacement of carbon atoms by one or more of the following elements: O, S, Se, Te, N, P, As, Sb, Bi, Si, Ge, Sn, Pb.
Examples:
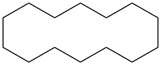
cyclotetradecane (PIN)
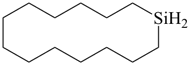
silacyclotetradecane (PIN)
[replacement (‘a’) prefix = ‘sila’]
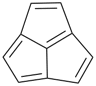
cyclopenta[cd]pentalene (PIN)
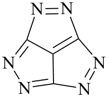
1,2,3,4,5,6-hexaazacyclopenta[cd]pentalene (PIN)
[replacement (‘a’) prefix = ‘aza’]
P-13.2.1.2 In specific instances, a heteroatom may be replaced by a carbon atom or by another heteroatom. The former is illustrated in the nomenclature of cyclic polyboranes (see IR-6.2.4.4, ref. 12) and both are found in natural products (see RF-5, ref. 9 and P-101.4) and must be applied only when specifically prescribed because the nomenclature of organic compounds is normally based on carbon atoms.
Examples:
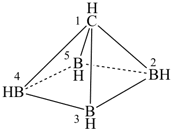
1-carba-nido-pentaborane(5) (PIN)
[replacement (‘a’) prefix = ‘carba’; carbon replacing boron; see P-68.1.1.2.1]
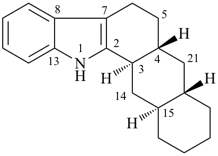
(4βH)-4-carbayohimban
[replacement (‘a’) prefix = ‘carba’; carbon replacing nitrogen; see P-101.4]
P-13.2.2 By prefixes or infixes signifying replacement of oxygen atoms or oxygen-containing groups.
P-13.2.2.1 This type of replacement is called ‘functional replacement’. The affixes represent the group(s) being introduced. Functional replacement nomenclature is described in P-15.5.
Examples:
(CH3)2P(O)-OCH3
methyl dimethylphosphinate (PIN) |  | (CH3)2P(=NH)-OCH3
methyl P,P-dimethylphosphinimidate (PIN)
[replacement infix = imid(o); =NH replaces =O]methyl P,P-dimethyl(imidophosphinate)
(replacement prefix = ‘imido’; =NH replaces =O)
|
C6H5-P(O)(OH)2
phenylphosphonic acid (PIN) |  | C6H5-P(≡N)-OH
phenylphosphononitridic acid (PIN) 
[replacement infix = ‘nitrid(o)’; ≡N replaces both =O and –OH]phenyl(nitridophosphonic acid)
(replacement prefix = ‘nitrido’; ≡N replaces both =O and –OH) |
P-13.2.2.2 The affixes ‘thio’, ‘seleno’, and ‘telluro’ indicate replacement of an oxygen atom of a characteristic group by another chalcogen atom.
Examples:
P-13.2.2.3 In specific instances, the prefixes ‘thio’, ‘seleno’, and ‘telluro’ indicate a skeletal modification. This replacement occurs with the cyclic parent hydrides having retained names, i.e., morpholine (see Table 2.3), pyran (see Table 2.2), chromene, isochromene, and xanthene (see Table 2.8), chromane and isochromane (see Table 3.1).
Example:
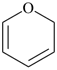
2H-pyran (PIN)
(not 2H-oxine, see P-22.2.2.1.1) | 
| 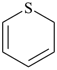
2H-thiopyran (PIN)
(replacement prefix = ‘thio’;
S replacing O)2H-thiine (Hantzsch-Widman name)
(see P-22.2.2.1.1) |
P-13.3 THE ADDITIVE OPERATION
The additive operation involves the formal assembly of a structure from its component parts without loss of any atoms or groups. This operation can be expressed in several ways, as shown in the following subsections.
P-13.3.1 By an additive prefix
Examples:
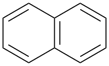
naphthalene (PIN) |  | 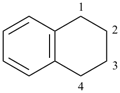
1,2,3,4-tetrahydronaphthalene (PIN)
(‘hydro’ = prefix designating addition
of one hydrogen atom) |
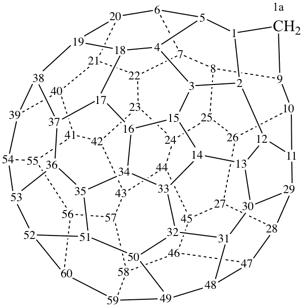
1aH-1(9)a-homo(C60-Ih)[5,6]fullerene (PIN)
1,9-seco(C60-Ih)[5,6]fullerene (PIN)
P-13.3.2 By an additive suffix
Examples:
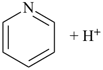
pyridine (PIN) |  |
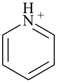
pyridin-1-ium (PIN)
(‘ium’ = suffix designating the
addition of one H+) |
CH3-BH2 + H–
methylborane (PIN) |  | CH3-BH3–
methylboranuide (PIN)
(‘uide’ = suffix designating the
addition of one H–) |
P-13.3.3 By a separate word
P-13.3.3.1 With the name of a neutral parent structure
Examples:
CH3-C≡N
acetonitrile (PIN) |  | CH3-C≡NO
acetonitrile oxide (PIN)
|
C6H5-CH=CH2
styrene
ethenylbenzene (PIN) |
 | 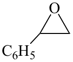
styrene oxide
phenyloxirane (PIN) |
P-13.3.3.2 With one or more substituent prefix name(s)
Here the separate word is a class or subclass name representing the characteristic group or the kind of characteristic group to which the substituents are linked (see also functional class nomenclature, P-15.2).
Examples:
CH3–
methyl (preferred prefix) | + | –OH
alcohol (class name) |  | CH3-OH
methyl alcohol
methanol (PIN) |
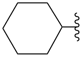
cyclohexyl
(preferred prefix) | + | 
cyclohexyl
(preferred prefix) | + | 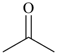
ketone
(class name) |  | 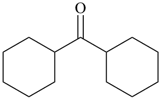
dicyclohexyl ketone
dicyclohexylmethanone (PIN) |
|
|
CH3–
methyl
(preferred prefix) | + | C6H5–
phenyl
(preferred prefix) | + | –O–
oxy
(preselected prefix) |  | CH3-O-C6H5
methyl phenyl ether
anisole (PIN)
methoxybenzene |
C6H5-CH2–
benzyl (preferred prefix) | + | –CN
cyanide
(class name) |  | C6H5-CH2-CN
benzyl cyanide
phenylacetonitrile (PIN) |
P-13.3.4 By adding substituent groups together, in an operation called ‘concatenation’
Examples:
CH3-CH2-CH2-CH2-CH2–
pentyl
(preferred prefix) | + | –O–
oxy
(preselected prefix) |  | CH3-CH2-CH2-CH2-CH2O–
pentyloxy
(preferred prefix)
|
Cl–
chloro
(preselected prefix) | + | –CO–
carbonyl
(preferred prefix) |  | Cl-CO–
chlorocarbonyl
carbonochloridoyl
(preferred prefix) |
–NH–
azanediyl
(preselected prefix) | + | –CH2-CH2–
ethane-1,2-diyl
(preferred prefix) | + | –NH–
azanediyl
(preselected prefix) |  | –NH-CH2-CH2-NH–
ethane-1,2-diylbis(azanediyl)
(preferred prefix) |
P-13.3.5 By adding molecular entities together
Chemical species AB in which two molecular entities A and B are combined directly with no loss of atoms from either A or B can be named as adducts (see P-14.8) by citing the names of A and B linked with an ‘em’ dash.
Example:
CO
carbon monoxide (PIN) | + | CH3-BH2
methylborane (PIN) |  | CO • BH2-CH3
carbon monoxide—methylborane (1/1) (PIN)  |
P-13.4 THE SUBTRACTIVE OPERATION
The subtractive operation involves the removal of an atom or group implicit in a name. This operation can occur with no other change, with introduction of unsaturation, or with formation of substituent groups, radicals, or ions. Several prefixes are used to indicate subtractive operations of many kinds in natural products. Subtraction can be expressed in several ways as shown in the following subsections.
P-13.4.1 By a suffix
Examples:
CH4
methane (PIN) | – | H•
monohydrogen
(preselected name) |  | CH3•
methyl
(PIN; a radical; the suffix ‘yl’
indicates loss of one hydrogen atom)
|
CH3-CH3
ethane (PIN) | – | H+
hydron
(preselected name) |  | CH3-CH2–
ethanide
(PIN; an anion; the suffix ‘ide’
indicates loss of a hydron)
|
CH3-CH2-CH2-CH3
butane (PIN) | – | H–
hydride
(preselected name) |  | CH3-CH2-CH2-CH2+
butylium
(PIN; the suffix ‘ylium’
indicates loss of a hydride ion) |
P-13.4.2 By a change of ending
Examples:
C6H5-SO2-OH
benzenesulfonic acid (PIN) | – | H+
hydron
(preselected name) |  | C6H5-SO2-O–
benzenesulfonate
(PIN; the ending ‘ate’
indicates loss of a hydron from an ‘ic acid’)
|
CH3-CH2-CH2-CH3
butane (PIN) | – | 2 H
hydrogen
(preselected name) |  | 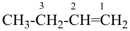
but-1-ene
(PIN; the ending ‘ene’
indicates loss of two hydrogen atoms) |
P-13.4.3 By the prefixes ‘dehydro’ and ‘nor’
P-13.4.3.1 The prefix ‘dehydro’
Example:
P-13.4.3.2 By the prefix ‘nor’
The prefix ‘nor’ is used to indicate removal of an unsubstituted saturated skeletal atom from a ring or a chain of a stereoparent structure with its attached hydrogen atom(s). It can also indicate the loss of a –CH= group from a mancude ring from a stereoparent structure (see P-101.3.1) and the loss of a carbon atom from a fullerene structure (see P-27.4.2).
Examples:
1,9-dinor(C60-Ih)[5,6]fullerene (PIN)
P-13.5 THE CONJUNCTIVE OPERATION
The conjunctive operation involves the formal construction of a name for a compound from the names of its components with subtraction of the same number of hydrogen atoms from each component at each site of the junction. This operation is expressed as noted in the following subsections.
P-13.5.1 By placing a multiplicative prefix ‘bi’, ‘ter’, ‘quater’, etc. (see P-14.2.3) before the name of the corresponding parent hydride.
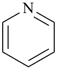
Example:
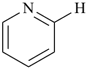 | + | 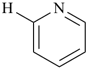 |  | 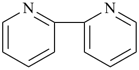 |
| pyridine (PIN) | | pyridine (PIN) | | 2,2′-bipyridine (PIN)
2,2′-bipyridyl (see P-28.2.1) |
P-13.5.2 By juxtaposition of component names (conjunctive nomenclature)
This method is used by Chemical Abstracts Service. It is not recommended for constructing preferred IUPAC names; substitutive nomenclature is the recommended operation (see P-51). This method is most commonly used when the two components to be joined are a ring or a ring system and a carbon chain (or chains) substituted by the principal characteristic group of the compound. In this method, both the principal characteristic group and the ring, or ring system, must terminate the chain; the rest of the structure attached to the chain, if any, is described by substituent prefixes, the locations of which are indicated by Greek letter locants, α, α1, β, β1, etc. (α designates the atom next to the principal characteristic group).
Examples:
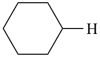 | + | 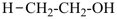 |  | 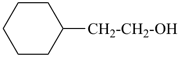 |
| cyclohexane (PIN) | | ethanol (PIN) | | cyclohexaneethanol
2-cyclohexylethan-1-ol (PIN) |
 |  | 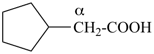 |  | 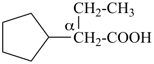 |
| cyclopentane (PIN) | | cyclopentaneacetic acid
cyclopentylacetic acid (PIN) | | α-ethylcyclpentaneacetic acid
2-cyclopentylbutanoic acid (PIN) |
P-13.5.3 Ring formation
The formation of a ring by means of a direct link between any two atoms of a parent structure with loss of one hydrogen atom from each is indicated by the prefix ‘cyclo’.
Examples:
 |  |  |
| propane (PIN) | | cyclopropane (PIN) |
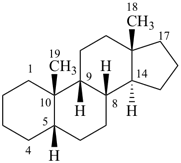 |  | 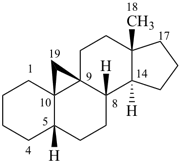 |
5β,9β-androstane
(fundamental parent structure) | | 9,19-cyclo-5β,9β-androstane
(see P-101.3.3) |
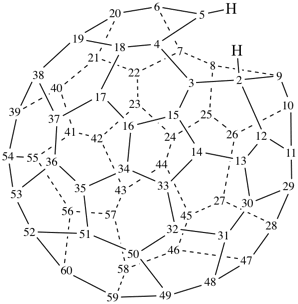
2H-2,9-cyclo-1-nor(C60-Ih)[5,6]fullerene (PIN)
P-13.6 THE MULTIPLICATIVE OPERATION
This operation allows the expression of multiple occurrences of parent structures connected by a symmetrical multivalent structure.
P-13.6.1 In substitutive nomenclature the multiplicative operation is used to name assemblies of identical parent structures linked by di- or polyvalent substituent groups. Identical parent structures are functionalized parent hydrides, functional parents, and rings or ring systems. It is, in fact, substitutive nomenclature in which identical parent structures are interconnected by a di- or polyvalent substituent group.
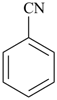 | + | –CH2– | + |  |  |  |
| benzonitrile (PIN) | | methylene
(preferred
prefix) | | benzonitrile (PIN) | | 2,2′-methylenedibenzonitrile (PIN) |
| 3 CH3-COOH | + | –N< |  | N(CH2-COOH)3 |
| acetic acid | | nitrilo
(preselected
prefix) | | 2,2′,2′′-nitrilotriacetic acid
N,N-bis(carboxymethyl)glycine
(see P-103.1, Table 10.4) |
 | + | –O– | + |  |  | 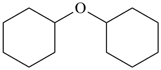 |
| cyclohexane (PIN) | | oxy
(preferred
prefix) | | cyclohexane (PIN) | | 1,1′-oxydicyclohexane (PIN) |
P-13.6.2 In functional class nomenclature the multiplicative operation is used to name assemblies of identical parent structures linked by a bi- or multivalent functional class name.
Examples:
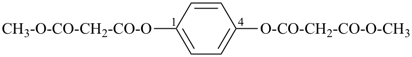
dimethyl 1,4-phenylene dipropanedioate (PIN)
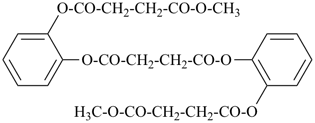
dimethyl butanedioylbis[oxy(2,1-phenylene)] dibutanedioate (PIN) 
P-13.7 THE FUSION OPERATION
The fusion operation involves the union of two rings or ring systems so that atoms or atoms and bonds are common to each. Spiro systems have one atom in common; fused ring systems have both atoms and bonds in common.
Examples:
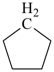 |  | 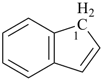 |  | 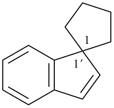 |
| cyclopentane (PIN) | | 1H-indene (PIN) | | spiro[cyclopentane-1,1′-indene] (PIN) |
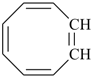 |  | 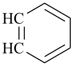 |  | 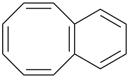 |
8-annulene
cycloocta-1,3,5,7-tetraene (PIN) | | benzene (PIN) | | benzo[8]annulene
(PIN; see P-25.3.2.1.1) |
P-13.8 OPERATIONS USED ONLY IN THE NOMENCLATURE OF NATURAL PRODUCTS
In the nomenclature of natural products several prefixes are used to indicate the loss of a group, i.e., the exchange of a group for hydrogen. The subtraction of the elements of water with concomitant bond formation can also be regarded as a subtractive operation. These operations are denoted by the following prefixes:
| ‘abeo’ | rearrangement of single bonds in a stereoparent structure (see P-101.3.5.1) |
| ‘anhydro’ | loss of H2O from two hydroxy groups with bond formation (see P-102.5.6.7) |
| ‘apo’ | removal of all of a side chain from a carotenoid system (see P-101.3.4.2) |
| ‘de’ | subtraction of an oxygen atom from an –OH group in carbohydrate nomenclature (see P-102.5.3) or exchange of a methyl group for a hydrogen atom (see P-101.7.5) |
| ‘des’ | removal of an amino acid residue from a peptide (see P-103.3.5.4) or of a terminal unsubstituted ring from a steroid skeleton (see P-101.3.6) |
| ‘retro’ | moving double bonds from a carotenoid system (see P-101.3.5.2) |
P-13.8.1 By the prefixes ‘de’ and ‘des’
P-13.8.1.1 The prefix ‘de’ (not ‘des’), followed by the name of a group or atom (other than hydrogen), denotes removal (or loss) of that group and addition of the necessary hydrogen atoms, i.e., exchange of that group with hydrogen atoms.
Example:
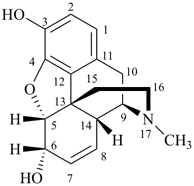 |  | 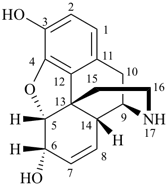 |
| I morphine | | II demethylmorphine
(exchange of methyl for H) |
I (5βH)-17-methyl-7,8-didehydrofuro[2′,3′,4′,5′:4,12,13,5]morphinan-3,6α-diol
4,5α-epoxy-17-methyl-7,8-didehydromorphinan-3,6α-diol
II (5βH)-7,8-didehydrofuro[2′,,3′,,4′,,5′,:4,12,13,5]morphinan-3,6α-diol
4,5α-epoxy-7,8-didehydromorphinan-3,6α-diol
As an exception, ‘deoxy’, when applied to hydroxy compounds, denotes the removal of an oxygen atom from an –OH group with the reconnection of the hydrogen atom. ‘Deoxy’ is extensively used as a subtractive prefix in carbohydrate nomenclature (see P-102.5.3).
Example:
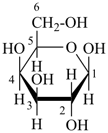 |  | 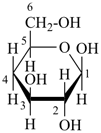 |
β-D-galactopyranose
(fundamental parent structure) | | 4-deoxy-β-D-xylo-hexopyranose
(not 4-deoxy-β-D-xylo-galactopyranose)
(2R,3R,4S,6S)-6-(hydroxymethyl)oxane-2,3,4-triol
(numbering based on the parent hydride oxane) |
P-13.8.1.2 The prefix ‘des’ signifies removal of an amino acid residue of a polypeptide, with rejoining of the chain (see P-103.3.5.4) or the removal of a terminal ring of a stereoparent (see P-101.3.6).
Examples:
| oxytocin |  | des-7-proline-oxytocin
(removal of the proline residue at
position 7 of oxytocin) |
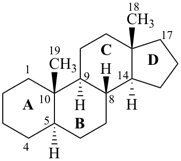 |  | 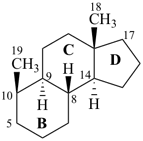 |
5α-androstane
fundamental parent structure) | | des-A-androstane
(see P-101.3.6)
(removal of ring A of 5α-androstane) |
P-13.8.2 By the prefix ‘anhydro’
Intramolecular ethers, formally arising by elimination of water from two hydroxy groups of a single molecule of a monosaccharide (aldose or ketose) or monosaccharide derivative, is indicated by the detachable prefix ‘anhydro’ preceded by a pair of locants identifying the two hydroxy groups involved. The prefix ‘anhydro’ is placed in a name in accordance with the principles of alphabetical order (see P-102.5.6.7.1).
Example:
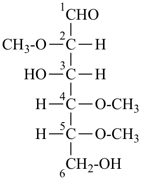 |  | 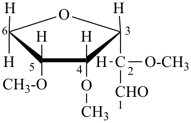 |
2,4,5-tri-O-methyl-D-mannose
(fundamental parent structure) | | 3,6-anhydro-2,4,5-tri-O-methyl-D-mannose
(the prefix ‘anhydro’ describes removal of H2O
from 2 ‘OH’ groups in the same structure) |
P-14 GENERAL RULES
P-14.0 Introduction
P-14.1 Bonding number
P-14.2 Multiplicative prefixes
P-14.3 Locants
P-14.4 Numbering
P-14.5 Alphanumerical order
P-14.6 Nonalphanumerical order
P-14.7 Indicated and ‘added indicated hydrogen’
P-14.8 Adducts
P-14.0 INTRODUCTION
Rules described in this section are of general application for naming types of compounds and individual compounds. They must be closely followed to construct preferred IUPAC names as well as names for general use.
P-14.1 BONDING NUMBER
The concept of a standard valence state for an atom is fundamental to organic nomenclature. Since most organic names are derived by formal exchange of hydrogen atoms of a parent structure for other atoms or groups, it is necessary to know exactly how many hydrogen atoms are implied for skeletal atoms of the parent structure. For example, does the name phosphane refer to PH3 or PH5 ? This is a problem when an element can occur in more than one valence state; in such cases, the standard state is normally not specified, but any other valence state is noted by citation of an appropriate bonding number. More details are given in the publication ‘Treatment of Variable Valence in Organic Nomenclature (Lambda Convention)’ (ref. 13). In these Recommendations, this convention is called simply the ‘λ-convention’.
P-14.1.1 Definition.
The bonding number ‘n’ of a skeletal atom is the sum of the total number of bonding equivalents (valence bonds) of that skeletal atom to adjacent skeletal atoms, if any, in a parent hydride and the number of hydrogen atoms.
Examples:
| H2S | for S, n = 2 |
| H6S | for S, n = 6 |
| (C6H5)3PH2 | for P, n = 5 |
|  | for N, n = 3 |
P-14.1.2 Standard bonding numbers. The bonding number of a skeletal atom is standard when it has the value given in Table 1.3.
Table 1.3 Standard bonding numbers for the elements of Groups 13, 14, 15, 16, and 17
Standard bonding
number (n) | Element |
| 3 | B | Al | Ga | In | Tl |
| 4 | C | Si | Ge | Sn | Pb |
| 3 | N | P | As | Sb | Bi |
| 2 | O | S | Se | Te | Po |
| 1 | F | Cl | Br | I | At |
P-14.1.3 Nonstandard bonding numbers
A nonstandard bonding number of a neutral skeletal atom of a parent hydride is indicated by the symbol ‘λn’, cited in conjunction with an appropriate locant. Note that the ‘n’ in the symbol ‘λn’ is italicized but the numbers in a specific symbol, e.g., ‘λ4’, are not (for the use of italicized ‘n’ in the symbol ‘λn’, see the General rules for symbols in physical quantities, Section 1.3 in ref. 14).
Examples:
CH3-SH5
methyl-λ6-sulfane (PIN)
(C6H5)3PH2
triphenyl-λ5-phosphane (PIN)
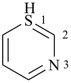
1λ4,3-thiazine (PIN)
P-14.2 MULTIPLICATIVE PREFIXES
Three types of multiplicative prefixes are used in names to denote multiplicity of identical features in structures (characteristic groups, substituent groups, multiple bonds) and correspondingly of affixes (suffixes, infixes, and prefixes) in names. They are always placed before the part of the name to which they relate.
P-14.2.1 Basic multiplicative prefixes denote simple features and, in general, are the first choice among such prefixes to specify multiplicity (ref. 15). They are listed in Table 1.4.
Table 1.4 Basic numerical terms (multiplicative prefixes)
| Number | Numerical
Term | | Number | Numerical
Term | | Number | Numerical
Term | | Number | Numerical
Term |
| 1 | mono, hen | | 11 | undeca | | 101 | henhecta | | 1001 | henkilla |
| 2 | di,do | | 20 | icosa | | 200 | dicta | | 2000 | dilia |
| 3 | tri | | 30 | triaconta | | 300 | tricta | | 3000 | trilia |
| 4 | tetra | | 40 | tetraconta | | 400 | tetracta | | 4000 | tetralia |
| 5 | penta | | 50 | pentaconta | | 500 | pentacta | | 5000 | pentalia |
| 6 | hexa | | 60 | hexaconta | | 600 | hexacta | | 6000 | hexalia |
| 7 | hepta | | 70 | heptaconta | | 700 | heptacta | | 7000 | heptalia |
| 8 | octa | | 80 | octaconta | | 800 | octacta | | 8000 | octalia |
| 9 | nona | | 90 | nonaconta | | 900 | nonacta | | 9000 | nonalia |
| 10 | deca | | 100 | hecta | | 1000 | kilia | | | | |
P-14.2.1.1 The prefix mono
P-14.2.1.1.1 When alone, the numerical term for the number ‘1’ is ‘mono’ and that for ‘2’ is ‘di’. In association with other numerical terms, the number ‘1’ is represented by ‘hen’ (except in the case of ‘undeca’) and the number ‘2’ by ‘do’ (except in the cases of ‘dicta’ and ‘dilia’). The numerical term for the number ‘11’ is ‘undeca’.
P-14.2.1.1.2 The prefix ‘mono’ is not used in systematically formed names to indicate the presence of one nomenclatural feature, for example suffixes, prefixes, endings. It is used in functional class nomenclature to designate a monoester of a diacid, for example phthalic acid monomethyl ester, and in terminology, to emphasize singleness, for example, monocyclic and mononuclear in contrast to bicyclic and polynuclear.
P-14.2.1.2 Derivation of basic numerical terms
After ‘undeca-’ (for the number eleven), composite numerical terms are formed systematically by citing the basic terms in the order opposite to that of the constituent digits in the arabic numbers. The composite terms are formed by direct joining of the basic terms, without hyphen(s). The letter ‘i’ in ‘icosa’ is elided after a vowel.
Examples:
| 486 | hexaoctacontatetracta |
| | 6 | | 80 | | 400 | |
| 14 | tetradeca | | 21 | henicosa | | 22 | docosa |
| 23 | tricosa | | 24 | tetracosa | | 41 | hentetraconta |
| 52 | dopentaconta | | 111 | undecahecta | | 363 | trihexacontatricta |
P-14.2.2 Numerical terms for compound or complex features
Multiplicative prefixes for compound or complex entities, such as substituted substituents, are formed by adding the ending ‘kis’ to the basic multiplicative prefix ending in ‘a’, ‘tetrakis’, ‘pentakis’, etc. (ref. 15). The prefixes ‘bis’ and ‘tris’ correspond to ‘di’ and ‘tri’. The basic prefix ‘mono’ has no counterpart in this series.
Examples:
| 2 | bis | | 3 | tris | | 4 | tetrakis |
| 231 | hentriacontadictakis | | | |
P-14.2.3 Multiplicative prefixes for naming assemblies of identical units
The traditional prefixes used to denote the number of repeated identical units in unbranched ring assemblies (see P-28) are as follows:
| 2 | bi | | 5 | quinque | | 8 | octi |
| 3 | ter | | 6 | sexi | | 9 | novi |
| 4 | quater | | 7 | septi | | 10 | deci |
This list has been completed from 11 to 9999. The prefixes are formed by changing the ending ‘a’ of basic numerical prefixes into ‘i’, for example, ‘undeci’ for the number ‘11’, ‘hexadeci’ for the number ‘16’, ‘tetraconti’ for the number ‘40’.
P-14.3 LOCANTS
P-14.3.1 Types of locants
Traditional types of locants are arabic numbers, for example, 1, 2, 3; primed locants, for example, 1′, 1′′′, 2′′; locants including a lower case Roman letter, for example, 3a, 3b; italicized Roman letters, for example, O, N, P; Greek letters, for example, ‘α-, β-, γ-’ and compound locants, for example, ‘1(10)’ and ‘5(17)’.
The locants o, m, p are no longer recommended; the numerical locants ‘1,2-’, ‘1,3-’, and ‘1,4-’ must be used in substitutive names. However, as an exception, the three isomers of xylene and cresol are still recognized as o-, m-, and p-xylene and o-, m-, and p-cresol in general IUPAC nomenclature (see P-22.1.3 and P-63.1.1.2). The prefixes o-, m-, and p-tolyl are still recognized for general nomenclature (see P-29.6.2.3). No substitution is allowed.
Composite locants, for example, 32, 2a1, N2′, and O3 have been developed in recent years for various purposes and are included in these recommendations. They are used in phane nomenclature to indicate positions in amplificants (see P-26.4.3); for numbering in ring assemblies (see P-28.3); to denote interior positions in fused ring systems (see P-25.3.3.3); and in von Baeyer descriptors for spiro ring systems (see P-24.2.2). They are also used in steroid (ref. 16 and P-101.7.1.1), tetrapyrrole (ref. 17), and amino acid and peptide nomnclature (ref. 18 and P-103.2.1)).
Primes are added to differentiate between the same locant in the same or different parts of the structure, for example, 1′, 2′′, N′, and α′. In locants consisting of two or more characters, primes are generally added to the primary character. For example, in locants including a lower case Roman letter, used in fused rings, primes are added following the arabic number, for example, 3′a and 2′a1; this format follows the principle that in locants for fusion positions in a fused ring system a letter follows the previous peripheral locant. For composite locants used in phane nomenclature, the prime follows the superatom locant, as in 2′3 and 2′4a. In multiplicative nomenclature, primes may appear just after a letter locant, such as N′4 or following the composite letter locant, such as N2′.
P-14.3.2 Position of locants
Locants (numerals and/or letters) are placed immediately before that part of the name to which they relate, except in the case of the traditional contracted names when locants are placed at the front of their names.
Examples:
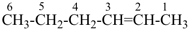
hex-2-ene (PIN)
(not 2-hexene)
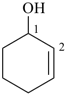
cyclohex-2-en-1-ol (PIN)
(not 2-cyclohexen-1-ol)
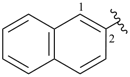
naphthalen-2-yl (preferred prefix)
2-naphthyl (contracted name)
(not naphth-2-yl)
P-14.3.3 Citation of locants
In preferred IUPAC names, if any locants are essential for defining the structure of the parent structure or of a unit of structure as defined by its appropriate enclosing marks, then all locants must be cited for the parent structure or that structural unit. For example, the omission of the locant ‘1’ in 2-chloroethanol, while permissible in general usage, is not allowed in preferred IUPAC names, thus the name 2-chloroethan-1-ol is the PIN. And in the following example, 1-phenyl-2-(phenyldiazenyl)-2-(phenylhydrazinylidene)ethan-1-one (PIN), locants are not used for the structural units defined by the parentheses even though locants are used for these substituents of the parent structure ethanone. Also, in preferred IUPAC multiplicative names and in preferred IUPAC names for ring assemblies locants are always cited, e.g., 1,1′-oxydibenzene (P-15.3.1.3) and 1,1′-biphenyl (P-28.2.1). For omission of locants from a PIN see P-14.3.4  .
.
P-14.3.4 Omission of locants
The practice of omitting locants when there is no ambiguity is widespread. However, for absolute clarity in preferred IUPAC names it is necessary to be prescriptive about when omission of locants is permissible.
Locants are omitted in preferred IUPAC names in the following cases.
P-14.3.4.1 Terminal locants are not cited in names for mono- and dicarboxylic acids derived from acyclic hydrocarbons and their corresponding acyl halides, amides, hydrazides, nitriles, esters, aldehydes, amidines, amidrazones, hydrazidines, and amidoximes, when unsubstituted or substituted on carbon atoms.
Examples:
HOOC-CH2-CH2-COOH
butanedioic acid (PIN)
HOOC-CH2-CH(Cl)-COOH
chlorobutanedioic acid (PIN)
CH3-CH2-CH2-CH2-CO–
pentanoyl (preferred prefix)
H2N-CO-CH(CH3)-CO-NH2
2-methylpropanediamide (PIN)
CH3-NH-CO-CH2-CO-NH-CH3
N1,N3-dimethylpropanediamide (PIN)
(not N1,N3-dimethylpropane-1,3-diamide)
P-14.3.4.2 The locant ‘1’ is omitted:
(a) in substituted mononuclear parent hydrides;
Examples:
CH3Cl
chloromethane (PIN)
SiH2Cl2
dichlorosilane (from silane, a preselected name)
(CH3)3Al
trimethylalumane
(b) in monosubstituted homogeneous chains consisting of only two identical atoms;
Examples:
CH3-CH2-OH
ethanol (PIN)
NH2-NH-Cl
chlorohydrazine (from hydrazine, a preselected name)
NH2-NH–
hydrazinyl (preselected prefix) (not hydrazin-1-yl)
(c) in monosubstituted homogeneous monocyclic rings;
Examples:
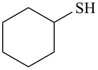
cyclohexanethiol (PIN)
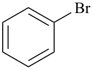
bromobenzene (PIN)
(d) in any dinuclear, unsubstituted trinuclear alkenes and alkynes and monounsaturated cycloalkenes and cycloalkynes; similarly, in unsubstituted monounsaturated compounds composed of homogeneous chains containing elements of Groups 13, 14, 15, and 16, and corresponding monounsaturated cyclic compounds. 
Examples:
CH2=CH2
ethene (PIN)
CH≡CH
acetylene (PIN)
CH3-CH=CH2
propene (PIN)
NH=NH
diazene (preselected name)
SiH≡SiH
disilyne (preselected name)
H2N-N=NH
triazene (preselected name)
P-14.3.4.3 The locant is omitted in monosubstituted symmetrical parent hydrides or parent compounds where there is only one kind of substitutable hydrogen.
Examples:
CH3-NH-CO-NH2
methylurea (PIN)
Cl-SiH2-O-SiH3
chlorodisiloxane (from disiloxane, a preselected name)
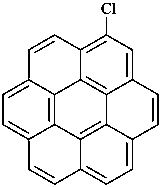
chlorocoronene (PIN)
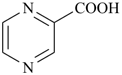
pyrazinecarboxylic acid (PIN)
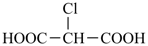
chloropropanedioic acid (PIN)
chloromalonic acid
P-14.3.4.4 Locants are omitted when no isomer can be generated by moving suffixes and/or prefixes (if any) from their position to another or by interchanging them between two different positions.
Examples:
CH3-CH=N-NH–
ethylidenehydrazinyl (preferred prefix)
(not 2-ethylidenehydrazin-1-yl)
HC≡Si-Si≡C-CH3
ethylidyne(methylidyne)disilane
(not 1-ethylidyne-2-methylidynedisilane)
C6H5-CH=SiH-Si(=CH-C6H5)–
dibenzylidenedisilanyl (preferred prefix)
(not 1,2-dibenzylidenedisilan-1-yl)
H2C=P-O-PH-O-P=CH-CH3
ethylidene(methylidene)triphosphoxane (PIN)
(not 1-ethylidene-5-methylidenetriphosphoxane)
H2Si=N-NH-Cl
chloro(silylidene)hydrazine (preselected name)
(not 1-chloro-2-silylidenehydrazine)
Br-S-S-CH3
(bromodisulfanyl)methane (PIN)
(not bromo(methyl)disulfane see P-63.3.1;
not 1-bromo-2-methyldisulfane)
O=C=CH–
oxoethenyl (preferred prefix)
Cl-N=N–
chlorodiazenyl (preferred prefix)
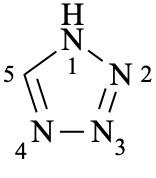
1H-tetrazole (PIN)
(not 1H-1,2,3,4-tetrazole) 
In the following examples locants are needed.
CH3-CH2-CH=SiH-Si(=CH-CH3)–
1-ethylidene-2-propylidenedisilan-1-yl (preferred prefix)
(by interchanging the two substituent groups another isomer is generated, i.e., 2-ethylidene-1-propylidenedisilan-1-yl)
CH3-CH=SiH-SiH2-Cl
1-chloro-2-ethylidenedisilane (PIN)
(another isomer is generated by moving the Cl atom to the other Si atom, i.e., 1-chloro-1-ethylidenedisilane)
Cl-CH=CH–
2-chloroethen-1-yl (preferred prefix)
2-chlorovinyl
As an exception the locant is not omitted from propa-1,2-diene, propan-2-one, butan-2-one, prop-2-enoic acid and prop-2-ynoic acid although unambiguous without a locant. 
P-14.3.4.5 All locants are omitted in compounds or substituent groups in which all substitutable positions are completely substituted or modified, for example, by hydro, in the same way. Except for hydrogen atoms attached to chalcogen atoms, such as in acids, alcohols, and to the carbon atoms of formyl groups (aldehydes), all hydrogen atoms are considered substitutable.
In case of partial substitution or modification, all numerical prefixes must be indicated. The prefix ‘per-’ is no longer recommended.
Examples:
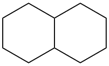
decahydronaphthalene (PIN)
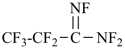
octafluoropropanimidamide (PIN)
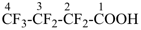
heptafluorobutanoic acid (PIN)
CF3-CF2-CH2-OH
2,2,3,3,3-pentafluoropropan-1-ol (PIN)
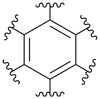
benzenehexayl (preferred prefix)
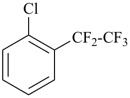
1-chloro-2-(pentafluoroethyl)benzene (PIN)
F2N-CO-NF2
tetrafluorourea (PIN)
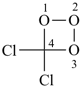
dichlorotrioxetane (PIN)
P-14.3.4.6 All locants are omitted for parent compounds when all substitutable hydrogen atoms have the same locant.
Examples:
F2CH-COOH
difluoroacetic acid (PIN)
(not 2,2-difluoroacetic acid)
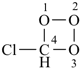
chlorotrioxetane (PIN)
(not 4-chloro-1,2,3-trioxetane) 
P-14.3.5 Lowest set of locants
The lowest set of locants is defined as the set that, when compared term by term with other locant sets, each cited in order of increasing value, has the lowest term at the first point of difference; for example, the locant set ‘2,3,5,8’ is lower than ‘3,4,6,8’ and ‘2,4,5,7’.
Primed locants are placed immediately after the corresponding unprimed locants in a set arranged in ascending order; locants consisting of a number and a lower-case letter with or without primes as 4a and 4′a (not 4a′) are placed immediately after the corresponding numeric locant and are followed by locants having superscripts.
Italic capital and lower-case letter locants are lower than Greek letter locants, which, in turn, are lower than numerals.
Examples:
| 2 is lower than 2′ |
| 3 is lower than 3a |
| 8a is lower than 8b |
| 4′ is lower than 4a |
| 4a is lower than 4′a |
| 12 is lower than 13 |
| 14 is lower than 2′ |
| 3a is lower than 3a1 |
| 1,1,1,4,2 is lower than 1,1,4,4,2 |
| 1,1′,2′,1′′,3′′,1′′′ is lower than 1,1′,3′,1′′,2′′,1′′′ |
| N,α,1,2 is lower than 1,2,4,6 |
Note: An exception must be noted in the field of carotenoid nomenclature, where 5,8,5′,8′- is used rather than 5,5′,8,8′- (see P-101.5.2); the latter would be recommended for systematic substitutive nomenclature
P-14.4 NUMBERING
When several structural features appear in cyclic and acyclic compounds, low locants
are assigned to them in the following decreasing order of seniority:
| Two important changes have been made to the 1979 recommendations (ref 1):
(1) heteroatoms in chains are now considered to be a part of the parent hydride; as such, they have seniority over suffixes for numbering as for heteroatoms in rings;
(2) hydro/dehydro prefixes are now classified as detachable prefixes but are not included in the category of alphabetized detachable prefixes; they are cited directly before the name of the parent hydride [see item (e) below]. |
(a) fixed numbering in chains, rings, or ring systems, i.e., when the numbering of a system is fixed, for example in purine, anthracene, and phenanthrene, this numbering must be used, both in PINs and in general nomenclature;
Examples:
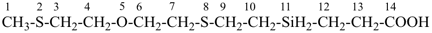
5-oxa-2,8-dithia-11-silatetradecan-14-oic acid (PIN)
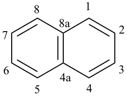
naphthalene (PIN)
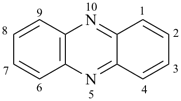
phenazine (PIN)
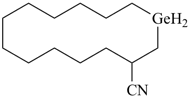
1-germacyclotetradecane-3-carbonitrile (PIN)
(b) indicated hydrogen for unsubstituted compounds; a higher locant may be needed at another position to accommodate a substituent suffix in accordance with structural feature (c); 
Examples:
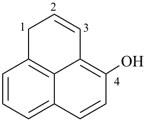
1H-phenalen-4-ol (PIN)
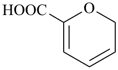
2H-pyran-6-carboxylic acid (PIN)
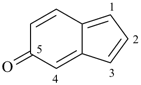
5H-inden-5-one (PIN)
(c) principal characteristic groups and free valences (suffixes);
Examples:
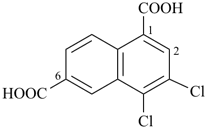
3,4-dichloronaphthalene-1,6-dicarboxylic acid (PIN)
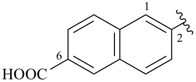
6-carboxynaphthalen-2-yl (preferred prefix)
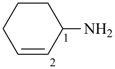
cyclohex-2-en-1-amine (PIN)
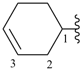
cyclohex-3-en-1-yl (preferred prefix)
(d) ‘added indicated hydrogen’ (consistent with the structure of the compound and in accordance with further substitution);
Examples:
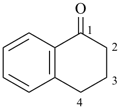
3,4-dihydronaphthalen-1(2H)-one (PIN)
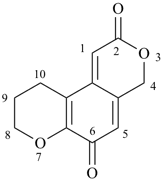
9,10-dihydro-2H,4H-benzo[1,2-b:4,3-c′]dipyran-2,6(8H)-dione (PIN)
(e) saturation/unsaturation:
(i) low locants are given to hydro/dehydro prefixes (see first example and P-31.2.2) and ‘ene’ and ‘yne’ endings;
(ii) low locants are given first to multiple bonds as a set and then to double bonds (see second and third examples and P-31.1.1.1);
Examples:
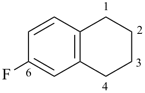
6-fluoro-1,2,3,4-tetrahydronaphthalene (PIN)
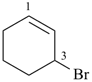
3-bromocyclohex-1-ene (PIN)
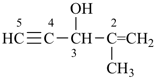
2-methylpent-1-en-4-yn-3-ol (PIN)
(not 4-methylpent-4-en-1-yn-3-ol)
(f) detachable alphabetized prefixes, all considered together in a series of increasing numerical order;
Example:
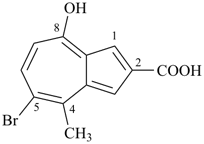
5-bromo-8-hydroxy-4-methylazulene-2-carboxylic acid (PIN)
(the locant set ‘4,5,8’ is lower than ‘4,7,8’)
(g) lowest locants for the substituent cited first as a prefix in the name;
Examples:
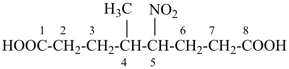
4-methyl-5-nitrooctanedioic acid (PIN)
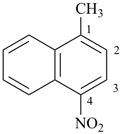
1-methyl-4-nitronaphthalene (PIN)
(not 4-methyl-1-nitronaphthalene)
(h) When a choice is needed between the same skeletal atom in different valence states, the one in a nonstandard valence state is assigned the lower locant. If a further choice is needed between the same skeletal atom in two or more nonstandard valence states, the one in the higher valence state is assigned the lower locant;
Examples:
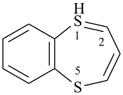
1λ4,5-benzodithiepine (PIN)
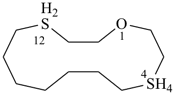
1-oxa-4λ6,12λ4-dithiacyclotetradecane (PIN)
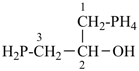
1-(λ5-phosphanyl)-3-phosphanylpropan-2-ol (PIN)
(λ5-phosphanyl is cited before phosphanyl and is given the lower locant)
(i) When there is a choice between equivalent numberings in an isotopically unmodified compound, the starting point and the direction of numbering of the analogous isotopically substituted compound are chosen so as to give lowest locants to the modified atoms or groups considered together as a set in increasing numerical order. If a choice still remains, the lower locant is given to the nuclide of higher atomic number. In the case of different nuclides of the same element, the lower locant is assigned to the nuclide of higher mass number;
Examples:
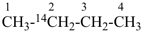
(2-14C)butane (PIN)
[not (3-14C)butane]
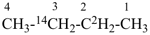
(3-14C,2,2-2H2)butane (PIN)
[not (2-14C,3,3-2H2)butane]
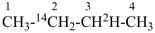
(2-14C,3-2H1)butane (PIN)
[not (3-14C,2-2H1)butane]
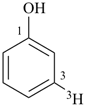
(3-3H)phenol (PIN)
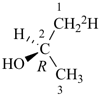
(2R)-(1-2H1)propan-2-ol (PIN)
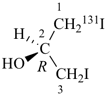
(2R)-1-(131I)iodo-3-iodopropan-2-ol (PIN)
(see P-82.2.2.1)
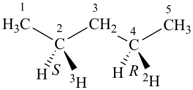
(2S,4R)-(4-2H1,2-3H1)pentane (PIN)
[not (2R,4S)-(2-2H1,4-3H1)pentane; isotopic modifications have seniority for low locants over stereodescriptors described in (j) below]
(j) When there is a choice for lower locants related to the presence of stereogenic centers or stereoisomers, the lower locant is assigned to CIP stereodescriptors Z, R, M, and r (pseudoasymmetry) that are preferred to E, S, P, and s, respectively, which are preferred to the non-CIP stereodescriptors cis, trans, or r (reference), c, and t (see P-91.2 for CIP and non-CIP stereodescriptors).
Examples:
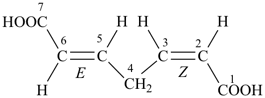
(2Z,5E)-hepta-2,5-dienedioic acid (PIN)
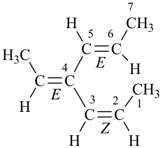
(the chain is numbered by assigning the low locant to the ‘Z’ double bond)
(2Z,4E,5E)-4-ethylidenehepta-2,5-diene (PIN) (low locants are assigned to the longest chain, then to the ‘Z’ double bond)
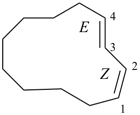
(1Z,3E)-cyclododeca-1,3-diene (PIN)
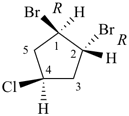 | or | 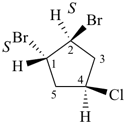 |
rel-(1R,2R)-1,2-dibromo-4-chlorocyclopentane (PIN)
1r,2t-dibromo-4c-chlorocyclopentane
(the preferred IUPAC name is denoted by CIP stereodescriptors; in the second name,
the relative configuration is expressed by the non-CIP stereodescriptors ‘1r,2t,4c’ rather than ‘1r,2t,4t’,
because a ‘cis’ arrangement, denoted by ‘c’, has priority over a ‘trans’ arrangement, denoted by ‘t’, in position ‘4’)
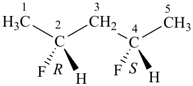
(2R,4S)-2,4-difluoropentane (PIN)
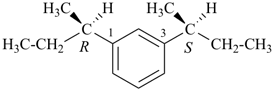
1-[(2R)-butan-2-yl]-3-[(2S)-butan-2-yl]benzene (PIN)
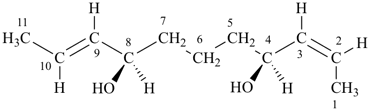
(2Z,4S,8R,9E)-undeca-2,9-diene-4,8-diol (PIN)
(the choice is between ‘E’ and ‘Z’ for position ‘2’, not between ‘R’ and ‘S’ for position ‘4’)
 |  |
| I | II |
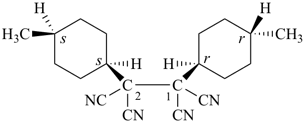
(I) 1-[(1r,4r)-4-methylcyclohexyl]-2-[(1s,4s)-4-methylcyclohexyl]ethane-1,1,2,2-tetracarbonitrile (PIN)
(the substituent denoted by the ‘r’ stereodescriptor receives the lowest locant, ‘1’;
the use of CIP stereodescriptors generates the preferred IUPAC name)
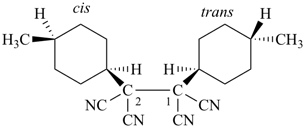
(II) 1-(cis-4-methylcyclohexyl)-2-(trans-4-methylcyclohexyl)ethane-1,1,2,2-tetracarbonitrile
(the ‘cis’ substituent receives the lowest locant, ‘1’) | |
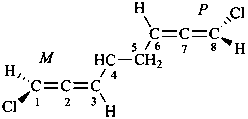
(1M,6P)-1,8-dichloroocta-1,2,6,7-tetraene (PIN)
P-14.5 ALPHANUMERICAL ORDER
Alphanumerical order has been commonly called ‘alphabetical order’. As these ordering principles do involve ordering both letters and numbers, in a strict sense, it is best called ‘alphanumerical order’ in order to convey the message that both letters and numbers are involved.
Alphanumerical order is used to establish the order of citation of detachable substituent prefixes (also the separately cited dehydro and hydro prefixes  ), and the numbering of a chain, ring, or ring system when a choice is possible.
), and the numbering of a chain, ring, or ring system when a choice is possible.
Alphanumerical order is applied as follows in organic nomenclature. Nonitalic Roman letters are considered first, unless used as locants or part of a compound or composite locant, for example, ‘N’ or ‘4a’ (see P-14.3), or in an isotopic descriptor. When all the Roman letters are identical, the set of locants for all initial locants for primary substituents, that is, locants appearing ahead of the first Roman letter of each primary substituent, are compared. Absence of locants is most preferred, followed by italic Roman letter locants, Greek letter locants (as in conjunctive names), if any, and arabic numerals in order from lowest to highest. Thus, the preferred order for alphanumerical order is: nonitalic Roman letters > italic letters > Greek letters.
For the sorting of nonalphanumerical characters, see P-14.6.
In these subsections the principles of alphanumerical order do not include Greek letters (except in conjunctive names) or isotopic or stereochemical descriptors.
P-14.5.1 Simple prefixes (i.e., those describing atoms and unsubstituted substituents) are arranged alphabetically; multiplicative prefixes, if necessary, are then inserted and do not alter the alphabetical order already established.
Examples:
1-ethyl-1-methylcyclohexane (PIN)
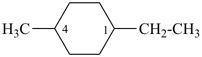
1-ethyl-4-methylcyclohexane (PIN)
[for numbering, see P-14.4 (g)]

3,3-dibromo-3-cyclohexylpropanoic acid (PIN)
(not 3-cyclohexyl-3,3-dibromopropanoic acid)
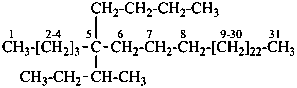
5-(butan-2-yl)-5-butylhentriacontane (PIN)
[butyl is not treated as butan-1-yl; for the use of enclosing marks, see P-16.5.1.2]
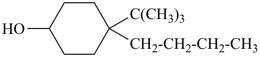
4-butyl-4-tert-butylcyclohexan-1-ol (PIN)
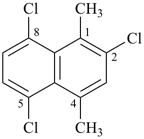
2,5,8-trichloro-1,4-dimethylnaphthalene (PIN)
[not 1,4-dimethyl-2,5,8-trichloronaphthalene; ‘trichloro’ is cited before ‘dimethyl’ as ‘chloro’ is earlier alphabetically than ‘methyl’]
[not 1,4,6-trichloro-5,8-dimethylnaphthalene; the locant set ‘1,2,4,5,8’ is lower than ‘1,4,5,6,8’ (see P-14.3.5)]
P-14.5.2 The name of a prefix for a substituent is considered to begin with the first letter of its complete name.
Examples:
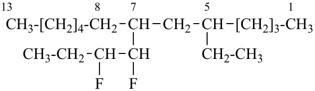
7-(1,2-difluorobutyl)-5-ethyltridecane (PIN)
[not 5-ethyl-7-(1,2-difluorobutyl)tridecane;
the compound substituent name in the PIN starts with ‘d’, and ‘d’ is earlier alphabetically than ‘e’]
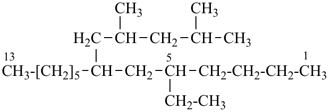
7-(2,4-dimethylpentyl)-5-ethyltridecane (PIN;
(‘dimethylpentyl’ begins with ‘d’ that precedes ‘e’ in alphabetical order)
P-14.5.3 When an alphanumerical ordering is required and Roman letters do not permit a decision for the order of citation, italicized letters are considered.
Examples:
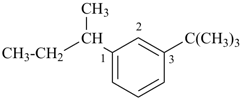
3-tert-butyl-1-(1-methylpropyl)benzene
1-(butan-2-yl)-3-tert-butylbenzene (PIN)
(for use of enclosing marks, see P-16.5.1.2)
[not 1-sec-butyl-3-tert-butylbenzene]
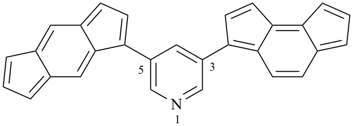
3-(as-indacen-3-yl)-5-(s-indacen-1-yl)pyridine (PIN)
[not 5-s-indacen-1-yl-3-as-indacen-3-ylpyridine]
And similarly, naphtho[1,2-f]quinolin-2-yl is alphanumerically preferred to naphtho[1,2-g]quinolin-1-yl (‘f’ before ‘g’).
P-14.5.4 When two or more prefixes consist of identical Roman letters, priority for order of citation is given to the group that contains the lowest locant(s) at the first point of difference.
Examples:
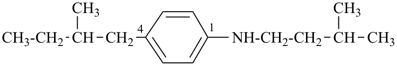
4-(2-methylbutyl)-N-(3-methylbutyl)aniline (PIN)
4-(2-methylbutyl)-N-(3-methylbutyl)benzenamine
(for ordering the substituents ‘2’ is lower than ‘3’; the fact that ‘N’ is lower than ‘4’ is irrelevant)
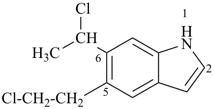
6-(1-chloroethyl)-5-(2-chloroethyl)-1H-indole (PIN)
(the locant ‘1’ is lower than ‘2’)
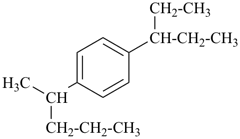
1-(pentan-2-yl)-4-(pentan-3-yl)benzene (PIN)
(the locant ‘2’ is lower than ‘3’; for the use of enclosing marks, see P-16.5.1.2)
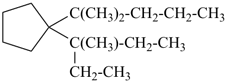
1-(2-methylpentan-2-yl)-1-(3-methylpentan-3-yl)cyclopentane (PIN)
[not 1-(3-methylpentan-3-yl)-1-(2-methylpentan-2-yl)cyclopentane;
the locant set ‘2,2’ is lower than ‘3,3’)
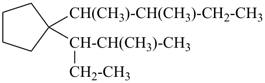
1-(2-methylpentan-3-yl)-1-(3-methylpentan-2-yl)cyclopentane (PIN)
[not 1-(3-methylpentan-2-yl)-1-(2-methylpentan-3-yl)cyclopentane;
the locant set ‘2,3’ is lower than ‘3,2’]
P-14.6 NONALPHANUMERICAL ORDER
Alphanumerical ordering resolves practically all of the ordering problems for organic names. However, there are the occasional names in which the alphanumerical character numberings are identical. In such cases, other characters, such as enclosing marks or punctuation marks, must be compared with letters and/or numbers, or with each other to select a preferred IUPAC name. Letters or numbers are always preferred to any other character. When necessary the order of priority for other characters is as follows, listed in decreasing priority order: braces > square brackets > parentheses (round brackets) > periods > commas > semi-colons > colons > hyphens.
Note: Previous recommendations did not provide rules for selection
of a preferred name beyond the ordering alphanumerical characters. For compounds whose alphanumerical characterics do not lead to a unique name, an ordering for nonalphanumerical characters, such as commas, enclosing marks, etc., is needed. The above paragraph provides this information.
Examples:
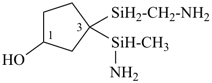
3-[amino(methyl)silyl]-3-[(aminomethyl)silyl]cyclopentan-1-ol (PIN)
{not 3-[(aminomethyl)silyl]-3-[amino(methyl)silyl]cyclopentan-1-ol;
alphanumerical characters are identical; at the fourth character of the name, the letter ‘a’ is preferred to an open parenthesis}
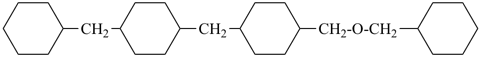
1-[(cyclohexylmethoxy)methyl]-4-{[4-(cyclohexylmethyl)cyclohexyl]methyl}cyclohexane (PIN)
{not 1-({4-[(cyclohexylmethoxy)methyl]cyclohexyl}methyl)-4-(cyclohexylmethyl)cyclohexane
the alphabetic letters and the primary substituent locants are identical for both names;
however, there is no locant for the first internal substituent in the PIN, but in the
alternative name the locant for the first internal substituent is ‘4’ and no locant is preferred to ‘4’}
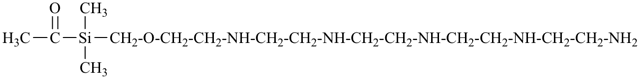
N1-(2-{[acetyldi(methyl)silyl]methoxy}ethyl)-N2-[2-({2-[(2-aminoethyl)amino]ethyl}amino)ethyl]ethane-1,2-diamine 
N1-{2-[(2-{[acetyldi(methyl)silyl]methoxy}ethyl)amino]ethyl}-N2-{2-[(2-aminoethyl)amino]ethyl}ethane-1,2-diamine 
[the parent structure must
be a diamine; alphanumerical orders of the first two names are the same;
at the third character the nonalphanumerical ‘{’ is preferred to ‘[’]
19-amino-3,3-dimethyl-5-oxa-8,11,14,17-tetraaza-3-silanonadecan-2-one (PIN)
P-14.7 INDICATED AND ‘ADDED INDICATED HYDROGEN’
P-14.7.1 Indicated hydrogen. Under certain circumstances it is necessary to indicate in the name of a mancude ring or ring system, i.e., one that contains the maximum number of noncumulative double bonds, one or more positions where no multiple bond is attached. When these positions are occupied by hydrogen atoms, the name can be made specific by indicating the position of one or more hydrogen atoms in the structure; this is accomplished by adding to the front of the name an italic capital ‘H’ preceded by an appropriate numerical locant for each of these atoms.
Examples:
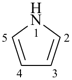 | | 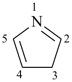 |
1H-pyrrole (PIN)
pyrrole | | 3H-pyrrole (PIN) |
In the first example, the ‘indicated hydrogen’ locates one hydrogen atom in position ‘1’ of the pyrrole ring; and in the second, the ‘indicated hydrogen’ indicates an ‘extra’ hydrogen atom at position ‘3’ , i.e., one hydrogen atom more than the number present if there were a double bond in the ring at that position. Indicated hydrogen of this type precedes the name of a parent hydride. In general nomenclature, indicated hydrogen may be omitted (see P-25.7.1.3) and 1H-pyrrole can be called just ‘pyrrole’. However, in a preferred IUPAC name a locant and the symbol ‘H’ must be cited.
Detailed procedures for using ‘indicated hydrogen’ are discussed in P-58.2.1.
P-14.7.2 ‘Added indicated hydrogen’. A second type of indicated hydrogen is called ‘added indicated hydrogen’. It describes hydrogen atoms added to a specified structure as the consequence of the addition of a suffix describing a structural modification. ‘Added indicated hydrogen’ is cited in parentheses after the locant of the structural feature to which it refers.
Example:
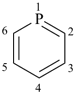 | | 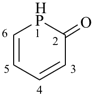 |
| phosphinine (PIN) | | phosphinin-2(1H)-one (PIN) |
‘Added indicated hydrogen’ is used to introduce a free valence, a radical or an ionic center, or a principal characteristic group into a fully unsaturated heteromonocyclic compound or fused polycyclic system in the absence of, or lack of, sufficient hydrogen atoms to accommodate the operation at the site of the operation. Such substituted compounds are named by using a suffix to denote an operation on either a –CH= group or a =C< atom, or on equivalent heteroatoms such as –N=, or groups such as =NH.
Detailed procedures for using ‘added indicated hydrogen’ are discussed in P-58.2.2.
Examples:
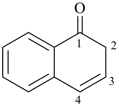
naphthalen-1(2H)-one (PIN)
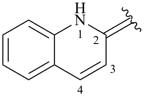
quinolin-2(1H)-ylidene (preferred prefix)
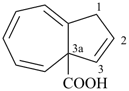
azulene-3a(1H)-carboxylic acid (PIN)
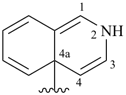
isoquinolin-4a(2H)-yl (preferred prefix)
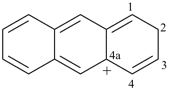
anthracen-4a(2H)-ylium (PIN)
‘Added indicated hydrogen’ is not cited for preferred IUPAC names when two identical characteristic groups that essentially remove one of the double bonds of the parent structure are cited as suffixes in a mancude compound.
Examples:
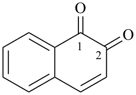
naphthalene-1,2-dione (PIN)
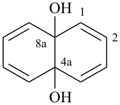
naphthalene-4a,8a-diol (PIN)
P-14.8 ADDUCTS
P-14.8.1 Organic adducts
An adduct is a chemical species, each molecular entity of which is formed by direct combination of separate molecular entities in such a way that there may be a change of connectivity but no loss of atoms from any of the molecular entities (see ref. 19).
In this subsection, only adducts formed from organic compounds are considered. Inorganic adducts are discussed in ref. 12, IR-5.5. In this subsection, preferred IUPAC names for two different types of organic adducts are considered, Lewis adducts and π-adducts. Salts of organic bases or acids of unknown structure are named in a similar manner, for which see Chapter P-7. In general nomenclature the definition of an adduct as given above may include other types of representations as adducts, for example, butadiene—hydrogen chloride.
Formulas for these adducts are written in the order that follows the general seniority of organic compounds given in P-41. Names are formed by citing the names of individual compounds in the order of the formula connected by long (em) dashes (—). The proportions of components are indicated after the name by an arabic number separated by a solidus from other numbers; arabic numbers and the solidus are placed in parentheses, separated from the name by a space.
Salts and Lewis adducts between boron compounds and the Group 15 elements in which the mode of attachment is specified by italic element symbols are considered in P-68.1.6.
|
For adducts composed solely of organic compounds, the individual components are cited in the order of seniority of classes (see P-41) in formulas, no longer according to the number of species in the adduct, nor in accordance with the alphanumerical order as recommended in the 1979 Recommendations (see Rule D-1.55, ref. 1) and in the revised Nomenclature of Inorganic Chemistry, 2005 Recommendations (ref. 12). For adducts composed of organic and inorganic compounds, organic compounds precede inorganic compounds in formulas. Names are formed by citing the names of individual components in the order of the formula. The use of order of seniority, a universal system, as a ranking criterion is preferred to the language-dependent alphanumerical order for both preferred IUPAC names and in general nomenclature. |
|
Examples:
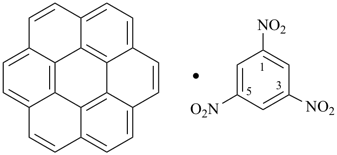
coronene—1,3,5-trinitrobenzene (1/1) (PIN)
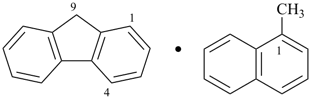
9H-fluorene—1-methylnaphthalene (1/1) (PIN)
(HOOC-CH2-NH-[CH2]2)2N-CH2-COOH • HN[CH2-P(O)(OH)2]2 • H2N-[CH2]2-NH2
2,2′-{[(carboxymethyl)azanediyl]bis(ethane-2,1-diylazanediyl)}diacetic acid—[azanediylbis(methylene)]bis(phosphonic acid)—ethane-1,2-diamine (1/1/1)
N,N′-{[(carboxymethyl)azanediyl]di(ethan-2,1-diyl)}diglycine—[azanediylbis(methylene)]bis(phosphonic acid)—ethane-1,2-diamine (1/1/1)
4,5-dichloro-3,6-dioxocyclohexa-1,4-diene-1,2-dicarbonitrile—1-methyl-1H-perimidine—iodomethane (1/1/1) (PIN)
benzene-1,2,3-triol—quinolin-8-ol (1/2) (PIN)
4-nitrobenzoic acid—quinolin-8-ol (1/1) (PIN)
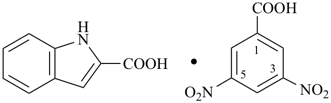
1H-indole-2-carboxylic acid—3,5-dinitrobenzoic acid (1/1) (PIN)
(C5H5)Fe(C5H4-CHO) • C6H6 • CBr4
ferrocenecarbaldehyde—benzene—tetrabromomethane (1/1/1)
4-aminobenzoic acid—1,3,5-trinitrobenzene (1/1) (PIN)
acetic acid—(7,7-dimethyl-2-oxobicyclo[2.2.1]heptan-1-yl)methanesulfonic acid—ethyl acetate—[1,1′-binaphthalene]-3,3′-diylbis(diphenyl-λ5-phosphanone) (1/1/1/1) (PIN)
{piperazin-1-ium 115-[(2,4-dinitrophenyl)diazenyl]-3,6,9,13,16,19-hexaoxa-1,11(1,3)-dibenzenacycloicosaphane-12-carboxylate}—ethyl acetate—dichloromethane (1/1/1) (PIN)
{piperazin-1-ium 13-[(2,4-dinitrophenyl)azo]-3,6,9,17,20,23-hexaoxatricyclo[23.3.1.111,15]triaconta-1(29),11(30),12,14,25,27-hexaene-29-carboxylate}—ethyl acetate—dichloromethane (1/1/1)
Solvates, including hydrates (see P-14.8.2), are treated as adducts and preferred IUPAC names must use the notation for denoting the proportion of components described above. In general nomenclature, hydrates may be named by adding the words ‘hydrate’ to the name preceded by an appropriate numerical prefix, such as ‘mono’,‘di’, ‘tri’, etc. Terms such as ‘hemi’ and ‘sesqui’ are also used.
Example:
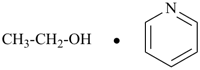
ethanol—pyridine (1/1) (PIN)
P-14.8.2 Mixed organic – inorganic adducts
Formulas for mixed adducts composed of organic and inorganic chemical species are written in the following order: organic components in order as described in P-14.8.1, inorganic components in order as described in Ref 12; water (if present), is cited last.
Although organic components are preferred IUPAC names, preferred IUPAC names cannot be assigned to mixed adducts because preferred IUPAC names have not yet been determined for inorganic components.
However, names are formed by citing the names of individual compounds, connected by long (em) dashes (—). The proportions of the components are indicated after the name by an arabic number(s) separated by a solidus enclosed in parentheses and separated from the name by a space. Hydrates may be named by adding the word ‘hydrate’ to the name preceded by an appropriate numerical prefix, such as ‘mono’,‘di’, ‘tri’, etc. Terms such as ‘hemi’ and ‘sesqui’ are also used.
Examples:
N-propyl-N-(pyridin-3-yl)-1H-indol-1-amine—hydrogen chloride (1/1)
N4-(7-chloroquinolin-4-yl)-N1,N1-diethylpentane-1,4-diamine—phosphoric acid (1/2)
N4-(7-chloroquinolin-4-yl)-N1,N1-diethylpentane-1,4-diamine—sulfuric acid (1/1)
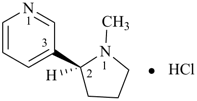
3-[(2S)-1-methylpyrrolidin-2-yl]pyridine—hydrogen chloride (1/1)
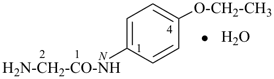
2-amino-N-(4-ethoxyphenyl)acetamide—water (1/1)
2-amino-N-(4-ethoxyphenyl)acetamide monohydrate
HOOC-COOH • H2N-CH2-CH2-NH2 • 3/2 H2O
oxalic acid—ethane-1,2-diamine—water (2/2/3)
oxalic acid—ethane-1,2-diamine sesquihydrate
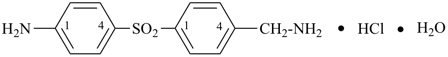
4-[4-(aminomethyl)benzene-1-sulfonyl]aniline—hydrogen chloride—water (1/1/1)
P-15 TYPES OF NOMENCLATURE
P-15.0 Introduction
P-15.1 Substitutive nomenclature
P-15.2 Functional class nomenclature
P-15.3 Multiplicative nomenclature
P-15.4 Skeletal replacement (‘a’) nomenclature
P-15.5 Functional replacement nomenclature
P-15.6 Conjunctive nomenclature
P-15.0 INTRODUCTION
‘Nomenclature’, in chemistry, is a system by which names are formed using various nomenclatural operations in accordance with a set of principles, rules, and conventions. There are fundamentally two types of nomenclature: (1) substitutive nomenclature, the principal nomenclature used in organic chemistry and the basis of IUPAC preferred organic names; and (2) additive nomenclature used in inorganic chemistry for generating coordination names. These two types are applied to name organic compounds and inorganic compounds, thus making nomenclature a matter of choice between them. For example, SiCl4 can be named silicon tetrachloride (binary name), tetrachloridosilicon (coordination name), and tetrachlorosilane (substitutive name). Although coordination nomenclature is not discussed in these recommendations, it is used in the nomenclature of organometallic compounds belonging to Groups 1 and 2, for example, dimethylmagnesium. Binary names are used for salts composed of an anionic or cationic organic part, for example sodium acetate and methanaminium chloride. Preferred IUPAC names are recommended when there is a choice within the limits of the nomenclature of organic compounds, for example a choice between two substitutive names (acetic acid and ethanoic acid), between a multiplicative name and a substitutive name (2,2′-oxydiacetic acid and [(carboxymethyl)oxy]acetic acid), or between a substitutive name and a functional class name (bromomethane and methyl bromide), but not between a coordination or binary name and an organic name, for example, when the choice could be between a substitutive name and coordination name (tetrachlorosilane and tetrachloridosilicon).
The nomenclature of organic compounds is considered as the set of different types of nomenclature based on the various operations described in P-13. The term nomenclature is usually associated with more than one kind of operation. Substitutive nomenclature may be regarded as based on substitutive, additive, and subtractive operations. Functional class nomenclature is essentially based on additive operations, but includes substituent group names formed by substitutive nomenclature. Multiplicative nomenclature is a subset of substitutive nomenclature based on cyclic parent hydrides, functionalized or not, functionalized acyclic parent hydrides, and heteroacyclic parent hydrides. Skeletal replacement nomenclature usually refers to replacement by ‘a’ terms and is thus often called just ‘a’ nomenclature. Similarly, conjunctive nomenclature, is restricted to conjunction operations involving rings or ring systems substituted by a chain bearing a principal group named substitutively or having a retained name. Finally, functional replacement nomenclature defines the rules for the use of prefixes and infixes to indicate replacement of oxygen atoms or oxygen groups by nitrogen atoms, nitrogen groups, chalcogen atoms, halogen atoms, peroxy groups, etc. (see P-15.5).
Thus, the term nomenclature is not normally associated with only a single type of operation, and name formation within a nomenclature system consists of a series of operations of various types, for example the subtractive operation in the formation of double bonds and the functional replacement operation in the replacement of oxygen atoms by chalcogen or nitrogen atoms.
The term nomenclature also applies to families or classes of compounds, for instance Nomenclature of Radicals and Ions, Phane Nomenclature for naming compounds composed of chains and/or ring systems, and Fullerene Nomenclature to describe all operations necessary to name polycyclic carbon cage compounds and their derivatives. This term is also used to describe families of compounds of natural origin, for example, Nomenclature of Natural Products is based on the concept of stereoparents. The nomenclature of carbohydrates, of α-amino acids, peptides, lipids, and of some other compounds of biochemical significance is generally considered to be Biochemical Nomenclature and, as such, is published comprehensively separately.
P-15.1 SUBSTITUTIVE NOMENCLATURE
Substitutive nomenclature is based on the selection of a parent structure having substitutable hydrogen atoms (see P-15.1.5) which are substituted by nomenclaturally significant structural fragments represented either by prefixes and/or suffixes or only by prefixes. In substitutive nomenclature there are three kinds of parent structures, parent hydrides, functionalized parent hydrides, and functional parent compounds.
P-15.1.1 Parent hydrides
Parent hydrides are unbranched acyclic, cyclic structures, or acyclic/cyclic structures to which only hydrogen atoms are attached. They may have retained names as described in Chapters P-2 and P-3, semisystematic names for natural products (see P-101), or names derived by the systematic methods described in Chapters P-2 and P-3.
Examples:
methane (PIN; retained name, P-21.1.1.1)
cyclohexane (PIN; P-22.1.1)
styrene (retained name, P-31.1.3.4); ethenylbenzene (PIN)
pyridine (retained name, PIN; P-22.2.1)
cholestane (retained name, Table 10.1, b)
Parent hydrides may be substituted by prefixes and/or suffixes. A complete substitutive name may be described schematically in Fig. 1.1.
| Prefixes | Parent | Endings | Suffixes |
Detachable
substitutive
prefixes | Saturation/
unsaturation
prefixes
(hydro/dehydro) | Nondetachable
structure
defining
prefixes | Name of
parent
hydride | Unsaturation
endings
(ane/ene/
yne) | Functional
suffixes | Cumulative
suffixes |
Fig. 1.1 Order of components in substitutive names based on parent hydrides
P-15.1.2 Functional parent compounds
P-15.1.2.1 Functional parent compounds used in systematic organic nomenclature are structures having retained names that imply the presence of at least one characteristic group and which have one or more hydrogen atoms attached to at least one of its skeletal atoms or in which at least one of its characteristic groups can form at least one kind of functional modification. The modification of these functional parent compounds is limited as only detachable prefixes and cumulative suffixes can be added. Furthermore, preferred IUPAC functional parent compound names cannot be modified by changing the degree of hydrogenation by means of the prefixes hydro/dehydro, by changing the ending ‘ane’ into ‘ene’ or ‘yne’, or by modifing the structure by nondetachable prefixes, such as ‘cyclo’.
Examples:
acetic acid (PIN; P-65.1.1)
aniline (PIN; P-62.2.1.1.1)
phosphonic acid (preselected name; P-67.1.1)
P-15.1.2.2 A second group of functional parent compounds is extensively used in the nomenclature of natural products, for which nondetachable prefixes, such as ‘cyclo’, ‘nor’, ‘homo’; hydro/dehydro prefixes; and the endings ‘ene’, ‘yne’, etc., as specified in Chapter P-10.
Examples:
D-glucose (Table 10.2)
alanine (Table 10.4)
A complete preferred IUPAC substitutive name based on a functional parent compound may be described schematically as shown in Fig. 1.2.
Detachable substitutive
prefixes | Name of functional parent
compound | Cumulative
suffixes |
Fig. 1.2 Order of components in a substitutive name based on a functional parent compound
P-15.1.2.3 A functionalized parent hydride is a parent hydride substituted by a characteristic group suffix, for example, ethanamine (see P-62.2.1.2) and cyclohexanol (see P-63.1.2), and should not be confused with a functional parent compound.
P-15.1.3 Suffixes (see also P-33)
There are, in general, two kinds of suffixes, functional suffixes and cumulative suffixes.
(1) Functional suffixes are generally simple suffixes used for describing characteristic groups that express classes. For example, the classes ketones, acids, amines, and esters are denoted by the suffixes -one, -carboxylic acid, -amine, and -carboxylate, respectively. Functional suffixes are exclusive suffixes, as the presence of one suffix denoting the principal characteristic group excludes all other suffixes describing other characteristic groups that must be designated as prefixes.
(2) Cumulative suffixes are used to designate radicals, ions, radical ions, and related species, for example, -yl, -ium, -ide, as well as substituent groups. Cumulative suffixes are not exclusive. They can be combined with each other as well as with functional suffixes, for example, -aminyl, -nitrilium, -sulfaniumyl.
Examples:
| CH4 |  | CH4•+ |
methane (PIN)
(parent hydride) | | methaniumyl (PIN)
(‘iumyl’ is a cumulative suffix) |
| CH3-CH3 |  | CH3-CH2-NH2 |  | CH3-CH2-NH3+ |
ethane (PIN)
(parent hydride) | | ethanamine (PIN)
(‘amine’ is a functional suffix) | | ethanaminium (PIN)
(‘ium’ is a cumulative
suffix) |
P-15.1.4 Position of the endings ‘ane’, ‘ene’, and ‘yne’
The modification of the ending ‘ane’ to ‘ene’ or ‘yne’ in acyclic, cyclic and polycyclic parent hydrides is used to describe the subtractive operation that inserts double and triple bonds into saturated parent hydrides. These endings are cumulative and can be combined with functional suffixes.
P-15.1.5 Prefixes and their order in names
There are two kinds of prefixes, nondetachable and detachable. Nondetachable prefixes describe structural modifications to the parent structure creating new parent structures, for example, replacement prefixes, which can be either skeletal replacement (‘a’) prefixes (see P-15.4) or ‘functional replacement prefixes’ (see P-15.5). Detachable prefixes are of two types: prefixes that describe saturation or unsaturation (‘hydro’ and ‘dehydro’); and prefixes that describe substitution, also called alphabetizable prefixes.
P-15.1.5.1 Nondetachable prefixes
P-15.1.5.2 Detachable hydro/dehydro prefixes
P-15.1.5.3 Detachable alphabetizable prefixes
Nondetachable prefixes, the detachable saturation prefixes (‘hydro/dehydro’), and the detachable alphatizable prefixes are cited in names as indicated in Fig. 1.1 (see P-15.1.1).
P-15.1.5.1 Nondetachable prefixes
Nondetachable prefixes are permanently attached to the name of the parent structure in a given order, which normally matches the order of operations used to modify the parent structure. Prefixes describing the first operation are attached directly to the name of the parent structure; those resulting from a second operation are placed in front of those already introduced, and so on (this technique may be termed ‘advancing backwards’ from the name of the parent structure). The order is precisely prescribed for each category, as indicated below:
P-15.1.5.1.1 Nondetachable prefixes creating new parent structures:
(a) alicyclic rings and ring systems by prefixes such as: ‘cyclo’, ‘bicyclo’, ‘tricyclo’, etc.; ‘spiro’, ‘dispiro’, etc.;
(b) fused ring systems by fusion prefixes: ‘benzo’, ‘naphtho’, ‘imidazo’, etc.;
(c) bridged fused ring systems by the addition of bridge prefixes: ‘methano’, ‘epoxy’, etc.;
(d) spiro compounds formed by combining names of cyclic compounds in (a), (b), and/or (c).
P-15.1.5.1.2 Replacement of atoms other than hydrogen by other atoms.
This type of replacement, called ‘skeletal replacement’, is essentially the replacement of carbon atoms by heteroatoms; it takes place with acyclic and cyclic hydrocarbons generating new parent structures by using skeletal replacement (‘a’) prefixes, i.e., ‘oxa’, ‘aza’, ‘thia’, etc.
P-15.1.5.1.3 Indicated hydrogen (see P-14.7) is also a nondetachable prefix and is introduced in front of all other nondetachable prefixes.
P-15.1.5.2 Detachable hydro/dehydro prefixes
| These two prefixes are introduced in names by an additive or subtractive operation; thus, they are not included in the category of detachable alphabetizable prefixes describing substitution (P-15.1.5.3). In names, they occupy a place between nondetachable prefixes and the detachable alphabetizable prefixes. These prefixes express modifications of the degree of hydrogenation of a ring or ring systen having the maximum number of noncumulative double bonds (a mancude structure) and are treated for numbering like the endings ‘ene’ and ‘yne’, which fulfill the same function. In names, the prefix ‘dehydro’ precedes the prefix ‘hydro’, when both are present. Simple numerical terms, such as ‘di-’, ‘tri-’, ‘tetra-’, etc., are used with ‘hydro’ and ‘dehydro‘. |
P-15.1.5.3 Detachable alphabetizable prefixes
These prefixes describe substituent groups denoting characteristic groups that are not cited as principal characteristic group or groups derived from parent hydrides and are cited before the ‘hydro-dehydro’ prefixes, if present (see P-31.2), or nondetachable prefixes, if present, as indicated in Fig. 1.1. They are alphabetized in accordance with P-14.5.
P-15.1.6 Other components of substitutive names
In addition to the components described above, the following nomenclatural indicators are added, as required:
P-15.1.6.1 Multiplicative prefixes placed before suffixes and prefixes to indicate multiple occurrences.
P-15.1.6.2 Locants used to indicate positions of the parent structure at which modifications represented by suffixes, prefixes, and endings occur.
P-15.1.6.3 Stereodescriptors placed at the front of the complete name or name fragment to which they apply (see Chapter P-9).
P-15.1.7 Construction of substitutive names
This subsection describes the formation of substitutive names and the application of four general sets of rules dealing with numbering (P-14.4), locants (P-14.3), multiplicative prefixes (P-14.2), and alphanumerical order (P-14.5). These four sets of rules are applied in constructing names of most organic compounds. In the first set of examples, alkanes and branched alkanes are used. In the second set, the general rule of numbering is exemplified by saturated and unsaturated acyclic compounds denoted by suffixes. The full question of name construction is considered in P-45.
P-15.1.7.1 Naming of alkanes and branched alkanes
P-15.1.7.1.1 The names of alkanes are either the retained names, methane, ethane, propane, and butane; or are names formed systematically by adding the ending ‘ane’ to a basic multiplicative prefix, with elision of the final letter ‘a’ of the multiplicative term (See Chapter P-2).
Examples:
| CH4 | CH3-CH3 | CH3-CH2-CH3 |
| methane (PIN) | ethane (PIN) | propane (PIN) |
| | | |
| CH3-CH2-CH2-CH3 | CH3-CH2-CH2-CH2-CH3 | CH3-[CH2]8-CH3 |
| butane (PIN) | pentane (PIN) | decane (PIN) |
P-15.1.7.1.2 Monovalent simple substituent groups derived from unbranched acyclic hydrocarbons (alkanes) by the removal of one hydrogen atom from a terminal carbon atom (subtractive operation) are named by replacing the ending ‘ane’ of the name of the hydrocarbon by ‘yl’ (see P-29.3.2.1) or, if one hydrogen atom is removed from a nonterminal carbon atom of a chain, by replacing the final ‘e’ of the name of the hydrocarbon by ‘yl’ (see P-29.3.2.2) (‘yl’ is a cumulative suffix, see Fig. 1.1, P-15.1.1).
Examples:
CH3-CH2-CH2-CH2–
butyl (preferred prefix)
CH3-CH2-CH2-CH2-CH2–
pentyl (preferred prefix)
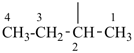
butan-2-yl (preferred prefix)
P-15.1.7.1.3 A saturated branched acyclic hydrocarbon is formed by substituting one or more substituent groups, formed as described in P-15.1.7.1.2, into the longest chain present in the structure (substitutive operation); it is named by prefixing the designations of the side chains, as formed in P-15.1.7.1.2, to the name of the longest chain [see P-15.1.7.1.4 for numbering].
Example:
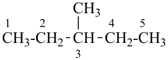
3-methylpentane (PIN)
P-15.1.7.1.4 The longest chain is numbered from one end to the other by arabic numbers, the direction being chosen so as to give the lower locants to the substituent groups (side chains) [see P-14.4 (f)]. The lower set of locants is defined as the set that, when compared term by term with other locant sets cited in order of increasing magnitude, has the lower term at the first point of difference (see P-14.3.5). The locants are placed immediately in front of the part of the name to which they refer. Identical simple substituent groups are indicated by multiplicative prefixes, such as ‘di’, ‘tri’, etc. [P-16.3.3 (b)]. For compound or complex substituent groups (see P-29.4 and P-29.5), the multiplicative prefixes ‘bis’, ‘tris’, ‘tetrakis-’, etc. (P-14.2.2) are used as described in P-16.3.5 (a).
Examples:
 | incorrect numbering
correct numbering |
| 2-methylpentane (PIN) | |
| | |
 | incorrect numbering
correct numbering |
2,3,5-trimethylhexane (PIN)
(not 2,4,5-trimethylhexane;
the locant set 2,3,5 is lower than 2,4,5) | |
P-15.1.7.1.5 If two or more different substituent groups are present, they are cited in names in alphanumerical order (see P-14.5). When two or more substituent groups occupy equivalent positions, the one to be assigned the lower locant is that one cited first in the name.
Examples:
 | incorrect numbering
correct numbering |
4-ethyl-2-methylhexane (PIN)
(not 3-ethyl-5-methylhexane;
the locant set 2,4 is lower than 3,5) | |
| | |
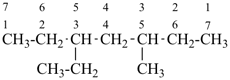 | incorrect numbering
correct numbering |
3-ethyl-5-methylheptane (PIN)
(not 5-ethyl-3-methylheptane;
the lower locant must be assigned to the
substituent group that is cited first) | |
P-15.1.7.2 The numbering rules
The following examples illustrate the rule for numbering described in P-14.4. This rule establishes an order of priority among different nomenclatural features for assignment of the lowest possible locants.
P-15.1.7.2.1 Alcohols are named by attaching the suffix ‘ol’ to the name of the parent hydride, with elision of the final letter ‘e’ in the parent hydride, if present. When alone in the structure, the characteristic group(s) must receive the lowest locant(s) possible, which is (are) cited immediately in front of the suffix (see P-14.3.2).
Example:
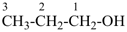
propan-1-ol (PIN)
P-15.1.7.2.2 Alkenes are acyclic branched or unbranched hydrocarbons having one double bond. When one double bond is present, an unbranched alkene is named by changing the ending ‘ane’ in the name of the alkane having the same number of carbon atoms to the ending ‘ene’ (see P-31.1). The double bond is assigned the lowest locant possible, which is placed immediately in front of the ending ‘ene’ (see P-14.3.2)
Example:
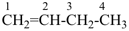
but-1-ene (PIN)
P-15.1.7.2.3 When several nomenclatural features are present in a structure, lowest locants are assigned in accordance with P-14.4. For example, in an unsaturated alcohol having one substituent group, lowest locants are assigned in the order: 
(a) characteristic group cited as suffix (-ol);
(b) unsaturation (‘ene‘ ending, for example);
(c) detachable alphabetized prefixes (a methyl group, for instance);.
The following examples illustrate the application of this rule
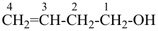 | |
| but-3-en-1-ol (PIN) | |
| | |
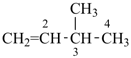 | |
| 3-methylbut-1-ene (PIN) | |
| | |
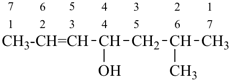 | incorrect numbering
correct numbering |
6-methylhept-2-en-4-ol (PIN)
(not 2-methylhept-5-en-4-ol) | |
P-15.1.8 Substitution rules for parent structures with retained names
The following rules describe the substitutability of parent structures having retained names found in P-22.1.2, P-22.1.3, Tables 2.2, 2.3, 2.7, 2.8, Tables 3.1, 3.2, and for functional parent compounds given in P-34. These rules do not apply to functional parent compounds used in the nomenclature of natural products (see Chapter P-10), where specific rules apply.
P-15.1.8.1 Type 1 Unlimited substitution by substituent groups cited either as suffixes or prefixes; this applies mainly to parent hydrides
P-15.1.8.2 Type 2 Limited substitution generalized as follows:
Type 2a Substitution limited to substituent groups cited as prefixes in accordance with the seniority of functional groups explicitly expressed or implied in the functional parent compound name;
Type 2b Substitution limited to substituent groups that are compulsory prefixes (see Table 5.1);
Type 2c Substitution for parent structures not covered by Types 2a or 2b.
P-15.1.8.3 Type 3 Substitution of any kind is not allowed
P-15.1.8.1 Substitution rules for Type 1 retained names
Type 1 retained names of parent hydrides described in Chapters P-2 and P-3 have unlimited substitution by substituent groups cited either as suffixes or prefixes.
Examples:
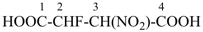
2-fluoro-3-nitrobutanedioic acid (PIN)
(butane is a retained name)
5-aminoazulen-2-ol (PIN; azulene is a retained name)
(not 2-hydroxyazulen-5-amine)
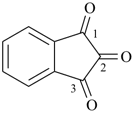
indane-1,2,3-trione
1H-indene-1,2,3-trione (PIN)
P-15.1.8.2 Substitution rules for Type 2 retained names
Substitution of parent hydrides with retained names and of functional parent compounds whose retained names explicitly or implicitly express the presence of a characteristic group normally expressed as a suffix, such as ‘-one’, or a functional class, such as ‘ether’, is limited in different ways as described in the following subsections. Rules for the substitution of inorganic oxo acids used as functional parents and parent structures are described in P-67.
P-15.1.8.2.1 Substitution rules for Type 2a retained names
Type 2a retained names include functional parent compounds whose name expresses or implies a characteristic group expressed as suffix in systematic names.
P-15.1.8.2.1.1 Substitution by substituent groups, expressed as prefixes, having a lower seniority than that denoted by the suffix is allowed.
Examples:
H2N-CH2-CO-CH3
aminoacetone
1-aminopropan-2-one (PIN)
HS-CH2-COOH
sulfanylacetic acid (PIN)
A suffix explicitly or implicitly present cannot be expressed as a prefix;
Examples:
HOOC-CH2-COOH
propanedioic acid (PIN)
malonic acid
(not 2-carboxyacetic acid)
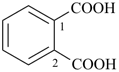
benzene-1,2-dicarboxylic acid (PIN)
phthalic acid
(not 2-carboxybenzoic acid)
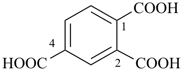
benzene-1,2,4-tricarboxylic acid (PIN)
(not 2,4-dicarboxybenzoic acid;
not 4-carboxyphthalic acid)
P-15.1.8.2.1.2 The senior characteristic group or functional class name must be expressed in the name.
Examples:
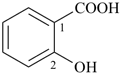
2-hydroxybenzoic acid (PIN)
(not 2-carboxyphenol)
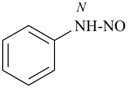
phenylnitrous amide (PIN)
N-nitrosoaniline 
P-15.1.8.2.2 Substitution rules for Type 2b retained names
Type 2b retained names include parent compounds explicitly or implicitly devoid of suffixes; acetylene (PIN) and allene are examples. Substitution of these parent compounds is possible by using specifically designated prefixes only.
The following characteristic groups cited can be used to substitute parent structures of Type 2b (ring and the side chain if required): halides –Br, –Cl, –F, –I, pseudohalides –N3, –NCO (and chalcogen analogues), –NC, substituent groups derived from the halogen oxo acids –ClO, –ClO2, –ClO3 (similarly for groups in which Cl is replaced by Br or I), –NO2 and –NO, and –OR (R = alkyl groups), and chalcogen analogues, and –SO-R and –SO2-R, and Se and Te analogues (see Table 5.1).
Examples:
Br-C≡C-H
bromoacetylene
bromoethyne (PIN)
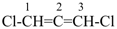
1,3-dichloroallene
1,3-dichloropropa-1,2-diene (PIN)
P-15.1.8.2.3 Substitution rules for Type 2c retained names
Type 2c retained names are functional parent compounds that are not included in Type 2b, for example, hydroxylamine (see P-68.3.1.1.1), formic acid (see P-65.1.8), and anisole (see P-34.1.1.4).
Examples:
H2N-O-CH3
O-methylhydroxylamine (PIN)
[not methoxyamine (see P-68.3.1.1.1.2)]
HS-CO-OH
carbonothioic S-acid (PIN)
[not sulfanylformic acid (see P-65.1.8.1)]
P-15.1.8.3 Substitution rules for Type 3 retained names
No substitution of any kind is allowed for retained names of parent hydrides and functional parent compounds of type 3. However, functionalization of characteristic groups, such as the formation of esters, anhydrides, and salts is allowed
Examples:
Cl-CH2-CH2-COOH
3-chloropropanoic acid (PIN)
(not 3-chloropropionic acid)
CH3-CH2-CO-O-CH2-CH3
ethyl propanoate (PIN)
ethyl propionate
P-15.2 FUNCTIONAL CLASS NOMENCLATURE
P-15.2.0 Introduction
Functional class nomenclature was quite important in the early days of organic chemistry when many compounds were named using class names. The procedures were identical with those of substitutive nomenclature except that suffixes were never used. Substituent groups, called ‘radicals’ in early nomenclature, were used in association with a name denoting the class; this nomenclature was called ‘radicofunctional nomenclature’. With time, substitutive nomenclature replaced functional class nomenclature in all but a few instances. In the context of IUPAC preferred names, substitutive nomenclature is the primary way for naming organic compounds; functional class nomenclature is reduced to a strict minimum.
The notion of functional class nomenclature is also applied to compounds that are named using a class name, but not necessarily preceded by a substituent group name. To that purpose, ‘functional modifiers’ are used to indicate a functional change, for example the change of acid to anhydride, as in acetic anhydride, or the formation of derivatives of ketones, for example, butan-2-one oxime.
It is convenient to classify the main operation involved in functional class nomenclature as an additive one, as is done in P-13.3.3.2. However it is also possible (and probably more relevant from a historical point of view) to regard the process as one of specifying the substituent groups (‘radicals’) present in compounds for which a class name is given. For instance, the name ‘methyl alcohol’ (for CH3-OH) consists of the name ‘methyl’ for the substituent group CH3– and the class name ‘alcohol’ (for R-OH).
Functional class nomenclature is discussed in relation to the traditional use of substituent group names and the use of functional modifiers. Some names are formed on the basis of a class name, but are not considered as belonging to the functional class nomenclature. They are called ‘descriptive’ names, for example, ‘propanoic acid methyl ester’, and are never considered as IUPAC preferred names. The order of seniority of classes (see P-41) is given in the context of preferred IUPAC names.
P-15.2.1 Functional class nomenclature using substituent group names
P-15.2.2 Functional class nomenclature using functional modifiers
P-15.2.3 Seniority of functional classes
P-15.2.4 Polyfunctional compounds
P-15.2.1 Functional class nomenclature using substituent group names
P-15.2.1.1 Functional class names are formed by expressing the functional class name of the compound as one word and the remainder of the molecule as a monovalent group name cited as a separate word, placed in front of the class name. Preferred IUPAC functional class names are restricted to esters (P-65.6.3.2), acyl halides (P-65.5.1), and pseudohalides (see P-65.5.2). Alkyl glycosides receive also functional class names that are classified as approved names in carbohydrate nomenclature (see P-102.5.6.2).
Examples:
CH3-CH2-CO-O-CH3
methyl propanoate (PIN)
CH3-CO-Cl
acetyl chloride (PIN)
C6H5-CO-CN
benzoyl cyanide (PIN)
C6H5-CH2-NCS
benzyl isothiocyanate
(isothiocyanatomethyl)benzene (PIN)
C6H5-NC
phenyl isocyanide
isocyanobenzene (PIN)
CH3-OH
methyl alcohol
methanol (PIN)
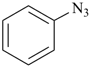
phenyl azide
azidobenzene (PIN)
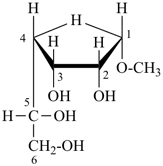
methyl α-D-gulofuranoside
P-15.2.1.2 When the functional class name refers to a characteristic group that is divalent, the two substituent groups attached to it are both expressed. When they are the same, appropriate numerical prefixes are used. When different, they are written as separate words, in alphanumerical order, locants being added as needed.
Examples:
CH3-O-CO-CH2-CH2-CO-O-CH3
dimethyl butanedioate (PIN)
dimethyl succinate
CH3-CO-O-CH2-CH2-O-CO-CH3
ethane-1,2-diyl diacetate (PIN)
ethylene diacetate
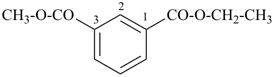
ethyl methyl benzene-1,3-dicarboxylate (PIN)
CH3-CH2-CO-CH2-CH3
diethyl ketone
pentan-3-one (PIN)
CH3-CH2-CO-CH3
ethyl methyl ketone
(not methyl ethyl ketone)
butan-2-one (PIN)
P-15.2.2 Functional class nomenclature using functional modifiers
Many derivatives of principal characteristic groups or functional parent compounds (see P-34) may be named by modifiers consisting of one or more separate words placed after the name of the parent structure. This method is most useful in an indexing environment, but it is the only method used for naming acyclic anhydrides (see P-65.7.1), N-oxides, N-sulfides, N-selenides, and N-tellurides (see P-62.5) in the context of preferred IUPAC names.
Examples:
CH3-CO-O-CO-CH3
acetic anhydride (PIN)
(the term ‘acid’ is replaced by ‘anhydride’)
(CH3)3NO
N,N-dimethylmethanamine N-oxide (PIN)
trimethylazane N-oxide
trimethylamine N-oxide
(traditional name, but cannot be substituted)
Functional modifiers are still acceptable for general nomenclature purposes, but the preferred IUPAC names are substitutive names for azines, oximes, hydrazones, semicarbazones, carbohydrazones, acetals, and hemiacetals.
Examples:
CH3-CH2-CH=N-OH
propanal oxime
(the term ‘oxime’ is added to the name of the carbonyl compound)
N-propylidenehydroxylamine
N-hydroxypropan-1-imine (PIN)
CH3-CH2-CH=N-NH2
propanal hydrazone
propylidenehydrazine (PIN)
(CH3)2C=N-NH-CO-NH2
acetone semicarbazone
2-(propan-2-ylidene)hydrazine-1-carboxamide (PIN)
CH3-CH2-CH(O-CH3)2
propanal dimethyl acetal
1,1-dimethoxypropane (PIN)
CH3-CH2-CH2-CO-OCH3
butanoic acid methyl ester
methyl butanoate (PIN)
P-15.2.3 Seniority of functional classes.
When two classes are present and both named by functional class nomenclature, the senior class must be chosen in accordance with the seniority order of classes (see P-41). The relevant order for preferred IUPAC names is anhydrides, esters, acyl halides. The seniority order of halides and pseudohalides is discussed in P-65.5. The senior class is expressed by functional class nomenclature and the lower ranking classes are expressed by prefixes, as usual, in that part of the name that is constructed by substitutive nomenclature.
Examples:
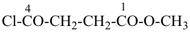
methyl 4-chloro-4-oxobutanoate (PIN)
methyl 3-(chlorocarbonyl)propanoate
methyl 3-carbonochloridoylpropanoate
Cl-CH2-OH
chloromethyl alcohol
chloromethanol (PIN)
(an alcohol is senior to a halide)
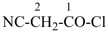
cyanoacetyl chloride (PIN)
(an acyl chloride senior to a nitrile)
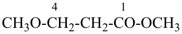
methyl 4-methoxybutanoate (PIN)
(an ester senior to an ether)
P-15.2.4 Polyfunctional compounds
Functional class names for polyfunctional compounds are not recommended. Substitutive nomenclature is preferred.
HO-CH2-CH2-CO-CH3
4-hydroxybutan-2-one (PIN; substitutive name)
2-hydroxyethyl methyl ketone (functional class name)
P-15.3 MULTIPLICATIVE NOMENCLATURE
P-15.3.0 Introduction
P-15.3.1 General methodology
P-15.3.2 Construction of multiplicative names
P-15.3.3 Identical structural units linked by unsymmetrical multiplicative groups
P-15.3.4 Limitations of multiplicative nomenclature
P-15.3.0 Introduction
Multiplicative nomenclature is used to name assemblies of identical parent structures. There are two subsets of multiplicative nomenclature:
(1) A subset of substitutive nomenclature in which the identical structural units are linked by di- or polyvalent substituent groups (see P-15.3.1);
(2) A subset of functional class nomenclature in which the identical structural units are linked by a di- or polyvalent nonacidic residue (this subset is only used to name esters, for which see P-65.6.3.2, and leads to names such as ‘ethane-1,2-diyl diacetate’ for CH3COO-CH2-CH2-OCOCH3).
In the subset of multiplicative nomenclature as a subset of substitutive nomenclature, two or more identical units, for example, parent structures, linked by di- or polyvalent substituent groups (called ‘multiplicative atoms or groups’ or ‘linking atoms or groups’) can be named in two ways:
(a) by multiplicative nomenclature, in which two or more parent structures are connected by symmetrical or unsymmetrical single or concatenated substituent groups; and
(b) by substitutive nomenclature (see P-15.1) in which one of the parent structures is chosen as the senior parent structure and the remainder of the structure is expressed by substituent prefixes.
For example, the following compound can be named 4,4′-sulfanediyldibenzoic acid (PIN) [numbering shown in (I)] using multiplicative nomenclature, a name that includes both of the benzoic acid groups in the name of the parent structure.
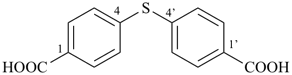 | | 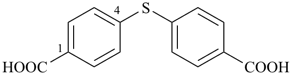 |
| (I) | | (II) |
Alternatively, it can be named 4-[(4-carboxyphenyl)sulfanyl]benzoic acid by substitutive nomenclature [numbering shown in (II)], a name that includes only one of the benzoic acid groups in the name of the parent structure; the other benzoic acid group is expressed as a prefix.
In this section, the general principles for construction of multiplicative names are discussed. The rules for the use of multiplicative nomenclature in constructing preferred IUPAC names are given in P-51.3.
| Multiplicative nomenclature is now extended to cyclic structures with or without characteristic groups; chains composed only of carbon atoms continue to be excluded. In past recommendations, multiplicative nomenclature was limited to compounds having characteristic groups expressed as suffixes or implied by a retained name, and for heterocyclic parent hydrides. The system now has also been expanded to allow substitution on the central unit of a multiplying group (P-15.3.1.2.1.2) and the use of nonsymmetrical central units under specific conditions (P-15.3.3.1). |
|
P-15.3.1 General methodology
P-15.3.1.1 Identical parent structures
P-15.3.1.2 Multiplicative substituent groups
P-15.3.1.3 Multiplicative name formation
P-15.3.1.1 Identical structural units
There are four types of identical parent structures in multiplicative nomenclature:
(a) cyclic parent hydrides, mancude or saturated;
(b) mononuclear or polynuclear acyclic parent hydrides with the exception of saturated or unsaturated acyclic hydrocarbons;
(c) cyclic or acyclic parent hydrides substituted by characteristic groups expressed as suffixes, i.e., functionalized parent hydrides (see P-15.1.2.3);
(d) functional parent compounds having substitutable hydrogen atoms, for example, acetic acid or phosphonic acid (see P-15.1.2.1).
Identical parent structures must be attached to multiplicative substituent groups by identical bonds (single, double, or triple) and be identically substituted.
P-15.3.1.2 Multiplicative substituent groups
There are two types of multiplicative substituent groups:
P-15.3.1.2.1 Simple multiplicative atoms or groups
P-15.3.1.2.2 Concatenated multiplicative groups
P-15.3.1.2.1 Simple multiplicative atoms or groups
P-15.3.1.2.1.1 Any simple polyvalent substituent group (see P-29.1 for definition) may be used as a multiplicative substituent group when attached to two or more identical structural units.
–CH2–
methylene (preferred prefix)
(not methanediyl)
–O–
oxy (preselected prefix)
–S–
sulfanediyl (preselected prefix)
thio
–OO–
peroxy (preselected prefix)
(dioxy is no longer recommended)
–SS–
disulfanediyl (preselected prefix)
dithio
–N<
nitrilo (preselected prefix)
| The prefix ‘nitrilo’, –N< ,is used as the preselected prefix only when the three bonds are attached to different atoms; it is no longer to be used for the structure –N=, which now is named as the preselected prefix ‘azanylylidene’. |
1,4-phenylene (also 1,2- and 1,3-isomers;
preferred prefixes)
–OC-CH2-CH2-CO–
butanedioyl (preferred prefix)
succinyl
ethane-1,1,2-triyl (preferred prefix)
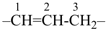
prop-1-ene-1,3-diyl (preferred prefix)
P-15.3.1.2.1.2 Substitution, expressed by prefixes or implied, is allowed on simple multiplicative groups, symmetrical or unsymmetrical, generating substitutive compound or complex (see P-29.1 for definitions) multiplicative groups.
Examples:
ClCH<
chloromethylene (preferred prefix;
a compound multiplicative group)
chloromethanylylidene (preferred prefix;
a compound multiplicative group)
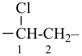
1-chloroethane-1,2-diyl (preferred prefix;
a compound multiplicative group)
CH3-N<
methylazanediyl (preferred prefix;
a compound multiplicative group)
(no longer methylimino)
| The name azanediyl (preselected prefix) derived from the preselected parent hydride name ‘azane’ is recommended for the multiplicative substituent group –NH– ; the name imino (also a preselected prefix) is used only for =NH as a substituent. |
|
ClCH2-P<
(chloromethyl)phosphanediyl (preferred prefix;
a complex multiplicative group)
2-methylpropane-1,3-diyl (preferred prefix)
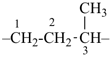
butane-1,3-diyl (preferred prefix; see P-29.3.2.2)
1-methylpropane-1,3-diyl (see P-29.4.1)
2-(dimethylamino)propane-1,3-diyl (preferred prefix)
P-15.3.1.2.2 Concatenated multiplicative groups
P-15.3.1.2.2.1 Concatenation is the method used for the formation of multipart di- and polyvalent multiplicative substituent groups. A concatenated multiplicative group is formed by first citing the central multiplicative substituent group, followed by a multiplicative prefix, such as ‘di’, ‘tri’, etc. or ‘bis’, ‘tris’, etc., and then, in order, and in the direction toward the identical parent structures, the names of successive di- or polyvalent substituent groups.
Examples:
–O-CH2-O–
methylenebis(oxy)
(preferred prefix)
(not methylenedioxy; dioxy could be ambiguous,
because it could be ‘peroxy’ or twice ‘oxy’ [see P-15.3.1.2.1.1, P-16.3.6 (b).]
–CH2-O-CH2–
oxybis(methylene) (preferred prefix)
benzene-1,2,4-triyltris(oxy) (preferred prefix)
ethane-1,1,2-triyltrinitrilo (preferred prefix)
–CH2-NH-CO-CH2-CO-NH-CH2–
propanedioylbis(azanediylmethylene) (preferred prefix)
Note: When two or more successive multiplicative groups follow the central multiplicative group they are not prefixed by separate multiplicative prefixes. Accordingly, the preferred IUPAC name for the example just above is NOT propanedioylbis(azanediyl)bis(methylene).
P-15.3.1.2.2.2 When polyvalent substituent groups of the ‘yl/ylidene’ type that have no locants and are cited in a prescribed order are used in a multipart multiplicative substituent group, concatenation occurs by adding a monovalent free valence of the ‘yl’ type to another monovalent free valence of the ‘yl’ type, and similarly for free valences of the ‘ylidene’ type.
Examples:
–CH=N-O-N=CH–
oxybis(azanylylidenemethanylylidene) (preferred prefix)
[not oxybis(nitrilomethanylylidene)]
–O-N=CH-CH=N-O–
ethanediylidenebis(azanylylideneoxy) (preferred prefix) 
–O-N=C=N-O–
methanediylidenebis(azanylylideneoxy) (preferred prefix)
=CH-N=C=N-CH=
methanediylidenebis(azanylylidenemethanylylidene) (preferred prefix)
[not methanediylidenebis(nitrilomethanylylidene)]
P-15.3.1.2.2.3 Substitution on the central substituent group and on successive substituent groups of a concatenated multiplicative substituent group is allowed provided that the sequence of atoms and bonds, starting at the central substituent group, are identical in each of the branches.
Examples:
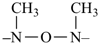
oxybis(methylazanediyl) (preferred prefix)
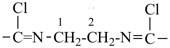
ethane-1,2-diylbis[azanylylidene(chloromethanylylidene)] (preferred prefix)
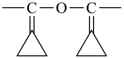
oxybis(cyclopropylidenemethylene) (preferred prefix)
Note: The preferred prefix given here would be ambiguous if the suffix ‘ylidene’ were not limited to the indication of double bonds (see P-29.2); it could refer to a structure in which the cyclopropane bonds went to the CH2 group and the oxygen atom as single bonds; however, the latter structure would have the preferred IUPAC prefix oxybis(cyclopropane-1,1-diylmethylene)
P-15.3.1.2.2.4 Numbering of the components of a multipart di- or trivalent multiplicative substituent group, when necessary, is achieved by attributing lowest locants to the atoms that are at the end of the component nearest to the multiplied parent structure, except where the component has a fixed numbering. The locants attached to the multiplied parent structure are cited last. When there is no choice the locants are cited in increasing numerical order.
Examples:
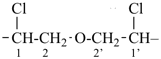
oxybis(1-chloroethane-2,1-diyl) (preferred prefix)
oxybis(1-chloroethylene)
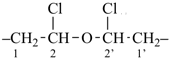
oxybis(2-chloroethane-2,1-diyl) (preferred prefix)
oxybis(2-chloroethylene)
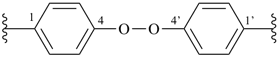
peroxydi(4,1-phenylene) (preferred prefix)
[not dioxydi(4,1-phenylene)]
methylenedi(1,3,5-triazine-6,2,4-triyl) (preferred prefix) 
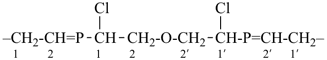
oxybis[(1-chloroethane-2,1-diyl)phosphanylylideneethan-1-yl-2-ylidene]
(preferred prefix; the numbering of ethan-1-yl-2-ylidene is fixed)
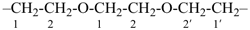
ethane-1,2-diylbis(oxyethane-2,1-diyl) (preferred prefix)
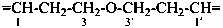
oxydi(propan-3-yl-1-ylidene) (preferred prefix)
hydrazinediylidenedi(propan-1-yl-3-ylidene) (preferred prefix;
the numbering of propan-1-yl-3-ylidene is fixed)
P-15.3.1.3 Multiplicative name formation
Multiplicative names are formed in accordance with the number of occurrences of identical structural units as defined in P-15.3.1.1 and the relationship of the linking multiplicative substituent group to the identical structural units.
When a compound contains identical structural units as defined in P-15.3.1.1 linked by a symmetrical simple, compound, complex, or concatenated multiplicative group (a di- or polyvalent substituent group), it is named by stating successively:
(a) the locants for the positions of substitution of the linking multiplicative substituent atom or group to the identical parent structural unit (the locant 1 is omitted when alone in the name of a mononuclear parent hydride);
(b) the name of the linking multiplicative substituent atom or group;
(c) the numerical prefix ‘di’ , ‘tri’, etc.; and/or ‘bis-’, ‘tris-’, etc., with no elision of the final vowel before the name of the identical parent structural unit;
(d) the name of one of the identical structural units including the principal characteristic group and substituents, if any, enclosed in appropriate enclosing marks (see P-16.5).
The numbering of the identical parent structural unit is retained and, when there is a choice, the locants of the point of substitution by the linking multiplicative substituent groups on the identical parent structure are as low as possible.
P-15.3.2 Construction of multiplicative names
P-15.3.2.1 Assemblies of identical structural units (see P-15.3.1.1)
P-15.3.2.2 Locants for nitrogen atoms in identical structural units
P-15.3.2.3 Use of multiplicative prefixes ‘bis’, ‘tris’, etc.
P-15.3.2.4 Substituted identical structural units (see P-15.3.1.1)
P-15.3.2.1 Assemblies of identical structural units (see P-15.3.1.1)
Retained and systematic names can be used as identical parent structural units. Primes, double primes, etc., are used to distinguish among the locants of the identical parent structural unit. When functionalized parent hydrides, i.e. parent structures substituted by groups expressed as suffixes (see P-15.1.2), have locants, they are enclosed in parentheses and preceded by a numerical prefix ‘di-’, ‘tri-’, etc. Unsubstituted functional parent compounds are preceded by the numerical prefix ‘di-’, ‘tri-ְ, etc., but are not enclosed in parentheses, provided there is no ambiguity as described in P-15.3.2.3. Compound and complex multiplicative substituent groups are enclosed in parentheses, brackets or braces, as required (see P-16.5)
Examples:
1,1′-peroxydibenzene (PIN)
(not dioxydibenzene)
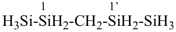
1,1′-methylenebis(disilane) (PIN)
1,1′-methylenebis(1-azacyclododecane) (PIN)
2,2′-sulfanediyldi(ethan-1-ol) (PIN)
2,2′-thiodi(ethan-1-ol)
8,8′-oxydi(spiro[4.5]decane) (PIN)
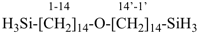
[oxydi(tetradecane-14,1-diyl)]bis(silane) (PIN)
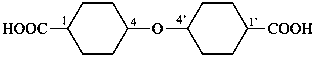
4,4′-oxydi(cyclohexane-1-carboxylic acid) (PIN)
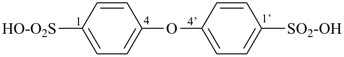
4,4′-oxydi(benzene-1-sulfonic acid) (PIN)
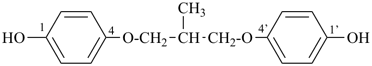
4,4′-[(2-methylpropane-1,3-diyl)bis(oxy)]diphenol (PIN)
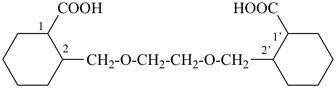
2,2′-[ethane-1,2-diylbis(oxymethylene)]di(cyclohexane-1-carboxylic acid) (PIN)
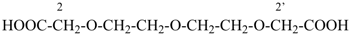
2,2′-[oxybis(ethane-2,1-diyloxy)]diacetic acid (PIN)
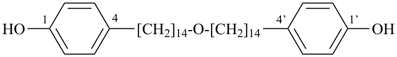
4,4′-[oxydi(tetradecane-14,1-diyl)]diphenol (PIN)
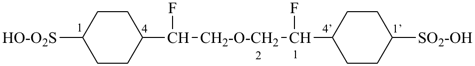
4,4′-[oxybis(1-fluoroethane-2,1-diyl)]di(cyclohexane-1-sulfonic acid) (PIN)
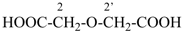
2,2′-oxydiacetic acid (PIN)
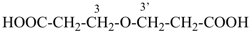
3,3′-oxydipropanoic acid (PIN)
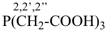
2,2′,2′′-phosphanetriyltriacetic acid (PIN)
(HO)2P(O)-CH2-NH-CH2-P(O)(OH)2
[azanediylbis(methylene)]bis(phosphonic acid) (PIN)
[oxydi(pyridazine-4,3,5-triyl)]tetramethanol (PIN)
(the locant set ‘3,4,5’ is lower than ‘4,5,6’ and the locant set ‘3,5’ is the lowest locant set of the pyridazine assigned to the junction to the multiplied parent structure)
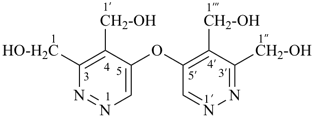
[oxydi(pyridazine-5,3,4-triyl)]tetramethanol (PIN)
(the locant set ‘3,4,5’ is lower than ‘4,5,6’; the locant set ‘3,4’ is the lowest locant set of the pyridazine assigned to the junction to the multiplied parent structure)
H2N-CH2-CH2-O-CH2-CH2-NH2
2,2′-oxydi(ethan-1-amine) (PIN)
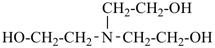
2,2′,2′′-nitrilotri(ethan-1-ol) (PIN)
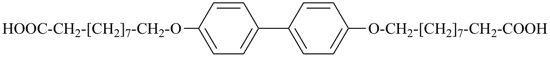
10,10′-[[1,1′-biphenyl]-4,4′-diylbis(oxy)]di(decanoic acid) (PIN).
[Note: A double set of square brackets appears in the above name because square brackets are required for substituent names derived from ring assembly names (see P-16.5.2.1) and brackets are needed to enclose the multiplicative substitutive name]
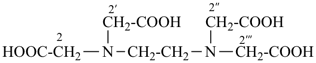
2,2′,2′′,2′′′-(ethane-1,2-diyldinitrilo)tetraacetic acid
N,N′-(ethane-1,2-diyl)bis[N-(carboxymethyl)glycine]
P-15.3.2.1.1 If there is a choice, the greater number of primes is given to the locants of the parent structure having the higher numbered point of attachment to the multiplicative substituent group. Such names are not acceptable as preferred IUPAC names but may be used in general nomenclature; in these cases preferred IUPAC names are generated by substitutive nomenclature principles (see P-51.3.3).
Examples:
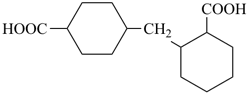
2,4′-methylenedi(cyclohexane-1-carboxylic acid) (multiplicative name)
2-[(4-carboxycyclohexyl)methyl]cyclohexane-1-carboxylic acid (PIN)
[not 4-[(2-carboxycyclohexyl)methyl]cyclohexane-1-carboxylic acid; the substituent locant ‘2’ is lower than ‘4’ (see P-45.2.2)]
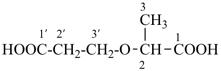
2,3′-oxydipropanoic acid (multiplicative name)
2-(2-carboxyethoxy)propanoic acid (PIN)
[not 3-(1-carboxyethoxy)propanoic acid; the substituent locant ‘2’ is lower than ‘3’ (see P-45.2.2)]
P-15.3.2.2 Locants for nitrogen atoms in identical parent structures
P-15.3.2.2.1 In multiplicative nomenclature, the use of primes on the italic letter N is used to differentiate among nitrogen atoms of identical structural units that contain one characteristic group with one or more nitrogen atoms.
Examples:
N,N′-methylenediethanamine
N,N′-diethylmethanediamine (PIN)
N′,N′′′-methylenediacetohydrazide (PIN)
P-15.3.2.2.2 In multiplicative nomenclature, a superscript arabic numeral indicating the locant of the parent structure at which the characteristic group is attached, and an appropriate number of primes, for example, N1, N2′, N4′′, etc., is used to distinguish among the nitrogen atoms of identical structural units containing two or more characteristic groups with one or more nitrogen atoms.
Examples:
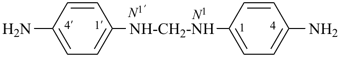
N1,N1′-methylenedi(benzene-1,4-diamine) (PIN)
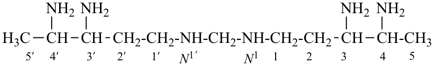
N1,N1′-methylenedi(pentane-1,3,4-triamine) (PIN)
[Note: The attachment locant of the second of the identical parent structures is N1; this is the locant that is primed]
For parent structures with multiple nitrogen atoms, such as di- or tricarboximidamides (P-66.4.1.4) and cyclophanamines (see P-62.2.5.3), even more complicated locant structures are required, such as primed letter locants with superscript numbers or with superscripted superscript numbers.
P-15.3.2.3 Use of multiplicative prefixes ‘bis’, ‘tris’, etc.
Identical parent structures for which the numerical prefixes ‘di-’, ‘tri-’, etc. cannot be used because of potential ambiguity (see P-16.3.6) are enclosed in parentheses or square brackets, as required, and preceded by the numerical terms ‘bis-’, ‘tris-’, etc.
Examples:
(benzene-1,3,5-triyl)tris(silane) (PIN)
(not benzene-1,3-5-triyltrisilane; trisilane is H3Si-SiH2-SiH3)
H3Si-[CH2]14-O-[CH2]14-SiH3
[oxydi(tetradecane-14,1-diyl)]bis(silane) (PIN)
[for ‘di’ before ‘tetradecane-14,1-diyl’ see P-16.3.4 (c)]
P-15.3.2.4 Substituted identical parent structures (see P-15.3.2.1)
When identical parent structures linked by symmetrical multiplicative groups contain substituents other than a principal characteristic group, if any, on the identical parent structures, these substituent groups are cited as prefixes in one of two ways.
P-15.3.2.4.1 Substituent groups on the identical parent structures other than the principal characteristic group, if any, are cited as prefixes associated with the identical parent structure when they fulfill the following two conditions:
(1) the linking bonds (single or multiple) between the central substituent group of the multiplicative group and all subsequent structural units are identical; and
(2) the locants of all substituent groups on the identical parent structures, including suffixes, are identical.
The identical parent structures, together with their prefixes and suffixes, if any, are treated as a compound or complex group, enclosed in parentheses, square brackets, or braces according to the nesting order given in P-16.5, and designated by the appropriate numerical prefix ‘bis’, ‘tris’, ‘tetrakis’, etc.
Examples:
1,1′-oxybis(4-bromobenzene) (PIN)
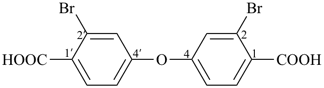
4,4′-oxybis(2-bromobenzoic acid) (PIN)
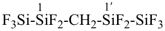
1,1′-methylenebis(pentafluorodisilane) (PIN)
N,N′-oxybis(N-methylmethanamine) (PIN)
N,N′-[ethenyl(methyl)silanediyl]bis(N-methylacetamide) (PIN)
1,1′-methylenebis[3-bromo-4-(chloromethyl)benzene] (PIN)
3,3′-oxybis[5-(1-chloroethyl)pyridine] (PIN)
(benzene-1,3,5-triyl)tris(trimethylsilane) (PIN)
3,3′-sulfanediylbis(N,N-dimethylpropanamide) (PIN)
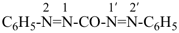
bis(phenyldiazenyl)methanone (PIN)
[not 1,1′-carbonylbis(2-phenyldiazene)]
P-15.3.2.4.2 When conditions (1) and (2) as defined in P-15.3.2.4.1, above, are not met, the substituent groups other than those identified by suffixes, if present, are cited as prefixes at the front of the name of the assembly. These prefixes are assigned the lowest locants available after priority has been given to the principal characteristic groups and the linking multiplicative substituent groups. Such names are not acceptable as preferred IUPAC names but may be used in general nomenclature; in these cases preferred IUPAC names are generated by substitutive nomenclature principles (see P-51.3.3).
Examples:
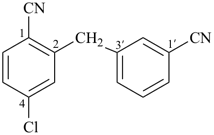
4-chloro-2,3′-methylenedibenzonitrile (multiplicative name; numbering shown)
4-chloro-2-[(3-cyanophenyl)methyl]benzonitrile (PIN)
[not 3-[(5-chloro-2-cyanophenyl)methyl]benzonitrile;
the PIN has the greater number of substituents; see P-45.2.1)
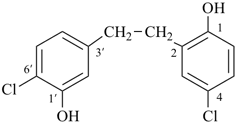
4,6′-dichloro-2,3′-(ethane-1,2-diyl)diphenol (multiplicative name; numbering shown)
4-chloro-2-[2-(4-chloro-3-hydroxyphenyl)ethyl]phenol (PIN)
[not 2-chloro-5-[2-(5-chloro-2-hydroxyphenyl)ethyl]phenol;
the locant set for the substituents in the PIN, ‘2,4’, is lower than ‘2,5’; see P-14.4 (f), P-45.2.2]
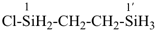
1-chloro-1,1′-(ethane-1,2-diyl)bis(silane) (multiplicative name; numbering shown)
chloro(2-silylethyl)silane (PIN)
[not [2-(chlorosilyl)ethyl]silane;
the parent silane of the PIN has more substituents; see P-45.2.1]
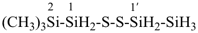
2,2,2-trimethyl-1,1′-(disulfanediyl)bis(disilane) (multiplicative name; numbering shown)
2-(disilanyldisulfanyl)-1,1,1-trimethyldisilane (PIN)
[not [2-(2,2,2-trimethyldisilan-1-yl)disulfanyl]disilane;
the parent disilane in the PIN has more substituents; see P-45.2.1)]
[not 6,6-dimethyl-3,4-dithia-1,2,5,6-tetrasilaheptane;
four heterounits are required for a skeletal replacement name (see P-51.4)]
2′,5-dichloro-2,4′-oxydipyridine (multiplicative name; numbering shown)
2-chloro-4-[(5-chloropyridin-2-yl)oxy]pyridine (PIN)
[not 5-chloro-2-[(2-chloropyridin-4-yl)oxy]pyridine;
the locant set for the substituents ‘2,4’ in the PIN is lower than ‘2,5’; see P-45.2.2]
P-15.3.2.4.3 When a choice is possible, unprimed locants are assigned to the identical parent structure having the substituent cited first in alphanumerical order.
Examples:
3-bromo-3′-chloro-1,1′-methylenedibenzene (multiplicative name; numbering shown)
1-bromo-3-[(3-chlorophenyl)methyl]benzene (PIN)
[not 1-[(3-bromophenyl)methyl]-3-chlorobenzene;
‘bromochloro’ is preferred alphanumerically to ‘bromophenyl’; see P-45.5]
5-bromo-5′-fluoro-2,2′-oxydibenzoic acid (multiplicative name; numbering shown)
2-(4-bromo-2-carboxyphenoxy)-5-fluorobenzoic acid (PIN)
[not 5-bromo-2-(2-carboxy-4-fluorophenoxy)benzoic acid;
‘2,5’ is preferred to ‘5,2’; see P-45.2.3]
P-15.3.3 Identical units linked by unsymmetrical multiplicative groups
P-15.3.3.1 Unsymmetrical central multiplicative substituent groups are allowed if they are formed from a multivalent substituent group to which subsequent groups are attached by identical bonds (single or multiple). There is no limit to the number of individual groups in the full central substituent used as a multiplier.
Examples:
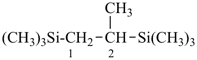
(propane-1,2-diyl)bis(trimethylsilane) (PIN)
trimethyl[1-(trimethylsilyl)propan-2-yl]silane
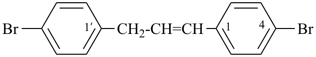
1,1′-(prop-1-ene-1,3-diyl)bis(4-bromobenzene) (PIN)
1-bromo-4-[3-(4-bromophenyl)prop-1-en-1-yl]benzene
[not 1-bromo-4-[3-(4-bromophenyl)prop-2-en-1-yl]benzene;
the correct substitutive name has lower locants in the parent substituent group]
P-15.3.3.2 Choice between multiplicative names
P-15.3.3.2.1 A preferred name multiplies the most identical parent structures.
Examples:
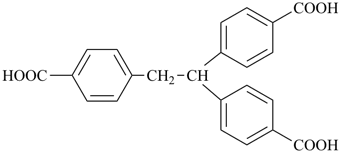
4,4′,4′′-(ethane-1,1,2-triyl)tribenzoic acid (PIN)
4,4′-[2-(4-carboxyphenyl)ethane-1,1-diyl]dibenzoic acid
(the preferred IUPAC name multiplies more identical parent structures. ‘3’ vs. ‘2’)
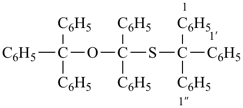
1,1′,1′′-({[diphenyl(triphenylmethoxy)methyl]sulfanyl}methanetriyl)tribenzene (PIN)
[not 1,1′-{(triphenylmethoxy)[(triphenylmethyl)sulfanyl]methylene}dibenzene;
not 1,1′,1′′-({diphenyl[(triphenylmethyl)sulfanyl]methoxy}methanetriyl)tribenzene;
the preferred IUPAC name is lower alphanumerically; ‘diphenyltriphenylmethoxy’ is lower than ‘diphenyltriphenylmethyl’]
P-15.3.3.2.2 Seniority order of classes (see P-41) is used when a choice has to be made between a parent structure and a component of a multiplicative group.
Example:
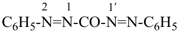
bis(phenyldiazenyl)methanone (PIN)
[not 1,1′-carbonylbis(2-phenyldiazene);
not
1,1′-[carbonylbis(diazenediyl)]dibenzene;
ketone is senior to ‘diazene’ which is senior to the carbocycle ‘benzene’, see P-41]
P-15.3.4 Limitations of multiplicative nomenclature
Multiplicative nomenclature is not used in the following circumstances. When multiplicative nomenclature is not possible, substitutive nomenclature is used to generate preferred IUPAC names.
P-15.3.4.1 Disallowed multiplicative substituent groups
P-15.3.4.2 Disallowed identical units
P-15.3.4.1 Disallowed multiplicative substituent groups.
Three different types of multiplicative substituent groups are not allowed in multiplicative nomenclature.
P-15.3.4.1.1 Unsymmetrical substituent groups consisting of two or more different components:
Examples:
| (1) | –CH2-OO– as in | H3Si-SiH2-CH2-OO-SiH2-SiH3 | |
| [(disilanylmethyl)peroxy]disilane (PIN)
[not [(disilanylperoxy)methyl]disilane;
‘disilanylmethylperoxy’ precedes ‘disilanylperoxymethyl’ in alphanumerical order (see P-14.5]
[not 3,4-dioxa-1,2,6,7-tetrasilaheptane; four hetero units are required for a skeletal replacement name (see P-51.4)] |
| (2) | –CH2-NH– as in |  |
| 4-[(4-hydroxyanilino)methyl]phenol (PIN)
[not 4-{[(4-hydroxyphenyl)methyl]amino}phenol;
‘hydroxyanilinomethyl’ precedes ‘hydroxyphenylmethylamino’ in alphanumerical order (see P-14.5)] |
| (3) | –O-CH2-CH2-S– as in | H3Si-SiH2-O-CH2-CH2-S-SiH2-SiH3 |
| 3-oxa-6-thia-1,2,7,8-tetrasilaoctane (PIN)
{[2-(disilanyloxy)ethyl]sulfanyl}disilane
[not [2-(disilanylsulfanyl)ethoxy]disilane;
‘disilanyloxy’ precedes ‘disilanylsulfanyl’ in alphanumerical order; see P-14.5] |
P-15.3.4.1.2 Unsymmetrical substituent groups having terminal atoms with different types of free valencies:
Examples:
| (1) | –CH= as in |  |
| (cyclohexylidenemethyl)benzene (PIN)
[not (phenylmethylidene)cyclohexane; benzene is senior to cyclohexane (see P-44.4.1.1)] | |
| (2) | –CH2-CH=N-CH2-CH= as in |  |
| 4-(2-{[2-(4-hydroxycyclohexyl)ethylidene]amino}ethylidene)cyclohexan-1-ol (PIN)
[not 4-(2-{[2-(4-hydroxycyclohexylidene)ethyl]imino}ethyl)cyclohexan-1-ol;
‘(hydroxycyclohexyl)ethylidene’ precedes ‘(hydroxycyclohexylidene)ethyl’ in alphanumerical order; see P-14.5] | |
P-15.3.4.1.3 Unsymmetrically substituted component units:
Example:
| (1) | 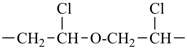 as in as in |  |
| 4-{2-[2-(4-carboxyphenyl)-1-chloroethoxy]-1-chloroethyl}benzoic acid (PIN)
[not 4-{2-[2-(4-carboxyphenyl)-2-chloroethoxy]-2-chloroethyl}benzoic acid;
‘1-chloroethoxy’ precedes ‘2-chloroethoxy’ in alphanumerical order; see P-45.5] |
P-15.3.4.2 Disallowed identical units
Acyclic hydrocarbons, saturated or unsaturated, are not allowed as parent structures in multiplicative nomenclature [see P-15.3.1.1 (b)].
Example:
1-{[2-(ethylsulfanyl)-1-(propylsulfanyl)ethen-1-yl]sulfanyl}propane (PIN; substitutive name)
(not 1,1′-{[2-(ethylsulfanyl)ethene-1,1-diyl]bis(sulfanyl)}dipropane;
alkanes are still not allowed to be identical units)
P-15.4 SKELETAL REPLACEMENT (‘a’) NOMENCLATURE
P-15.4.0 Introduction.
Skeletal replacement (‘a’) nomenclature is a subset of replacement nomenclature that also includes functional replacement nomenclature (see P-13.2). Functional replacement nomenclature is discussed in P-15.5. Just as functional replacement is considered a nomenclature, skeletal replacement is also considered a nomenclature.
In the nomenclature of organic compounds, skeletal replacement based on the replacement of carbon atoms by other atoms included in the general scope of the nomenclature of organic compounds is described under the title of skeletal replacement (‘a’) nomenclature, because the heteroatoms replacing carbon atoms are denoted by nondetachable prefixes ending in ‘a’. Skeletal replacement (‘a’) nomenclature also includes replacement of boron atoms by other atoms, including carbon (see P-68.1.1.3.1), and the replacement of heteroatoms by other atoms, including carbon, to modify fundamental structures of natural products as described in P-101.4. Skeletal replacement denoted by methods other than ‘a’ prefixes is described for the replacement of nitrogen atoms by phosphorus and arsenic atoms in certain heterocycles (see Table 2.9) and oxygen atoms by sulfur, selenium, and tellurium atoms in other specific heterocycles (see Table 2.8).
This section deals primarily with skeletal replacement in hydrocarbon parent hydrides. Skeletal replacement (‘a’) nomenclature is used in two ways:
(a) to generate names for heterocyclic parent hydrides by replacing carbon atoms in corresponding cyclic hydrocarbons; and
(b) to generate simpler names for heteroacyclic structures than those formed by following the principles of substitutive nomenclature, for example, in naming polyamines, polyethers, etc., by replacing carbon atoms in corresponding acyclic hydrocarbons.
| In previous recommendations, a heteroacyclic chain had to be terminated by carbon atoms (Rule R-2.2.3.1 in the 1993 Guide); but in these recommendations a heterocyclic chain now may terminate on any of the following heteroatoms: P, As, Sb, Bi, Si, Ge, Sn, Pb, B, Al, Ga, In, Tl, and C (see P-15.4.3.1).
|
|
The selection of preferred IUPAC names involving skeletal replacement (‘a’) nomenclature is discussed in P-51.4.
P-15.4.1 General rules
P-15.4.1.1 Nondetachable prefixes, called ‘a’ prefixes, are used to designate the replacing skeletal atoms with their standard bonding number. Those related to these recommendations are listed in Table 1.5.
Table 1.5 Skeletal replacement (‘a’) prefixes
Standard
Bonding
Numbers | 3 | 4 | 3 | 2 | 1 |
| B | bora | C | carba | N | aza | O | oxa | F | fluora |
| Al | alumina | Si | sila | P | phospha | S | thia | Cl | chlora |
| Ga | galla | Ge | germa | As | arsa | Se | selena | Br | broma |
| In | inda | Sn | stanna | Sb | stiba | Te | tellura | I | ioda |
| Tl | thalla | Pb | plumba | Bi | bisma | Po | polona | At | astata |
P-15.4.1.2 For naming and numbering purposes, the following decreasing order of element seniority is followed: F > Cl > Br > I >At > O > S > Se > Te > Po > N > P > As > Sb > Bi > C > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl (see Appendix 1). Once a structure modified by skeletal replacement (‘a’) prefixes has been named and numbered, it is considered to be a new parent hydride. As locants assigned to heteroatoms are essential, all locants must be cited as defined in P-14.3.3.
P-15.4.1.3 The symbol λn is used to describe heteroatoms having nonstandard bonding numbers (P-14.1.3). In names, it is placed immediately after the locant (without an intervening hyphen) denoting the heteroatom.
Example:
6λ5-phosphaspiro[4.5]decane (PIN)
P-15.4.1.4 Anionic and cationic skeletal replacement (‘a’) prefixes used to designate anionic and cationic heteroatoms are derived from the neutral prefixes given in Table 1.5. These skeletal replacement prefixes are all preselected.
Examples:
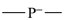 | 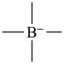 |  |
| phosphanida | boranuida | sulfanuida
λ4-sulfanida |
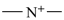 | 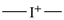 | 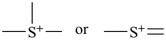 |
| azanylia | iodonia | thionia |
The selection of preferred IUPAC names involving ionic skeletal replacement (‘a’) prefixes is fully discussed in P-72.4 and P-73.4.
P-15.4.2 Skeletal replacement (‘a’) nomenclature is applied in three ways in naming heterocyclic parent hydrides.
P-15.4.2.1 Skeletal replacement (‘a’) nomenclature is applied simply in the generation of individual heteromonocyclic parent hydrides (see P-22.2.3), heterocyclic von Baeyer parent hydrides (see P-23.3), heterocyclic spiro parent hydrides composed of only monocyclic rings (see P-24.2.4.1), and fullerenes (see P-27.5). These heterocyclic compounds are used as identical parent structures in multiplicative nomenclature, but not as components in fusion nomenclature or ring assemblies.
P-15.4.2.2 When individual heterocycles are components of heterocyclic spiro ring systems not covered in P-15.4.2.1 above [see P-24.3.4, P-24.4.3 (in part), and P-24.5.2 (in part)], heterocyclic phane systems (see P-26.5.2), or heterocyclic ring assemblies (see P-28.4), skeletal replacement (‘a’) nomenclature is applied in a two-step procedure. First the structure is named as a total or partial hydrocarbon system. Then skeletal replacement (‘a’) prefixes are added and cited at the front of the name. Names formed by this method are used as identical parent structures in multiplicative nomenclature.
P-15.4.2.3 Heteromonocyclic mancude components named by skeletal replacement (‘a’) nomenclature are modified when used as component rings in fusion nomenclature [see P-25.2.2.1, P-25.2.2.4 (in part), and P-25.3.2.1.2]
P-15.4.3 Skeletal replacement (‘a’) nomenclature for acyclic parent hydrides
P-15.4.3.1 Skeletal replacement names are formed by placing skeletal replacement (‘a’) prefixes in front of the name of the unbranched parent structure in accord with their seniority order given in P-15.4.1.2. Multiplicative prefixes ‘di’, ‘tri’, ‘tetra’, etc, indicate a multiplicity of identical heteroatoms, and locants are used to designate their positions. The chain must be terminated by a C atom or one of the following heteroatoms: P, As, Sb, Bi, Si, Ge, Sn, Pb, B, Al, Ga, In, or Tl.
| Termination of a hetero acyclic chain by P, As, Sb, Bi, Si, Ge, Sn, Pb, B, Al, Ga, In, or Tl is a change from earlier recommendations where the chain could only be terminated by carbon atoms.
|
|
Examples:
2,8-dioxa-4,5-dithia-11-selenadodecane (PIN)
2-oxa-4-thia-1,5-disilapentane (PIN)
P-15.4.3.2 Numbering of heteroacyclic parent hydrides
P-15.4.3.2.1 Unbranched chains are numbered continuously from one end to the other so as to give a lower set of locants to heteroatoms considered together as a set without regard to kind, and then, if there is a choice, to heteroatoms cited first in the seniority order given in P-15.4.1.2.
| In these recommendations, skeletal replacement (‘a’) nomenclature generates new acyclic parent hydrides whose numbering is fixed, as it is for rings and ring systems; this is a major modification to Rule C-0.6 (ref. 1). Then suffixes, endings, and prefixes are added in accordance with this fixed numbering.
|
|
Examples:
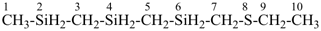
8-thia-2,4,6-trisiladecane (PIN)
(the locant set ‘2,4,6,8’ is lower than ‘3,5,7,9’; see P-14.3.5)
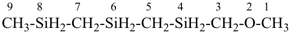
2-oxa-4,6,8-trisilanonane (PIN)
(the heteroatom locant sets, ‘2,4,6,8‘, are identical;
therefore, the low locant is given to ‘O’ over ‘Si’; see Appendix 1)
P-15.4.3.2.2 Free valences of substituent groups receive locants according to the fixed numbering of the heterochain.
Examples:
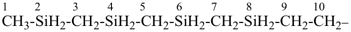
2,4,6,8-tetrasiladecan-10-yl (preferred prefix)
[Note the use of ‘decan-10-yl’ not ‘dec-10-yl’; see P-29.3.2.2]
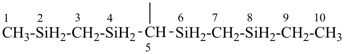
2,4,6,8-tetrasiladecan-5-yl (preferred prefix)
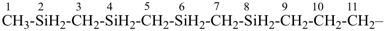
2,4,6,8-tetrasilaundecan-11-yl (preferred prefix)
P-15.4.3.2.3 Characteristic groups cited as suffixes are given locants in accordance with the fixed numbering of the heterochain.
Examples:
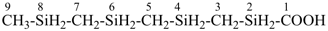
2,4,6,8-tetrasilanonan-1-oic acid (PIN)
(locant 1 is not omitted, in accordance with P-15.4.3.2.1)
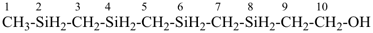
2,4,6,8-tetrasiladecan-10-ol (PIN)
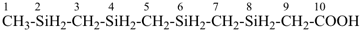
2,4,6,8-tetrasiladecan-10-oic acid (PIN)
P-15.4.3.2.4 Double and triple bonds are given locants in accordance with the fixed numbering of the heterochain, and, if there is a choice, according to the general priorities for multiple bonds (see P-31.1.2.2.2).
Example:
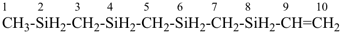
2,4,6,8-tetrasiladec-9-ene (PIN)
P-15.4.3.2.5 Heteroatoms with nonstandard valences
(a) A nonstandard bonding number of a neutral skeletal heteroatom in a parent hydride is indicated by the symbol λn, where ‘n’ is the bonding number following the appropriate locant (see P-14.1.3).
Example:
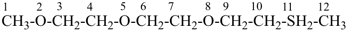
2,5,8-trioxa-11λ4-thiadodecane (PIN)
(‘O’ is preferred to ‘S’ for low locant; see Appendix 1)
(b) When there is a choice, low locants are assigned to heteroatoms having a higher bonding number.
Example:
2λ4,5,8,11-tetrathiadodecane (PIN)
P-15.4.3.3 The use of anionic and cationic skeletal replacement (‘a’) prefixes is fully discussed in P-72.4 and P-73.4, respectively.
P-15.5 FUNCTIONAL REPLACEMENT NOMENCLATURE
P-15.5.1 Definition
P-15.5.2 General methodology
P-15.5.3 Scope of functional replacement nomenclature
P-15.5.1 Definition
Functional replacement nomenclature is a method by which oxygen atoms of characteristic groups and in functional parent compounds are replaced by halogen, chalcogen, and/or nitrogen atoms.
P-15.5.2 General methodology
The replacement of oxygen atoms or hydroxy groups by other atoms or groups can be described by nondetachable prefixes attached to, or infixes inserted into, names of characteristic groups, parent hydrides and functional parent compounds having retained or systematic names. A list of prefixes and infixes is given in Table 1.6. Prefixes and infixes are used for designating the replacing atom(s) –OO–, –S–, –Se–, –Te–, etc. For example, thio indicates the replacement of oxygen atoms by sulfur in the suffixes sulfonothioyl and carbothioyl and in the functional parent thioacetic acid. Similarly, peroxo or peroxy indicates the replacement of an oxygen atom by the –OO– group in the suffix peroxoic acid and in the name peroxyacetic acid. When two or more prefixes or infixes are present indicating the presence of two or more different replacement groups, they are cited in names in alphabetical order, for example, cyclohexanecarboselenothioic Se-acid (see P-65.1.5.1) and 3-amido-2-imidodiphosphonic chloride (see P-67.2.3).
P-15.5.3 Scope of functional replacement nomenclature
Prefixes and infixes given in Table 1.6 are used in accordance with specific rules describing replacement in:
P-15.5.3.1 Replacement in heterocyclic parent hydrides
P-15.5.3.2 Replacement in characteristic groups expressed as suffixes in substitutive nomenclature
P-15.5.3.3 Replacement in characteristic groups expressed as prefixes in substitutive nomenclature
P-15.5.3.4 Replacement in functional parent compounds
P-15.5.3.1 Replacement in heterocyclic parent hydrides
Prefixes are used to modify a limited group of parent hydrides as follows. See Table 2.2 for pyran, Table 2.3 for morpholine, Table 2.8 for chromene, isochromene and xanthene, and Table 3.1 for chromane and isochromane.
Example:
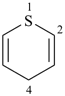
4H-thiopyran (PIN)
4H-thiine
P-15.5.3.2 Replacement in characteristic groups expressed as suffixes in substitutive nomenclature.
Replacement in suffixes is limited to –OO–, =S and –S–, =Se and –Se–, =Te and –Te–, =NH and =NNH2 and any combination of these affixes (see Table 1.6). Suffixes including a carbon atom and those corresponding to sulfonic and sulfinic acids and their analogues are modified by infixes. Other suffixes are modified by prefixes. For examples and the order of seniority of suffixes modified by functional replacement see Tables 4.2 and 4.3 (Chapter P-4), respectively.
Table 1.6 Prefixes and infixes in functional replacement nomenclature
| Prefix | Infix | Replacement atom
or group | Replacing
atom or group |
| amido | amido | –OH | –NH2 |
| azido | azido | –OH | –N3 |
| bromo | bromido | –OH | –Br |
| chloro | chlorido | –OH | –Cl |
| cyanato | cyanatido | –OH | –OCN |
| cyano | cyanido | –OH | –CN |
| dithioperoxy* | dithioperoxo* | –O– | –S-S– |
| fluoro | fluorido | –OH | –F |
| hydrazido | hydrazido | –OH | –NH-NH2 |
| hydrazono | hydrazono | =O | =N-NH2 |
| imido | imido | =O | =NH |
| iodo | iodido | –OH | –I |
| isocyanato | isocyanatido | –OH | –NCO |
| isocyano | isocyanido | –OH | –NC |
| isothiocyanato* | isothiocyanatido* | –OH | –NCS |
| nitrido | nitrido | =O and –OH | ≡N |
| peroxy | peroxo | –O– | –O-O– |
| seleno | seleno | =O or –OH | =Se or –SeH |
| telluro | telluro | =O or –OH | =Te or –TeH |
| thio | thio | =O or –OH | =S or –SH |
| thiocyanato* | thiocyanatido* | –OH | –SCN |
| thioperoxy* | thioperoxo* | –O– | –OS– or –SO– |
* Selenium and tellurium analogues are named using ‘seleno’ and ‘telluroְ in place of ‘thioְ.
Examples:
–CO-OOH
carboperoxoic acid (preferred suffix)
–CO-SH
carbothioic S-acid (preferred suffix)
–CS-OH
carbothioic O-acid (preferred suffix)
–C(=NH)-OH
carboximidic acid (preferred suffix)
–SO-OOH
sulfinoperoxoic acid (preselected suffix)
–S(=NNH2)-OH
sulfinohydrazonic acid (preselected suffix)
–(C)O-SH
thioic acid (preferred suffix)
–(C)S-NH2
thioamide (preferred suffix; no contraction to thiamide)
=S
thione (preselected suffix)
–SeH
selenol (preselected suffix)
P-15.5.3.3 Replacement in characteristic groups expressed as prefixes in substitutive nomenclature
Functional replacement is used to modify prefixes containing oxygen atoms by the prefixes ‘thio’, ‘seleno’, and ‘telluro’. Prefixes are described in appropriate sections of Chapter P-6 and listed in Appendix 2.
Examples:
–C{O/S}H
thiocarboxy (preferred prefix)
=S
thioxo
sulfanylidene (preselected prefix)
P-15.5.3.4 Replacement in functional parent compounds
Functional replacement is used to modify carboxylic acids and oxoacids, by prefixes and suffixes according to specific rules given in P-15.5.3.4.1 and P-15.5.3.4.2 below. It is also used to modify two retained names expressing functional parent structures, urea and semicarbazone, as described in P-15.5.3.4.3 below. Functional replacement is not used to replace oxygen atoms in ketones, alcohols, or derivatives such as acetals and ketals, etc.; systematic names are recommended instead.
Examples:
CH3-CS-CH3
propane-2-thione (PIN)
(not thioacetone)
C6H5-SH
benzenethiol (PIN)
(not thiophenol)
P-15.5.3.4.1 In general nomenclature, retained names of monocarboxylic acids may be modified by the prefixes peroxy, thio, seleno, and telluro to indicate the replacement of an oxygen atom by the replacing atom(s) –OO–, –S– or =S, –Se– or =Se, and –Te– or =Te (see P-65.1.4.1 and P-65.1.5.2).
Examples:
CH3-CO-SH
thioacetic S-acid
ethanethioic S-acid (PIN)
CH3-CH2-CS-OH
thiopropionic O-acid
propanethioic O-acid (PIN)
C6H5-CS-SH
dithiobenzoic acid
benzenecarbodithioic acid (PIN)
P-15.5.3.4.2 Names of mononuclear and polynuclear oxoacids are modified by prefixes and infixes listed in Table 1.6 according to the rules described in P-67.
Example:
CH3-P(=NH)(OH)(SH)
P-methylphosphonimidothioic acid (PIN)
P-15.5.3.4.3 Retained names of acyclic polynitrogen functional parent compounds described in P-66.1.6 and P-68.3.1 are modified by prefixes ‘thio’, ‘seleno’, and ‘telluro’.
Examples:
H2N-CS-NH2
thiourea (PIN; P-66.1.6.1.3)
carbonothioic diamide
H2N-NH-CSe-NH2
selenosemicarbazide
hydrazinecarboselenoamide (PIN, see P-68.3.1.2.4)
P-15.6 CONJUNCTIVE NOMENCLATURE
P-15.6.0 Introduction
Conjunctive nomenclature is based essentially on the conjunctive operation, by which a compound is formally constructed by subtracting the same number of hydrogen atoms from each component at each site where joined. It is traditionally reserved for naming compounds having a principal group attached to an acyclic component that is also directly attached by a carbon-carbon bond to a cyclic component. This method can be used in general nomenclature as an alternative to substitutive nomenclature, but it is not recommended for generating preferred IUPAC names (see P-51.5). The names have been modified (ref. 2) so that the placement of locants in the conjunctive names is consistent with the placement of locants in names as established in these recommendations (see P-14.3.2). Other aspects of conjunctive nomenclature used in CAS index nomenclature are discussed here briefly, including its limitations (P-15.6.2).
P-15.6.1 Name formation
P-15.6.1.1 Names are formed by juxtaposition of component names. The name of the cyclic component is cited first followed by the systematic or retained name of the component to which the principal characteristic group is attached.
Examples:
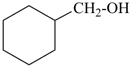
cyclohexanemethanol
cyclohexylmethanol (PIN)
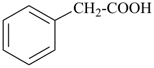
benzeneacetic acid
phenylacetic acid (PIN)
P-15.6.1.2 When necessary, the position of attachment of the side chain to the cyclic component is given by the appropriate locant placed before the name of the cyclic component, unless locants for structural features referring to the cyclic component, such as heteroatoms and indicated hydrogen, are already there; in this case the locant follows the name of the cyclic component. Since the acyclic component must terminate at the cyclic component, it is not necessary to give its locant of attachment. Carbon atoms of the side chain are indicated by Greek letters proceeding from the principal characteristic group to the cyclic component; these locants are used in the name only to locate other substituents on the side chain. The carbon atom of the characteristic group (in acids, aldehydes, nitriles, etc.) is omitted when allocating Greek letter locants.
Examples:
naphthalene-2-propanol
3-(naphthalen-2-yl)propan-1-ol (PIN)
3-(2-naphthyl)propan-1-ol
1,3-thiazole-2-acetic acid
(1,3-thiazol-2-yl)acetic acid (PIN)
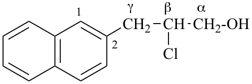
β-chloronaphthalene-2-propanol
2-chloro-3-(naphthalen-2-yl)propan-1-ol (PIN)
5,6-dimethyl-2H-isoindole-2-acetic acid
(5,6-dimethyl-2H-isoindol-2-yl)acetic acid (PIN)
P-15.6.1.3 For all purposes in conjunctive nomenclature the side chain is considered to extend only from the principal group to the cyclic component. Any other chain members, even those extending the side chain terminally, are named as substituents, appropriate prefixes and locants being placed before the name of the cyclic component.
Examples:
γ-methylnaphthalene-2-propanol
3-(naphthalen-2-yl)butan-1-ol (PIN)
3-(2-naphthyl)butan-1-ol
α-propylbenzenepropanol
1-phenylhexan-3-ol (PIN)
P-15.6.1.4 When a cyclic component carries at least two identical side chains, multiplicative prefixes ‘di’, ‘tri’, etc., are used to indicate their number; these prefixes are placed before the name of the side chain and superscript arabic numbers are used for all locants on the side chain instead of primes.
Examples:
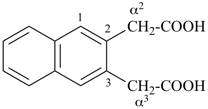
naphthalene-2,3-diacetic acid
2,2′-(naphthalene-2,3-diyl)diacetic acid (PIN)
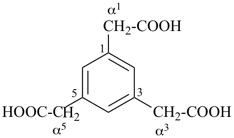
benzene-1,3,5-triacetic acid
2,2′,2′′-(benzene-1,3,5-triyl)triacetic acid (PIN)
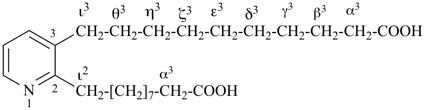
pyridine-2,3-di(decanoic acid)
10,10′-(pyridine-2,3-diyl)di(decanoic acid) (PIN)
P-15.6.1.5 When different side chains are attached to a cyclic component:
(a) the chain that contains the principal characteristic group is chosen for naming by the conjunctive method; or
(b) if there is more than one side chain containing the principal characteristic group, the conjunctive name that expresses the greater number of the principal characteristic group is chosen. When necessary, side chains are selected by applying the seniority order for selecting the principal chain (see P-44.3).
Examples:
2-(3-hydroxypropyl)quinoline-3-acetic acid
[2-(3-hydroxypropyl)quinolin-3-yl]acetic acid (PIN)
(a carboxylic acid is senior to an alcohol)
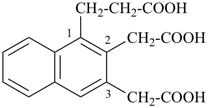
1-(2-carboxyethyl)naphthalene-2,3-diacetic acid
3-[2,3-bis(carboxymethyl)naphthalen-1-yl]propanoic acid (PIN)
P-15.6.1.6 When the side chain is linked to two different cyclic components, the senior ring or ring system is selected in accord with the seniority order of rings and ring systems (see P-44.2).
Example:
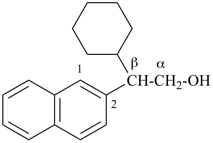
β-cyclohexylnaphthalene-2-ethanol
(naphthalene is senior to cyclohexane)
2-cyclohexyl-2-(naphthalen-2-yl)ethan-1-ol (PIN)
P-15.6.2 Limitations of conjunctive nomenclature
Conjunctive nomenclature is not used when:
(a) a double bond links the acyclic component to the ring system;
(b) a double bond or a heteroatom is present in the side chain;
(c) two identical characteristic groups are located on the side chain provided that the condition of maximum number of principal characteristic groups is not violated;
(d) characteristic groups having higher priority for citation as principal group are directly attached to the cyclic component;
(e) two or more characteristic groups of the same kind as the principal group are located on the ring or ring system.
In the cases listed above, normal substitutive nomenclature must be used.
Examples:
cyclopentylideneacetic acid (PIN)
phenylcarbamic acid (PIN)
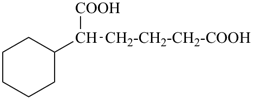
2-cyclohexylhexanedioic acid (PIN)
2-(hydroxymethyl)benzene-1,4-diol (PIN)
β1,5-dihydroxybenzene-1,3-diethanol
1-[3-hydroxy-5-(2-hydroxyethyl)phenyl]ethane-1,2-diol (PIN)
P-15.6.3 Analysis of conjunctive name construction
Note: The PIN is the substitutive name.
Example 1:
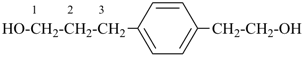 Analysis:
Analysis:
| Principal group | –OH | ol |
| | | |
| Parent: | | |
| ring: | C6H6 | benzene |
| chain: | CH3-CH2-CH3 | propane |
| Conjunctive parent including suffix: | |
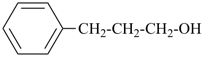 | | benzenepropanol |
| Prefix: | –CH2-CH2-OH | |
| Prefix components: | –OH
–CH2-CH3 | hydroxy
ethyl |
| | | |
| Prefix name | | 2-hydroxyethyl |
Together with other rules, this analysis leads to the conjunctive name:
4-(2-hydroxyethyl)benzenepropanol
3-[4-(2-hydroxyethyl)phenyl]propan-1-ol (PIN)
Example 2:
| Principal group | –CHO | carbaldehyde or al |
| | | |
| Parent: | | |
| ring | benzene |
| chain | heptane |
| Conjunctive parent including suffix: | |
 | | benzeneheptanal |
| Prefix: | –CHO | formyl |
Together with other rules, this analysis leads to the conjunctive name:
3-formylbenzeneheptanal
3-(7-oxoheptyl)benzaldehyde (PIN)
P-16 NAME WRITING
P-16.0 Introduction
P-16.1 Spelling
P-16.2 Punctuation
P-16.3 Multiplicative prefixes and ‘di’, ‘tri’, etc. vs. ‘bis’, ‘trisְ, etc.
P-16.4 Other numerical terms
P-16.5 Enclosing marks
P-16.6 Italicization
P-16.7 Elision of vowels
P-16.8 Addition of vowels
P-16.9 Primes
P-16.0 INTRODUCTION
Names are written in accordance with a symbolism specific to the nomenclature of organic compounds in order to avoid ambiguity and to establish an unequivocal relationship between a name and the corresponding structure. The recommended symbolism is particularly important in the formation of IUPAC preferred names. As usual, IUPAC recognizes the needs of other languages to introduce modifications specific to a particular language, but it is hoped that, whenever possible, the following conventions will be applied to construct IUPAC names for general use as well as for IUPAC preferred names.
In the 1979 and 1993 recommendations, names were written with a capital initial letter. This practice has been abandoned in recent publications in order to ensure that names of organic compounds are not considered as proper nouns; the usual practice of capitalizing letters at the beginning of a sentence must however be respected.
P-16.1 SPELLING
The spelling of elements is that given in the IUPAC Nomenclature of Inorganic Chemistry (ref. 12, Table I, pp. 248-249), for example, sulfur not sulphur, aluminium not aluminum, and caesium not cesium.
P-16.2 PUNCTUATION
P-16.2.1 Commas
P-16.2.2 Full stops
P-16.2.3 Colons and semicolons
P-16.2.4 Hyphens
P-16.2.5 Spaces
P-16.2.1 Commas are used:
(a) to separate locants, numerals, or italicized letters;
Examples:
1,2-dibromoethane (PIN, P-61.3.1)
N,N-diethylfuran-2-carboxamide (PIN, P-66.1.1.3.1.1)
(b) to separate numbers, as well as italicized letters, in fusion descriptors when they indicate the presence of separate attached components; however, italicized letters denoting peri-fused junctions are not separated by commas.
Examples:
dibenzo[c,g]phenanthrene [PIN, P-25.3.4.2.1 (c)]
6H-pyrrolo[3,2,1-de]acridine (PIN, P-25.3.1.3)
P-16.2.2 Full stops
Full stops are used to separate numbers that denote lengths of bridges in polyalicyclic names constructed according to the von Baeyer system (see P-23.2.5.1) and of chain lengths between spiro atoms in von Baeyer spiro names (see P-24.2.1).
Examples:
bicyclo[3.2.1]octane (PIN, P-23.2.3)
6-oxaspiro[4.5]decane (PIN, P-24.2.4.1.1)
P-16.2.3 Colons and semicolons
Colons separate related sets of locants; if a higher level of separation is required, semicolons are used.
Examples:
benzo[1′′,2′′:3,4;4′′,5′′:3,4]dicyclobuta[1,2-b:1′,2′-c′]difuran (PIN, see P-25.3.7.3)
11,21:22,31-tercyclopropane (PIN, see P-28.3.1)
P-16.2.4 Hyphens
P-16.2.4.1 Hyphens are used in substitutive names:
(a) to separate locants from words or word fragments;
Example:
2-chloro-2-methylpropane (PIN, P-61.3.1)
(b) after parentheses, if the closing parenthesis is followed by a locant;
Examples:
1-(chloromethyl)-4-nitrobenzene (PIN, P-61.5.1)
N1-(2-aminoethyl)-N1,N2,N2-trimethylethane-1,2-diamine (PIN, P-62.2.4.1.3)
(c) to separate adjacent locants from a subsequent opening enclosing mark;
Examples:
1-(3,4-dihydroquinolin-1(2H)-yl)ethan-1-one (PIN, P-64.3.2)
N-acetyl-N-(3-chloropropanoyl)benzamide (PIN, P-66.1.2.1)
(d) to separate italic letters from Roman letters;
Examples:
di-tert-butyl (P-61.2.3)
as-indacene (P-25.1.1)
P-16.2.4.2 No hyphen is placed after a numerical prefix cited in front of a compound substituent enclosed by parentheses, even if that substituent begins with locants;
Example:
N,1-bis(4-chlorophenyl)methanimine (PIN, P-62.3.1.1)
P-16.2.4.3 Hyphens separate the two parts of a fusion descriptor, i.e., numbers and italicized letters.
Example:
naphtho[1,2-a]azulene (PIN, P-25.3.1.3)
P-16.2.4.4 Hyphens separate stereodescriptors from the rest of the name or the part of a name to which they relate.
Example:
(2E)-but-2-ene (PIN, P-93.4.2.1.1)
P-16.2.4.5 A long hyphen (an ‘em’ dash) is used to separate the names of components in adducts.
Example:
carbon monoxide—methylborane (PIN, P-13.3.5)
P-16.2.5 Spaces are a very important type of punctuation for many kinds of names in the English language. If a space is required in a name, it must be used. On the other hand, the use of spaces where they are not required, for example, in substitutive names that must be written continuously from one end to the other using hyphens to connect the different parts, may be misleading. Spaces are used in:
(a) names of acids and salts;
Examples:
acetic acid (PIN, P-65.1.1.1)
sodium 4-ethoxy-4-oxobutanoate (PIN, P-65.6.3.3.5)
(b) functional class names;
Examples:
ethyl acetate (PIN, P-65.6.3.2.1)
2-(carbonocyanidothioyl)benzoyl chloride (PIN, P-65.5.4)
butyl ethyl sulfoxide (P-63.6)
1-(ethanesulfinyl)butane (PIN)
(c) names formed using a functional modifier;
Examples:
cyclohexanone ethyl methyl ketal (P-66.6.5.1.1)
1-ethoxy-1-methoxycyclohexane (PIN)
pentan-2-one oxime (P-68.3.1.1.2)
(pentan-2-ylidene)hydroxylamine
N-hydroxypentan-2-imine (PIN)
(d) additive names;
Examples:
ethene oxide (P-63.5)
oxirane (PIN)
trimethylphosphane oxide
trimethyl-λ5-phosphanone (PIN, P-68.3.2.3.1, P-74.2.1.4)
P-16.3 MULTIPLICATIVE PREFIXES ‘DI’, ‘TRI’, ETC. vs.‘BIS’, ‘TRIS’, ETC.
P-16.3.1 Multiplicative prefixes are derived from Greek and Latin number names (see P-14.2) and are the principal method for describing a multiplicity of identical features of a structure in chemical names. They are intimately interconnected with enclosing marks, particularly parentheses.
P-16.3.2 General methodology.
The general method to best determine whether ‘di’, ‘tri’, etc. or ‘bis’, ‘tris’, etc. should be used involves the following steps, not necessarily in the order given:
(a) determine whether the component to be multiplied is simple or compound. Simple components are unsubstituted parent hydrides, such as naphthalene ; unsubstituted prefixes, such as ethyl or tert-butyl; functionalized parent hydrides, such as benzenesulfonic acid; or retained names, such as acetic acid. All of these are multiplied by the multiplicative prefixes ‘di’, ‘tri’, etc.;
(b) determine whether these simple nomenclatural components require parentheses (see P-16.3.4) and insert them into the name;
(c) any component which is substituted automatically requires use of the multiplicative forms ‘bis’, ‘tris’, etc.;
(d) determine whether a simple nomenclature component would be ambiguous if multiplied by the multiplicative prefixes ‘di’, ‘tri’, etc., with or without the parentheses (see P-16.3.4).
P-16.3.3 The basic numerical prefixes ‘di’, ‘tri’, ‘tetra’, etc. are used to indicate a multiplicity of:
(a) functional and cumulative suffixes, basic or modified by functional replacement, with the exception of ‘thioic acid’ and ‘dithioic acid’ described in P-16.3.5 (b);
Examples:
diol (see P-63.1.2)
dicarboxylic acid (see P-65.1.1.2.1)
disulfonic acid (see P-65.3.1)
diyl (see P-71.2.3)
diide (see P-72.2.2.1)
diium (see P-73.1.1.2)
diperoxoic acid (see P-65.1.4.1)
dicarboperoxoic acid (see P-65.1 4.1)
diimidic acid (P-65.1.3.1.1)
dicarbohydrazonic acid (P-65.1.3.2.1)
disulfonoperoxoic acid (see P-65.3.1.2)
disulfinimidic acid (see P-65.3.1.4)
disulfonohydrazonimidic acid (see P-65.3.1.4)
dithioamide (see P-66.1.4.1.1)
diimidamide (see P-66.4.1.1)
dicarbohydrazonamide (P-66.4.2.1)
dicarboximidohydrazide (P-66.4.2.1)
dicarboximidamide (see P-66.4.1.1)
(b) simple substituent prefixes, including parent hydrides with ‘ene’ and ‘yne’ endings (without locants), and characteristic groups
Examples:
dicyclohexyl (see P-44.1.2)
dimethyl (see P-61.2.3)
di-tert-butyl (see P-61.2.3)
diethenyl (see P-61.2.3)
diimino (see P-62.3.1.2)
dibromo (see P-61.3.1)
(c) skeletal ‘a’ replacement prefixes
Examples:
trisila (see P-15.4.3.2.1)
trioxa (see P-15.4.3.2.5)
(d) multiplied components with retained names and systematic names other than those described in P-16.3.4 (c), P-16.3.4 (d) and P-16.3.4 (e).
Examples:
diphenol (see P-15.3.2.1)
diacetic acid (see P-15.3.2.1; see also P-15.6.1.4)
dipropanoic acid (see P-15.3.2.1)
(e) simple functional modifiers
Examples:
dioxime (see P-68.3.1.1.2)
dihydrazide (see P-66.3.5.2)
P-16.3.4 Parentheses (round brackets) (see P-16.6.1) are used to enclose multiplied components that are:
(a) simple substituent prefixes having locants;
Examples:
di(propan-2-yl) (preferred prefix; see P-61.2.3)
tetra(naphthalen-2-yl) (preferred prefix; see P-61.2.3)
(b) simple substituent prefixes modified by ‘ene’ and ‘yne’ endings and that have locants;
Example:
di(prop-1-en-2-yl) (preferred prefix; see P-68.2.6.1)
(c) simple substituent prefixes and functionalized parent hydrides beginning with a multiplicative prefix;
| This is a change from earlier editions where ‘bi’ was used
|
|
Examples:
di(dodecyl) (preferred prefix; see P-68.2.6.1)
di(tridecyl) (preferred prefix, see P-52.2.8)
di(tetradecane-14,1-diyl) (multipicative preferred prefix: see P-15.3.2.1)
di(pentanal) (see P-68.2.6.2)
(d) simple substituent groups and functionalized parent hydrides beginning with ‘dec’ to distinguish multiplied groups from acyclic components containing 12 to 19 atoms (see P-15.1.2)
Examples:
di(decyl) (see P-57.1.1.1)
di(decanoic acid) (see P-15.3.2.1)
tri(decyl) [preferred prefix,
defines three –C10H21 groups, see P-61.2.3,
tridecyl defines the –C13H27 group, see P-57.1.1.1]
tetra(decanoic acid) (preferred suffix,
defines four decanoic acid suffixes;
tetradecanoic acid defines an acid suffix group with fourteen atoms)
(e) functionalized parent hydrides with locants;
Examples:
di(benzene-1-sulfonic acid) (see P-15.3.2.1)
di(cyclohexane-1-carboxylic acid) (see P-15.3.2.1)
(f) simple components containing brackets;
Examples:
8,8′-oxydi(spiro[4.5]decane) (PIN; see P-15.3.2.1)
di(bicyclo[3.2.1]octan-3-yl) (preferred prefix; see P-44.2.1.1)
di([4-2H]benzoyl) (preferred prefix, see P-83.1.2.2)
P-16.3.5 The numerical prefixes ‘bis’, ‘tris’, ‘tetrakis’, etc. are used to indicate a multiplicity of:
(a) compound or complex (i.e. substituted) prefixes (see P-35.3 and P-35.4);
Examples:
bis(2-chloropropan-2-yl) (preferred prefix; see P-61.3.1)
bis(dimethylamino) (preferred prefix; see P-62.5)
ethane-1,2-diylbis(oxymethylene) (preferred prefix; see P-15.3.2.1)
bis(bromomethyl) (preferred prefix, see P-61.3.1)
(b) ‘thioic acid’ and ‘dithioic acid’ suffixes, and their Se and Te analogues, as exceptions to suffixes described in P-16.3.3 and P-16.3.4;
Examples:
bis(thioic acid) [multiple preferred suffix,
describes two –C(O/S)H suffixes, see P-65.1.5.1;
whereas dithioic
acid describes a –CSSH suffix, see P-65.1.5.1]
bis(dithioic acid) (multiple preferred suffix,
describes two –CSSH suffixes, see P-65.1.5.1)
not didithoic acid
(c) before suffixes that are composed of two or more cumulative suffixes;
Examples:
bis(ylium) (multiple preferred suffix,
describes two ‘-ylium’ suffixes, see P-73.2.2.1.2;
whereas ‘diylium’ could describe a cation radical suffix)
bis(nitrilium) (preferred suffix,
describes two ‘nitrilium’ suffixes, see P-73.1.2.1;
whereas ‘dinitrilium’ might be interpreted as two ‘nitrile’ suffixes and one ‘ium’ suffix)
(d) substituted functional modifiers;
Examples:
bis(phenylhydrazone) (see P-68.3.1.2.2)
pentane-2,4-dione bis(O-phenyloxime) (see P-68.3.1.1.2)
(e) substituted parent compounds in multiplicative nomenclature.
Examples:
bis(2-bromobenzoic acid) (see P-15.3.2.4.1)
bis[5-(1-chloroethyl)pyridine] (see P-15.3.2.4.1)
P-16.3.6 The prefixes ‘bis’, ‘tris’, ‘tetrakis’, etc. are also used to avoid ambiguity:
(a) before a mononuclear subset of a polynyclear acyclic structure;
Examples:
bis(sulfanyl) (preferred prefix;
defines two –SH groups, see P-63.1.5;
whereas disulfanyl defines the –SSH group; see P-63.4.2.2)
tris(sulfanyl) (preferred prefix;
defines three –SH groups (see P-63.1.5)]
whereas trisulfanyl (preferred prefix; defines the –SSSH group; see P-68.4.1)
bis(λ4-sulfanyl) (preferred prefix;
defines two –SH3 groups) (not di(λ4-sulfanyl)
Note: And similarly for the analogous Se, Te, N, P, As, Sb, Bi, Si, Ge, Sn, Pb, B, Al, Ga, In, and Tl groups.
bis(phosphonic acid) [preselected name;
describes two phosphonic acids, see P-67.1.1.2;
diphosphonic acid describes the acid (HO)(O)PH-O-PH(O)(OH), see P-67.2.1)]
bis(hydrogen sulfate) [preselected name;
describes two (hydrogen sulfate) anions or acid esters, see P-67.1.3.2,
hydrogensulfate is an inorganic name for HSO4–, see ref. 12, IR 8.5]
tris(iodide) [preselected name
describes three iodide ions, see ref. 12, IR 5.4.2.3;
triiodide describes the (I3–) anion; see ref. 12, IR 5.4.2.3]
bis(phosphate) (preselected name)
Example: D-fructofuranose 1,6-bis(phosphate) (see P-102.5.6.1.2)]
(b) before the retained names of mononuclear divalent substituent groups, such as ‘oxy’, ‘thio’, and ‘methylene’ to distinguish multiplied groups from chains (see P-15.3.1.2.2.1);
Examples:
bis(oxy) [preferred prefix,
defines two –O– groups, see P-15.3.2.1;
whereas ‘dioxy‘ would define the –OO– group, used in the past, see P-15.3.2.1)]
bis(methylene) [preferred prefix,
defines two –CH2– groups, see P-29.4.2;
whereas dimethylene might be used to define the –CH2-CH2– group
properly named as ethane-1,2-diyl (preferred prefix) or ethylene; see P-29.6.2.3]
bis(azo) defines two –N=N– groups, see P-68.3.1.3.2.3;
whereas ‘diazo’ is one =N2 group, see P-61.4)
Example: 3,3′-[methylenebis(azo)]dipropanoic acid
bis(sulfonyl) (preferred prefix,
defines two –SO2– groups, see P-65.3.2.3;
whereas ‘disulfonyl’ is –SO2-SO2–)
Example: 3,3′-[oxybis(sulfonyl)]di(propan-1-ol) (PIN; see P-65.3.2.3)
bis(sulfinyl) (preferred prefix,
defines two –S(O)– groups, see P-65.3.2.3; disulfinyl is –S(O)-S(O)–)
Example: 3,3′-[azanediylbis(sulfinyl)]di(propan-1-amine) (PIN, see P-65.3.2.3)
(c) before skeletal replacement (‘a’) prefixes, such as ‘aza’, ‘oxa’, etc. that are used in the construction of Hantzsch-Widman names and in skeletal replacement (‘a’) nomenclature, to describe clearly the number of replacement atoms in a name or name component;
Examples:
benzo[1,2-c:3,4-c′]bis([1,2,5]oxadiazole) (PIN,
describes two ‘oxadiazole’ rings, see P-25.3.7.1;
whereas the name ‘benzo[1,2-c:3,4-c′]di[1,2,5]oxadiazole’ might describe
a five-membered ring with two oxygen atoms and two nitrogen atoms, see P-22.2.2.1.1)
bis(azacyclododecane) (PIN,
describes two ‘azacyclododecane’ rings, see P-22.2.3;
whereas the name diazacyclododecane describes
a cyclododecane ring with two nitrogen atoms, see P-22.2.3.2.2)
bis(1,2-oxazol-3-yl) [preferred prefix,
but di(isoxazol-3-yl), see P-16.3.2;
whereas di(1,2-oxazol-3-yl) might be interpreted
as a ‘dioxazole’ ring system, see P-22.2.2.1.1)]
(d) before attached components in fusion nomenclature to distinguish a multiplicative prefix from multiplied attached fusion components;
Examples:
bis(benzo[a]anthracen-1-yl) (preferred prefix,
describes two ‘benzo[a]anthracen-1-yl prefixes; see P-61.2.4;
whereas dibenzo[a]anthracen-1-yl might be interpreted
as a dibenzoanthracene ring system; see P-25.3.4.2.1)
bis(cyclobuta[1,2-c]furan) (PIN,
describes two cyclobuta[1,2-c]furan ring systems, see P-25.3.7.3;
whereas dicyclobuta[1,2-c]furan might be interpreted
as a dicyclobutafuran ring system, see P-25.3.6.1)
(e) before names beginning with a multiplicative prefix ‘di’.
Examples:
bis(diazonium) (preselected suffix, see P-73.2.2.3) (not didiazonium)
bis(diazenyl) (preselected prefix, see P-68.3.1.3.1) (not didiazenyl)
bis(disulfanyl) (preselected prefix, see P-29.3.2.2, P-63.1.5, and P-63.4.2.2) (not didisulfanyl)
tris(dihydrogen phosphate) (preselected name; see P-67.1.3.2) (not tridihydrogen phosphate)
P-16.4 OTHER NUMERICAL TERMS
P-16.4.1 The numerical prefixes ‘bi’, ‘ter’, ‘quater’, etc. are used mainly in naming ring assemblies (see P-28).
Examples:
1,1′-biphenyl (PIN, P-28.2.1)
12,22:26,32:36,42-quaterpyridine (PIN, P-28.3.1)
P-16.4.2 The prefix ‘mono’ is usually omitted in names of organic compounds. However, it is used to indicate that only one characteristic group of a parent structure has been modified. The ending ‘kis’ is never associated with ‘mono’.
Examples:
monoperoxyphthalic acid (P-65.1.4.2)
2-carbonoperoxoylbenzoic acid (PIN; see P-65.1.4.2)
carbon monoxide (PIN; see P-13.3.5)
P-16.4.3 The term ‘sesqui’ is used to mean one and a half times as great. It is not used in IUPAC names as a numerical prefix, but may be found as a general descriptive term, for example ‘sesquisiloxanes’.
P-16.5 ENCLOSING MARKS
Parentheses, ( ), (also called ‘curves’ or ‘round brackets’), brackets, [ ], (also called ‘square brackets’), and braces { }, (also called ‘curly brackets’) are used in chemical nomenclature to set off parts of a name dealing with specific structural features in order to convey the structure of a compound as clearly as possible. It is important to note that although they are used in the same way in names of organic and inorganic compounds, they are not used in the same way in inorganic formulas.
Enclosing marks must not be omitted from preferred IUPAC names. In general nomenclature, when there is no possible ambiguity, enclosing marks may be omitted to simplify a name.
P-16.5.1 Parentheses (also called curves or round brackets)
P-16.5.2 Brackets (also called square brackets)
P-16.5.3 Braces (also called curly brackets)
P-16.5.4 Multiple types of enclosing marks 
P-16.5.1 Parentheses (also called curves or round brackets)
P-16.5.1.1 Parentheses are used around compound (see P-29.1.2) and complex (see P-29.1.3) prefixes; after the multiplicative prefixes ‘bis’, ‘tris’, etc.; to enclose a multiplied parent compound consisting of a parent hydride and a substituent suffix or a multiplied substituent prefix even though preceded by a basic numerical prefix, such as ‘di’, ‘tri’, etc.
Examples:
Cl-CH2-SiH3
(chloromethyl)silane [PIN;
the name chloro(methyl)silane would describe the structure Cl-SiH2-CH3]
(HO-CH2-CH2-O)2CH-COOH
bis(2-hydroxyethoxy)acetic acid (PIN)
1,3,5-tri(decyl)cycohexane (PIN)
(not 1,3,5-tris(decyl)cyclohexane)
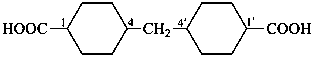
4,4′-methylenedi(cyclohexane-1-carboxylic acid) (PIN)
H{S/O}C-CH2-CH2-C{O/S}H
butanebis(thioic acid) (PIN)
–CH-[CH2]12-CH2-O-CH2-[CH2]12-CH2–
oxydi(tetradecane-14,1-diyl) (preferred prefix;
note the order of the substituent locants used in multiplicative nomenclature)
1,4-di(tridecyl)benzene (PIN)
P-16.5.1.2 Parentheses are used around simple substituent prefixes (see P-29.1) to separate locants of the same type referring to different structural elements, even though locants referring to a particular structural feature may not be explicitly cited. This is recommended for clarity since locants assigned to free valences were previously placed in front of the name, for example, 2-naphthyl.
Examples:
(naphthalen-2-yl)acetic acid (PIN)
(the locant ‘2’ for acetic acid is not cited, see P-14.3.4.6)
naphthalene-2-acetic acid
4-(pyridin-4-yl)benzamide (PIN)
[former name 4-(4-pyridinyl)benzamide]
4-(4-pyridyl)benzamide
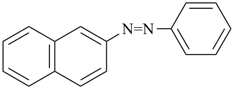
(naphthalen-2-yl)(phenyl)diazene (PIN; see P-68.3.1.3.2.2)
(2-naphthyl)phenyldiazene
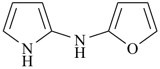
N-(furan-2-yl)-1H-pyrrol-2-amine (PIN)
(furan-2-yl)(1H-pyrrol-2-yl)amine
(2-furyl)(pyrrol-2-yl)amine
P-16.5.1.3 Parentheses for substituents of parent structures. 
P-16.5.1.3.1 For mononuclear parent hydrides with two or more substituents the first cited substituent never has enclosing marks unless it is a compound substituent group or includes a locant. The second and further substituents are each enclosed with parentheses even for simple substituents. When the simple substituent groups are accompanied by multiplicative prefixes such as ‘di’ and ‘tri’, the multiplicative prefixes are not included in the parentheses. 
Examples:
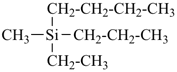
butyl(ethyl)(methyl)(propyl)silane (PIN)
CH3-SiH2Cl
chloro(methyl)silane (PIN)
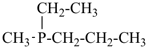
ethyl(methyl)(propyl)phosphane (PIN)
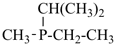
ethyl(methyl)(propan-2-yl)phosphane (PIN)
ethyl(isopropyl)(methyl)phosphane
ClCH2-SiH2-CH3
(chloromethyl)(methyl)silane (PIN; P-68.2.6.1)
BrCH2-SiH2-CH2Cl
(bromomethyl)(chloromethyl)silane (PIN; P-68.2.6.1)
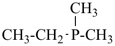
ethyldi(methyl)phosphane (PIN)
ethyldi(propan-2-yl)silane (PIN)
ethyldi(isopropyl)silane
ethyl(methyl)(propyl)silanecarboxylic acid (PIN) 
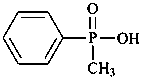
methyl(phenyl)phosphinic acid (PIN) 
cyclopropyl(phenyl)methanol (PIN) 
P-16.5.1.3.2 Simple substituents of a parent where only one possible position can be substituted do not require locants. Enclosing marks are used with second and subsequent simple substituents (see P-16.5.1.3.1). In some cases, such as acetic acid, alkyl substituents are not allowed as the resulting compound requires a different parent structure. Locants are required for related compounds where additional substitutable positions are available, for example acetamide.
Examples:
bromo(nitro)(phenyl)acetic acid (PIN) 
diethyl ethyl(methyl)propanedioate (PIN) 
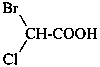
bromo(chloro)acetic acid (PIN) 
cyclopropyl(fluoro)acetonitrile (PIN) 
P-16.5.1.4 Parentheses are placed around substituent groups including the name of a parent hydride in order to avoid any confusion from having two parent hydrides in a substitutive name.
| This is a change from the 1993 Guide (ref. 2) to facilitate name interpretation in names as such as cyclohexanecarbonyl, in that they, even though they are simple prefixes, could give the illusion that two parent hydrides are present. |
|
Examples:
4-(cyclohexanecarbonyl)benzene-1-carbothioic acid (PIN)
di(benzenesulfinyl)acetic acid (PIN)
P-16.5.1.5 Parentheses are placed around a prefix denoting simple hydrocarbyl substituents adjacent to the term ‘amine’ in naming amines using ‘amine’ as the parent structure, when several different substituent groups are present. This requirement is necessary to insure the specificity of this kind of amine name and to distinguish them from older names. Other enclosing marks are used as needed.
Examples:
(2-chloroethyl)(propyl)amine (P-62.2.2.1)
N-(2-chloroethyl)propan-1-amine (PIN)
butyl(ethyl)(propyl)amine (P-62.2.2.1)
N-ethyl-N-propylbutan-1-amine (PIN)
butyl(ethyl)(methyl)amine (P-62.2.2.1)
N-ethyl-N-methylbutan-1-amine (PIN)
P-16.5.1.6 Parentheses are used to isolate a second locant of a compound locant, around locants denoting ring numbering in phane nomenclature, and to enclose fullerene identifiers.
Examples:
bicyclo[8.5.1]hexadeca-1(15),10-diene (PIN, P-31.1.1.1)
1(1,3),4(1,4)-dibenzenacycloheptaphane (PIN, P-26.4.2.2)
(C60-Ih)[5,6]fullerene (PIN, P-27.2.2)
P-16.5.1.7 Parentheses are used to enclose ‘added indicated hydrogen’ and its locant, stereodescriptors such as ‘E’, ‘Z’, ‘R’, ‘S’, etc., and descriptors for isotopically substituted compounds.
Examples:
phosphinin-2(1H)-one (PIN, P-14.7.2)
(2E)-but-2-ene (PIN, P-93.4.2.1.1)
(13C)methane (PIN, P-82.2.1)
P-16.5.1.8 Parentheses are used to clarify a modification made by functional replacement prefixes.
Example:
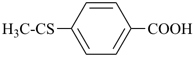
4-(thioacetyl)benzoic acid
4-(ethanethioyl)benzoic acid (PIN, P-65.1.5.1)
P-16.5.1.9 Parentheses (or other enclosing marks if parentheses are already present) are placed around a parent structure including the prefix ‘di’ or ‘bis’ before multiparent systems (P-25.3.7.1) substituted by one substituent group only cited as suffix.
Examples:
[benzo[1,2-c:3,4-c′]bis([1,2,5]oxadiazole)]-4-carboxylic acid (PIN; P-65.1.2.2.2)
(benzo[1,2:4,5]di[7]annulene)-2-carbonitrile (PIN; (P-66.5.1.1.3)
P-16.5.1.10 Parentheses are used to enclose terms modified by the numerical prefixes ‘bis’, ‘tris’, ‘tetrakis’, etc., discussed in P-16.3.6.
Example:
disilylmethyl (preferred prefix; see P-68.2.5)
bis(silanyl)methyl
P-16.5.1.11 Parentheses are used to enclose groups attached to a chain in linear formulas.
Examples:

propane-2-thiol (PIN)
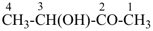
3-hydroxybutan-2-one (PIN)
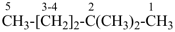
2,2-dimethylpentane (PIN)
P-16.5.1.12 Parentheses are used to enclose multiple dots and charges in radical ion structures.
Example:
[C6H5-C6H5](2•)(2–)
[1,1′-biphenyl]dielide (PIN)
biphenyl diradical
dianion biphenyl diradical ion(2–)
P-16.5.2 Brackets (also called square brackets)
P-16.5.2.1 Brackets enclose descriptors denoting fusion sites in fused ring systems and enclose numbers denoting the length of bridges and chains connecting spiro atoms in names of polyalicyclic ring systems constructed according to von Baeyer methods. They also enclose ring assembly names when these are followed by a principal group suffix or a cumulative suffix and enclose names of components in von Baeyer spiro names.
Examples:
naphtho[2,1-a]azulene (PIN, P-25.3.1.3)
bicyclo[3.2.1]octane (PIN, P-23.2.2)
spiro[4.5]decane (PIN, P-24.2.1)
[2,2′-bipyridin]-5-yl (preferred prefix, P-29.3.5)
spiro[cyclohexane-1,1′-indene] (PIN, P-24.5.1)
P-16.5.2.2 Brackets enclose locants that describe structural features of components, such as double bonds in bridges and heteroatoms of component rings in names of fused ring systems.
Examples:
6,7-(epiprop[1]en[1]yl[3]ylidene)benzo[a]cyclohepta[e][8]annulene (PIN, P-25.4.3.4.2)
5H-pyrido[2,3-d][1,2]oxazine [PIN, P-25.3.2.4 (d)]
P-16.5.2.3 Brackets enclose the numerals that describe ring size in annulenes and fullerenes.
Examples:
[10]annulene (P-22.1.2)
cyclodeca-1,3,5,7,9-pentaene (PIN)
(C60-Ih)[5,6]fullerene (PIN, P-27.2.2)
P-16.5.2.4 Brackets enclose substituent prefixes in which parentheses have already been used.
Example:
4-[(hydroxyselanyl)methyl]benzoic acid (PIN, P-63.4.2.2)
P-16.5.2.5 Brackets are used to enclose descriptors for isotopically labeled compounds.
Example:
[13C]methane (PIN, P-83.1.2.1)
P-16.5.2.6 Brackets are employed in formulae to indicate repetition of a group in a chain.
Example:
CH3-[CH2]68-CH3
heptacontane (PIN, P-21.2.1)
P-16.5.3 Braces (also called curly brackets)
P-16.5.3.1 Braces are used to enclose substituent prefixes in which brackets and parentheses have already been used.
Example:
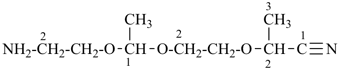
2-{2-[1-(2-aminoethoxy)ethoxy]ethoxy}propanenitrile (PIN)
P-16.5.4 Multiple types of enclosing marks 
When multiple types of enclosing marks are required, the nesting order is as follows: {[({[( )]})]}, etc. as illustrated in Fig.1.3. It must be noted, however, that the presence of square brackets and/or parentheses that are an integral part of the name of a parent structure does not affect the nesting order given here.
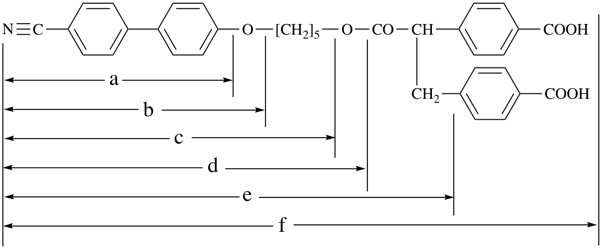
(a) = 4′-cyano[1,1′-biphenyl]-4-yl
(b) = (4′-cyano[1,1′-biphenyl]-4-yl)oxy
(c) = 5-[(4′-cyano[1,1′-biphenyl]-4-yl)oxy]pentyl
(d) = {5-[(4′-cyano[1,1′-biphenyl]-4-yl)oxy]pentyl}oxy
(e) = 3-({5-[(4′-cyano[1,1′-biphenyl]-4-yl)oxy]pentyl}oxy)-3-oxopropane-1,2-diyl
(f) = 4,4′-[3-({5-[(4′-cyano[1,1′-biphenyl]-4-yl)oxy]pentyl}oxy)-3-oxopropane-1,2-diyl]dibenzoic acid (PIN)
|
Fig. 1.3 Nesting order of enclosing marks
Example:
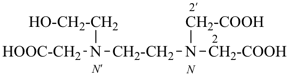
2,2′-({2-[(carboxymethyl)(2-hydroxyethyl)amino]ethyl}azanediyl)diacetic acid
N-(carboxymethyl)-N′-(2-hydroxyethyl)-N,N′-(ethane-1,2-diyl)diglycine
P-16.5.4.1 The presence of square brackets and/or parentheses that are an integral part of the name of a parent structure may affect the nesting order as described below. 
P-16.5.4.1.1 In determining the nesting order in a name the parentheses of added indicated hydrogen are ignored.
Example:
1-(3,4-dihydroquinolin-1(2H)-yl)ethan-1-one (PIN, P-64.3.2)
P-16.5.4.1.2 In determining the nesting order in a name the square brackets of ring fusion, spiro fusion, ring assembly, or the extended von Baeyer names are ignored.
Examples:
(7,7-dimethyl-2-oxobicyclo[2.2.1]heptan-1-yl)methanesufonic acid (PIN, P-14.8.1)
10,10′-[[1,1′-biphenyl]-4,4′-diylbis(oxy)]di(decanoic acid) (PIN, P-15.3.2.1)
trispiro{1-oxaspiro[2.3]hexane-2,3′:4,3′′:5,3′′′-tri(tetracyclo[3.2.0.02,7.04.6]heptane)} (PIN, P-24.7.4.1)
N-(dibenzo[b,d]furan-1-yl)acetamide (PIN, P-66.1.1.4.3)
P-16.5.4.1.3 In determining the nesting order in a name the parentheses used to indicate compound locants, phane amplification locants, stereochemistry, specific isotopic substitution, or coordination with multicenter bonding are taken into consideration.
Examples:
2-[bicyclo[6.6.1]pentadeca-8(15)-en-1-yl]ethan-1-ol (PIN)
trimethyl[12H-1(6)-pyrana-3,5(1,4),7(1)-tribenzenaheptaphan-74-yl]silane (PIN) (P-44.1.2.1)
[1(1′)E,3E,3′E]-3,3′-diethylidene-1,1′-bi(cyclobutylidene) (PIN, P-93.5.1.4.2.1)
[1(44)E,2S,26R]-bicyclo[24.20.1]heptatetraconta-1(46),44,45-trien-2-ol (PIN, P-93.5.2.3)
10-{[(3S)-1-phosphabicyclo[2.2.2]octan-3-yl]methyl}-10H-phenoxazine (PIN)
(3S)-2-[(2S)-2-{[(1S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (PIN)
1,2-di[(13C)methyl]benzene (PIN. P-82.2.1)
3-[ethyl(2-34S)trisulfanyl]propanoic acid (PIN, P-82.6.3.2)
[(2,3,5,6-η)-bicyclo[2.2.1]hepta-2,5-diene]tricarbonyliron (P-69.2.4)
{μ-[2(1-3,3a,8a-η):1(4-6-η)]azulene}-(pentacarbonyl-1κ3C,2κ2C)diiron(Fe—Fe) (P-69.2.5)
P-16.5.4.1.4 In determining the nesting order where square brackets are used for an isotopically labeled compound, by convention, parenthesis are used.
Example:
1-(amino[14C]methyl)cyclopentan-1-ol (PIN, P-83.1.2.1)
P-16.5.4.1.5 When the nesting order given in P-16.5.4 results in consecutive enclosing marks of the same level, the next level of enclosing mark is used. For example the following name formed by the application of the nesting order rules given above results in the appearance of consecutive parentheses twice.
2-{2-[(1-ethoxy-1-oxo-4-phenylbutan-2-yl)amino]propanoyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (without stereochemistry)
(3S)-2-[(2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (with stereochemistry)  With this example the stereochemistry needs to be inserted at three positions. Insertion of the third one into the name without stereochemistry results in two opening parenthesis next to each other. Hence to clarify the brackets these need to be adjusted as shown by the second name.
With this example the stereochemistry needs to be inserted at three positions. Insertion of the third one into the name without stereochemistry results in two opening parenthesis next to each other. Hence to clarify the brackets these need to be adjusted as shown by the second name.
Explanation: The order of insertion of stereochemistry for 2-{2-[(1-ethoxy-1-oxo-4-phenylbutan-2-yl)amino]propanoyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid is as shown below.
Stage 1 insert stereochemistry at the inner most position:
2-(2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid 
The substituent (2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl requires square brackets to surround it hence subsequent enclosing marks need to be adjusted.
Stage 2 insert stereochemistry at the next position:
2-[(2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
The substituent (2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl has a parenthesis on the left and brace on the right so to make clear it requires square brackets around it.
Stage 3 insert stereochemistry at the third position:
(3S)-2-[(2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
| This procedure is a change to alleviate the situation that can happen when one follows precisely the nesting order given above and it is necessary to insert independent enclosing marks to accommodate other factors, such as stereodescriptors, that two or more enclosing marks of the same type might line up consecutively resulting in confusion. |
|
P-16.6 ITALICIZATION
Italicizing marks letters that are not involved in the primary stage of alphanumerical ordering. In manuscripts, italics are conventionally indicated by underlining when an italic font is not available.
P-16.6.1 Lower case italic letters are used in descriptors for fusion sites in names of fused ring systems.
Example:
selenopheno[2,3-b]selenophene (PIN, P-25.3.1.3)
The letters o, m, and p have been used in place of ortho, meta, and para, respectively, to designate the 1,2-, 1,3-, and 1,4- isomers of disubstituted benzene. This usage is strongly discouraged and is not used in preferred IUPAC names.
Example:
o-xylene (P-22.1.3)
1,2-xylene (PIN)
1,2-dimethylbenzene
P-16.6.2 Italicized elements symbols, such as O, N, As, are locants indicating attachment of a substituent to these heteroatoms
Examples:
N,N-diethylethanamine (PIN, P-62.2.2.1)
O-ethyl hexaneselenoate (PIN, P-65.6.3.3.7.1)
P-16.6.3 The italic element symbol H denotes indicated or added hydrogen.
Examples:
1H-azepine (PIN, P-22.2.2.1.4)
quinolin-2(1H)-one (PIN, P-64.3.1)
P-16.6.4 Italic terms, syllables, and capital Roman letters are used in some structural descriptors and stereodescriptors, such as ‘cis’, ‘trans’, ‘R’, ‘S’, ‘E’, ‘Z’, ‘r’, ‘s’, ‘c’, ‘t’, and ‘retro’.
Examples:
‘tert’, but not ‘iso’ (P-29.6.3)
‘E’ and ‘Z’ (P-93.4.2.1.1); ‘cis’, and ‘trans’ (P-93.4.2.1.1); ‘r’, ‘c’, and ‘t’ (P-93.5.1.3);
‘R’ and ‘S’ (P-93.1.1), ‘R*’ (spoken R-star), ‘S*’ (spoken S-star), ‘r’, ‘s’, (P-93.5.1.1), ‘rel’ (P-93.1.2.1)
‘meso’ (P-102.5.6.5.2), ‘ambo’ (P-93.1.4), ‘rac’ (P-93.1.3)
‘M’ and ‘P’, ‘Ra’ and ‘Sa’, ‘Rp’ and ‘Sp’ (P-93.4.2.2 and P-93.5.5.1)
‘TPY-3’, ‘TS-3’, ‘SS-4’, ‘TBPY’, ‘SPY’, and ‘OC’ (P-93.3)
‘retro’, but not ‘abeo’, ‘apo’, ‘cyclo’, ‘de’, ‘des’, ‘homo’, ‘nor’ or ‘seco’ (P-101.3.1 through P-101.3.7)
P-16.7 ELISION OF VOWELS
P-16.7.1 Vowels are systematically elided as follows:
(a) the terminal letter ‘e’ in names of parent hydrides or endings ‘ene’ and ‘yne’ when followed by a suffix or ending beginning with ‘a’, ‘e’, ‘i’, ‘o’, ‘u’, or ‘y’; 
Examples:
pentanal (PIN, P-66.6.1.1.1)
cyclopentadec-1-en-4-yne (PIN, P-31.1.3.1)
methanium (PIN, P-73.1.1.2)
butan-2-one (PIN, P-64.2.2.1)
tetramethylboranuide (PIN, P-72.3)
sulfanyl (preselected prefix, P-29.3.1)
(b) in Hantzsch-Widman names, the final letter ‘a’ of an ‘a’ prefix when followed by a vowel;
Examples:
1,3-thiazole (PIN, P-22.2.2.1.3)
(not 1,3-thiaazaole)
1,6,2-dioxazepane (PIN, P-22.2.2.1.3)
(not 1,6,2-dioxaazaepane)
(c) the terminal letter ‘a’ in the names of numerical multiplicative prefixes when followed by a suffix beginning with ‘a’ or ‘o’;
Examples:
[1,1′-biphenyl]-3,3′,4,4′-tetramine (PIN, P-62.2.4.1.2)
(not [1,1′-biphenyl]-3,3′,4,4′;-tetraamine)
benzenehexol (PIN, P-63.1.2)
(not benzenehexaol)
(d) the terminal letter ‘a’ of an element ‘a’ prefix in ‘a(ba)n’ repeating unit names when followed by a vowel;
Examples:
disiloxane (preselected name, P-21.2.3.1)
(not disilaoxane)
tetrastannoxane (preselected name, P-21.2.3.1)
(not tetrastannaoxane)
(e) the terminal letter ‘o’ of a functional replacement infix when followed by a vowel;
Example:
N,P-diphenylphosphonochloridimidic acid (PIN, P-67.1.2.4.1.1)
(not N,P-diphenylphosphonochloridoimidic acid)
(f) the terminal letter ‘o’ of ‘benzo’ in names of ‘benzoheterocycles’ formed by fusion of a benzene ring to a heteromonocycle whose name begins with a vowel [an exception to P-16.7.2 (g)].
3-benzoxepine (PIN, P-25.2.2.4) 4H-3,1-benzoxazine (PIN, P-25.2.2.4)
P-16.7.2 There is no elision of terminal vowels in the following cases:
(a) in conjunctive names;
Example:
benzeneacetic acid (P-15.6.1.1)
[phenylacetic acid (PIN)]
(b) from replacement or numerical multiplicative prefixes in skeletal replacement (‘a’) nomenclature;
Example:
2,4,6,8-tetrasilaundecan-11-yl (preferred prefix, P-15.4.3.2.2)
(c) from multiplicative prefixes in homogeneous acyclic parent hydrides other than hydrocarbons and boron hydrides;
Example:
nonaazane (P-21.2.2)
(not nonazane)
(d) from numerical multiplicative prefixes in multiplicative parent compounds;
Example:
2,2′,2′′,2′′′-(ethane-1,2-diyldinitrilo)tetraacetic acid
N,N′-(ethane-1,2-diyl)bis[N-(carboxymethyl)glycine
(e) from numerical multiplicative prefixes before substituent prefix names;
Example:
5,6,7,8-tetraiodo-1,2,3,4-tetrahydroanthracene-9-carboxylic acid (PIN, see P-65.1.2.4)
(f) from component prefixes of compound (see P-29.1.2) and complex (see P-29.1.3) prefixes before following prefixes beginning with a vowel;
Examples:
chloroamino (preselected name, P-35.3.1)
aminooxy (preselected name, P-68.3.1.1.1.5)
(g) from prefixes designating attached components in fusion nomenclature; for example, the terminal letter ‘o’ of acenaphtho, benzo, perylo, phenanthro, and the terminal letter ‘a’ of anthra, cyclopropa, cyclobuta, are not elided before a vowel [see P-16.7.1 (f) for an exception involving ‘benzo’].
| This recommendation is in accordance with P-25.3.1.3 and Rule R-2.4.1.1 of the 1993 Guide (ref. 2) that abrogated the elision recommended by Rule A-21.4 in the 1979 edition (ref. 1).
|
|
Examples:
cyclopropa[de]anthracene (PIN, P-25.3.8.1)
naphtho[1,2-a]azulene (PIN, P-25.3.1.3)
P-16.8 ADDITION OF VOWELS
P-16.8.1 For euphonic reasons, in functional replacement nomenclature the vowel ‘o’ is inserted between consonants.
Examples:
ethanesulfonodiimidic acid (PIN, P-65.3.1.4)
(not ethanesulfondiimidic acid)
phenylphosphononitridic acid (PIN, P-67.1.2.4.1.1)
(not phenylphosphonnitridic acid)
P-16.8.2 For euphonic reasons, the letter ‘a’ is inserted between the root of the name for polyenes, polyynes, and polyenynes, and the numerical multiplicative prefix ‘di’, ‘tri’, etc., preceding the ending ‘ene’ or ‘yne’.
Examples:
buta-1,3-diene (PIN, P-31.1.1.2)
(not but-1,3-diene)
hexa-1,3-dien-5-yne (PIN, P-31.1.2.2.1)
(not hex-1,3-dien-5-yne)
P-16.9 PRIMES
In preferred IUPAC names, primes, double primes, etc., sometimes with superscripted arabic numbers that are the locants of the parent structure to which a group is attached, are added to locants and symbols without a space and are not italicized even after italic fonts. When more than three primes are required, they are cited in group of threes.
P-16.9.1 Primes (′), double primes (′′), triple primes (′′′), etc. are used to differentiate the nitrogen atoms of hydrazides (P-66.3), imidamides (amidines) (see P-66.4.1), hydrazonamides (amidrazones) (see P-66.4.2), and hydrazonohydrazides (hydrazidines) (see P-66.4.3).
Examples:
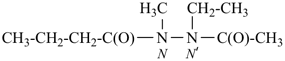
N′-acetyl-N′-ethyl-N-methylbutanehydrazide (PIN, P-66.3.3.2)
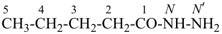
pentanehydrazide (PIN, P-66.3.1.1)
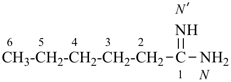
hexanimidamide (PIN, P-66.4.1.1)
(no longer hexanamidine)
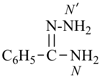
benzenecarbohydrazonamide (PIN, P-66.4.2.1)
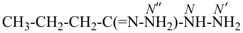
butanehydrazonohydrazide (PIN, P-66.4.3.1)
P-16.9.2 Superscript arabic numbers, which are the locants of the parent structure, are used to differentiate the nitrogen atoms of di- and polyamines, di- and polyimines, di- and polyamides, except for geminal amines, imines, and amides.
| Superscript arabic numbers are now used to differentiate the nitrogen atoms of symmetrical diamines, diimines, diamides, di(imidamides), di(amidines), di(hydrazonamides), di(amidrazones), di(hydrazonohydrazides), imidohydrazides, and polyimidopolycarbonic and inorganic oxoacids, where primes (′), double primes (′′), triple primes (′′′), etc. were formerly used.
|
|
Examples:
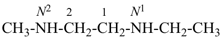
N1-ethyl-N2-methylethane-1,2-diamine (PIN, P-62.2.4.1.2)
N-ethyl-N′-methylethane-1,2-diamine
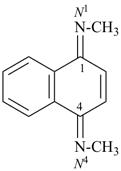
N1,N4-dimethylnaphthalene-1,4-diimine (PIN, P-62.3.1.1)
N,N′-dimethylnaphthalene-1,4-diimine (see also P-58.2.2.3)
N,N′-dimethyl-1,4-dihydronaphthalene-1,4-diimine
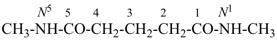
N1,N5-dimethylpentanediamide (PIN, P-66.1.1.3.1.1)
N,N′-dimethylpentanediamide
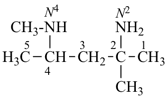
N4,2-dimethylpentane-2,4-diamine (PIN, P-62.2.4.1.2)
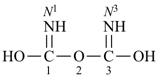
1,3-diimidodicarbonic acid (PIN, P-65.2.3.1.2.1)
Superscript arabic numbers are also used to differentiate the chalcogen atoms in polynuclear organic and inorganic oxoacids.
| The use of superscripted letter locants is a change from previous practice where numerical locants were placed in front of the letter locants such as 1-O and 3-O, as described in ref. 1, Rule C-213.1. |
|
Example:
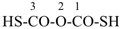
1,3-dithiodicarbonic S1,S3-acid
(not 1,3-dithiodicarbonic 1-S,3-S-acid)
P-16.9.3 Primes (′), double primes (′′), triple primes (′′′), etc., with superscripted numerical locants indicating the position of the suffix on the parent structure, are used to differentiate the nitrogen atoms in cases other than those listed in P-16.9.1 and P-16.9.2, above, especially when symmetry conditions are not fulfilled.
Examples:
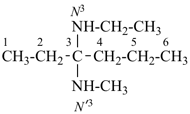
N3-ethyl-N′3-methylhexane-3,3-diamine (PIN, P-62.2.4.1.2)
N-ethyl-N′-methylhexane-3,3-diamine
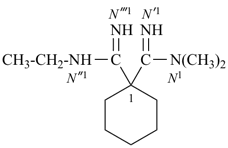
N′′1-ethyl-N1,N1-dimethylcyclohexane-1,1-dicarboximidamide (PIN, P-66.4.1.1 and P-66.4.1.4.2)
N′′-ethyl-N,N-dimethylcyclohexane-1,1-dicarboximidamide
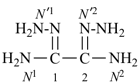
ethanedihydrazonamide (PIN, P-66.4.2.1)
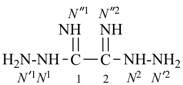
ethanediimidohydrazide (PIN, P-66.4.2.1)
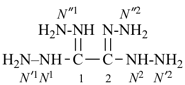
ethanedihydrazonohydrazide (PIN, P-66.4.3.1)
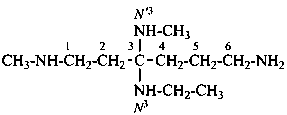
N3-ethyl-N1,N′3-dimethylhexane-1,3,3,6-tetramine (PIN, P-62.2.4.1.2)
N3-ethyl-N1,N′3-dimethyl(hexane-1,3,3,6-tetrayltetraamine)
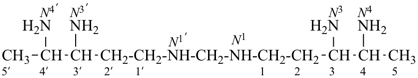
N1,N1′-methylenedi(pentane-1,3,4-triamine) (PIN, P-62.2.5.2)
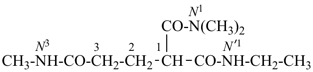
N′1-ethyl-N1,N1,N3-trimethylpropane-1,1,3-tricarboxamide (PIN, P-66.1.1.3.1.2)
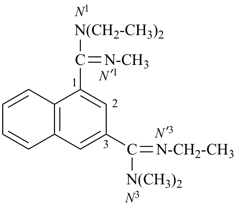
N1,N1,N′3-triethyl-N′1,N3,N3-trimethylnaphthalene-1,3-dicarboximidamide (PIN, P-66.4.1.4.1)
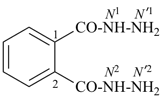
benzene-1,2-dicarbohydrazide (PIN, P-66.3.1.2.2)
P-16.9.4 Primes (′), double primes (′′), triple primes (′′′), etc., are also used to differentiate identical locants other than N locants, for example 1, 1′, 1′′, 4′a (not 4a′; for locants of a fused ring component,  primes are added after the number, not after the letter). Such usage occurs:
primes are added after the number, not after the letter). Such usage occurs:
(a) in multiplicative nomenclature to denote multiplied units and modify locants accordingly;
Example:
2,2′,2′′-nitrilotri(ethan-1-ol) (PIN, P-15.3.2.1)
(b) in spiro-fused compounds, to denote positions in polycyclic systems, identical or different;
Examples:
7,7′-spirobi[bicyclo[4.1.0]heptane] (PIN, P-24.3.1)
1H,1′H,1′′H,3′H-2,2′:7′,2′′-dispiroter[naphthalene] (PIN, P-24.4.1)
spiro[cyclohexane-1,1′-indene] (PIN, P-24.5.1)
(c) in ring assemblies, to number identical ring components;
Examples:
1,1′-bi(cyclopropane) (PIN, P-28.2.1)
1,1′-biphenyl (PIN, P-28.2.1)
3a,3′a-biindene (PIN, P-28.2.3)
P-16.9.5 Primes also occur:
(a) in fusion nomenclature, to identify first and higher attached components, identical attached components, and multiparent names;
Examples:
pyrido[1′′,2′′:1′,2′]imidazo[4′,5′:5,6]pyrazino[2,3-b]phenazine (PIN, P-25.3.4.1.1)
difuro[3,2-b:2′,3′-e]pyridine (PIN, P-25.3.4.1.2)
cyclopenta[1,2-b:5,1-b′]bis([1,4]oxathiine) (PIN, P-25.3.7.1)
(b) in fullerenes ortho-fused to organic ring or ring systems, to identify positions in the nonfullerene component;
Example:
3′H-cyclopropa[1,9](C60-Ih)[5,6]fullerene (PIN, P-27.6.1)
(c) in natural product nomenclature, to identify positions in ring(s) fused to a fundamental parent hydride;
Example:
naphtho[2′,1′:2,3]-5α-androstane (P-101.5.1.1)
P-16.9.6 Multiple primes
Long strings of primes may be needed with ring assemblies and polyspiro compound. It is difficult to count long streams of primes. For example, how many primes appear in this string: ′′′′′′′′ ? It is eight. In these recommendations, to simplify the counting process, long strings of primes are broken into groups of three as: ′′′ ′′′ ′′, which has the same number of primes as the string above. This is a change from the publication on spiro compounds (ref. 8) in which multiple primes were separated by a space after every four primes.
Continued with P-2 Parent Hydrides
Return to:
IUPAC Chemical Nomenclature home page.
Blue Book home page.




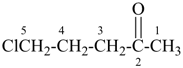
















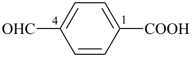
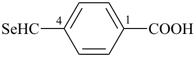






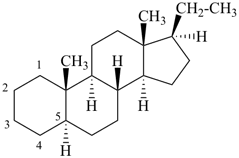
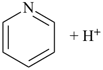

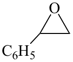



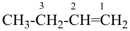
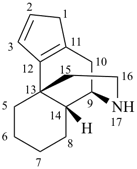






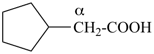
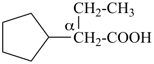
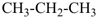









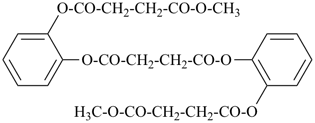
















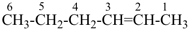






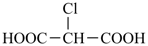




















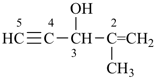

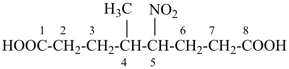



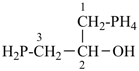
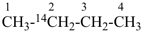
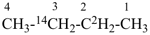
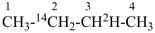

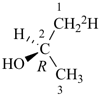

























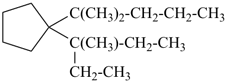
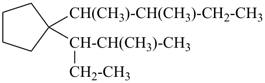

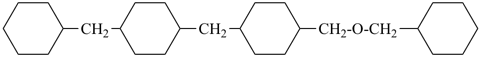
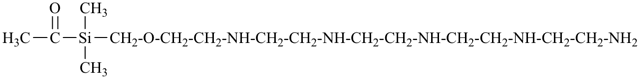
















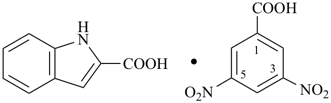









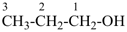
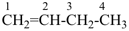
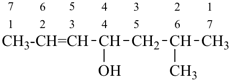









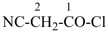



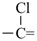
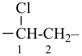
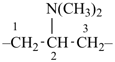

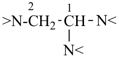

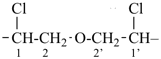
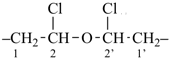
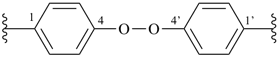

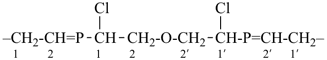
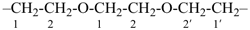
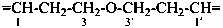
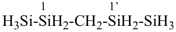


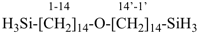
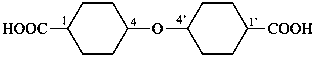
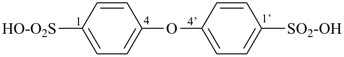

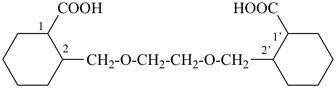
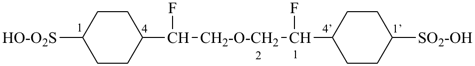
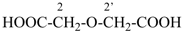
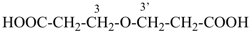
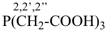


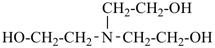

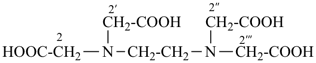

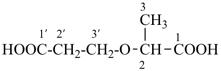
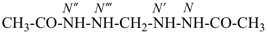
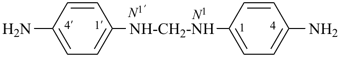
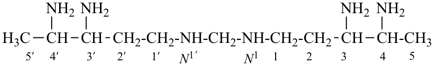

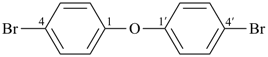

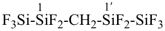
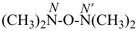
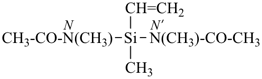



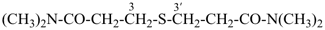
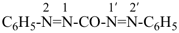


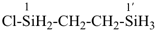
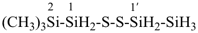



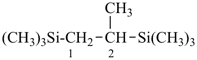
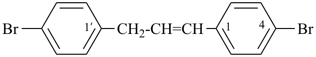



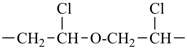 as in
as in