Replace structure with:

Division VIII Chemical Nomenclature and Structure Representation Division
World Wide Web version Prepared by G. P. Moss
School of Physical and Chemical Sciences, Queen Mary University of London,
Mile End Road, London, E1 4NS, UK
g.p.moss@qmul.ac.uk
For additional minor corrections click here Characters deleted from the printed text or which are modified are marked in red on the original text. Characters added to the corrected text are marked in red too.
Page xiii, contents, P-32.2. [corrected 31 December 2025]
For P-32.2 SUBSTITUENT GROUPS DERIVED FROM PARENT HYDRIDES MODIFIED BY THE PREFIX ‘HYDRO’
read P-32.2 SUBSTITUENT GROUPS DERIVED FROM PARENT HYDRIDES MODIFIED BY THE PREFIX ‘HYDRO’ AND ‘DEHYDRO’
Page xxx, 3 substitutive nomenclature (f), lines 1/2. [corrected 24 July 2019]
For Oximes are named systematically as ‘-ylidene’ derivatives of hydroxylamine and not by functional class nomenclature...
read Oximes are named systematically as ‘N-hydroxy’ derivatives of imines and not by functional class nomenclature...
Page xxxii, entry 7 (g), lines 1/2. [modified 3 March 2021]
For ...preferred IUPAC names; the preferred prefix is '’carbonimidamido'.
read ..preferred IUPAC names but may be used in general nomenclature; the preferred prefix is 'carbamimidoylamino'.
RO minor correction 13.2.2021 change to BBminor
Page xxxiii, Changes 7 (l), lines 1-4. [corrected 21 August 2019]
For ...from ‘triazane’ and ‘tetrazane’ are named systematically as hydrocarbons are named, for example ‘triazan-1-yl’, tetrazan- 2-yl. The prefixes ‘triazano’ and ‘tetrazano’ previously used for ‘triazan-1-yl’ and ’tetrazan-1-yl’ and also...
read ...from ‘triazane’ and ‘tetraazane’ are named systematically as hydrocarbons are named,for example ‘triazan-1-yl’, ‘tetraazan- 2-yl’. The prefixes ‘triazano’ and ‘tetraazano’ previously used for ‘triazan-1-yl’ and ’tetraazan-1-yl’ and also...
Page xxxiii, 8 (c), line 2. [corrected 5 July 2018]
For ...semicarbazone, carbadiazone, and the...
read ...semicarbazone, and the...
Page xxxv, 11. (d), line 1. [corrected 3 October 2018]
For 2H-1-benzopyran
read 2H-1-benzopyran
Page xxxvi, 12 seniority (b), lines 3-5. [corrected 24 July 2019]
For ...(see P-67.1.2.6) and compounds such as R-NH-OH are named as N-derivatives of hydroxylamine, NH2-OH (see P-68.3.1.1.1) and not as N-substituted amines as in previous recommendations.
read ...(see P-67.1.2.6) and compounds such as R-NH-OH are named as N-derivatives of the senior amine and not as N-derivatives of hydroxylamine, NH2-OH (see P-62.4, P-68.3.1.1) as in previous recommendations.
Page xxxvii, entry 17 (c), line 4. [modified 3 March 2021]
For ...the latter is ‘1,3-dihydroxydisulfoxane-1,3-dione’; and the...
read ...the latter is ‘1,3-dihydroxy-1λ4,3λ4-dithioxane-1,3-dione’; and the...
Page xxxvii, 18. (b), lines 3-5. [corrected 16 January 2019]
For ... or cationic centers at the first atom in the order of classes (P-41) at the skeletal atom first cited in the seniority order of classes: N > P > As > Pb > Al > Ga...
read ...or cationic centers at the skeletal atom first cited in the seniority order of classes (P-41): N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga...
Page xxxviii, glossary, Attached component, line 3.
For ...in benzo[g]quinoline and...
read ...in benzo[g]quinoline and...
Page xxxix, glossary entry 6 on this page, lines 2/3. [corrected 6 May 2020]
For ...cyclohexyloxy (C6H11-CO–)
read ...cyclohexyloxy (C6H11-O–)
Page xli, glossary entry 12 (corrected to 13) on this page, lines 2-4. [corrected 3 March 2021]
For ... skeletal heteroatoms or to one carbon atom and one skeletal heteroatom, except for nitrogen and halogens, or to a heteroatom belonging to a ring or ring system, for example piperidin-2-one and 1-silylethan-1-one.
read ... skeletal heteroatoms or an acyclic compound, other than esters or acid anhydrides, having a carbonyl group linked to one carbon atom and one skeletal heteroatom, except for nitrogen and halogens, or an acyclic compound having a carbonyl group linked to a heteroatom belonging to a ring or ring system, for example piperidin-2-one, 1-silylethan-1-one and 1-(piperidin-1-yl)ethan-1-one.
Page 4, P-12.1, paragraph 2, line 7. [corrected 26 January 2022]
For ...substitutive names ‘vinylbenzene’, ‘phenylethene’, and ‘phenylethylene’. The name...
read ...substitutive name ‘vinylbenzene’. The name...
Page 4, P-12.1, example 6. [corrected 26 January 2022]
Delete phenylethene and phenylethylene
Page 6, P-12.1, Table 1.2, Formula 3' parent.
For phosphane (PIN)
read phosphane (preselected name)
Page 6, P-12.1, Table 1.2, Formula 6, name. [corrected 31 July 2019]
For 1-ethoxy-2-[2-(methoxyethoxy)ethoxy]ethane
read 1-ethoxy-2-[2-(2-methoxyethoxy)ethoxy]ethane
Page 6, P-12.2, Table 1.2, Formula 7, name. [corrected 1 May 2019]
For 2-phenyloxirane
read phenyloxirane
Page 6, P-12.1, Table 1.2, Formula 8, parent.
For bornane (PIN)
read bornane
for bicycloheptane (PIN)
read bicyclo[2.2.1]heptane (PIN)
Page 6, P-12.1, table 1.2 example 9, name.
For 2-(1H-indol-1-yl)acetic acid
read (1H-indol-1-yl)acetic acid
Page 7, P-12.1, paragraph 4, line 8. [corrected 31 July 2019]
For 1-ethoxy-2-[2-(methoxyethoxy)ethoxy]ethane
read 1-ethoxy-2-[2-(2-methoxyethoxy)ethoxy]ethane
Page 7, P-12.1, paragraph 4 line 17.
For 2-(1H-indol-1-yl)acetic acid
read (1H-indol-1-yl)acetic acid
Page 9, P-12.3, line 2.
For napthhalene
read naphthalene
Page 11, P-13.2.1.2, example 2. [corrected 30 January 2019]
For 4βH-4-carbayohimban
read (4βH)-4-carbayohimban
Page 12, P-13.2.2.2, example 2.
For hexane(dithioic) acid (PIN)
read hexane(dithioic acid) (PIN)
Page 12, P-13.2.2.2, example 3. [corrected 28 October 2016]
For 4-methaneselenoylbenzoic acid (PIN)
read 4-(methaneselenoyl)benzoic acid (PIN)
Page 13, P-13.2.2.3, line 3.
For ...furan, pyran (see Table 2.2)...
read ...pyran (see Table 2.2)...
Page 15, P-13.3.3.1, example 2. [corrected 21 August 2019]
For 2-phenyloxirane
read phenyloxirane
Page 17, P-13.3.5, example. [Corrected 8 October 2025]
For carbon monoxide—methylborane (PIN)
read carbon monoxide—methylborane (1/1) (PIN)
Page 19, P-13.4.3.2, example 2 on this page. [corrected 3 October 2018]
For 1,9-dinor(C60-Ih)[5,6]fullerene (PIN)
read 1,9-dinor(C60-Ih)[5,6]fullerene (PIN)
Page 21, P-13.6.1, example 2.
For nitrilotriacetic acid
read 2,2′,2′′-nitrilotriacetic acid
Page 22, P-13.6.2, example 2. [corrected 9 April 2025]
For dimethyl butanedioylbis(oxy-2,1-phenylene) dibutanedioate (PIN)
read dimethyl butanedioylbis[oxy(2,1-phenylene)] dibutanedioate (PIN)
Page 24, P-13.8.1.2, example 1.
For (removal of the proline residue at positon 7 of oxytoxin
read (removal of the proline residue at positon 7 of oxytocin)
Page 25, P-13.8.2, example.
For 2,4,5-tri-O-methyl-D-mannitol
read 2,4,5-tri-O-methyl-D-mannose
for 3,6-anhydro-2,4,5-tri-O-methyl-D-mannitol
read 3,6-anhydro-2,4,5-tri-O-methyl-D-mannose
Page 26, P-14.1.2, Table 1.3, 2nd line last entry. [corrected 19 September 2018]
For Th
read Tl
Page 28, P-14.2.1.2, example 5.
For 23 tricos
read 23 tricosa
Page 30, P-14.3.4.1, line 3. [corrected 9 January 2019]
For ...hydrazides, nitriles, aldehydes...
read ...hydrazides, nitriles, esters, aldehydes...
Page 30, P-14.3.4.1, example 2. [corrected 1 May 2019]
For 2-chlorobutanedioic acid (PIN)
read chlorobutanedioic acid (PIN)
Page 31, P-14.3.4.2 (d). [corrected 9 April 2025]
For(d) in unsubstituted dinuclear and trinuclear...
read (d) in any dinuclear, unsubstituted trinuclear...
Page 31, P-14.3.4.2 (d), example 1.
For ethane
read ethene
Page 31, P-14.3.4.3, example 1.
Replace the structure with:
CH3-NH-CO-NH2
Page 32, P-14.3.4.3, example 1 on this page. [corrected 19 September 2018]
For choropropanedioic acid (PIN)
read chloropropanedioic acid (PIN)
Page 32, P-14.3.4.4, example 2.
For (not 1-ethylidyne-2-ethylidynedisilane)
read (not 1-ethylidyne-2-methylidynedisilane)
Page 32, P-14.3.4.4, example 4. [corrected 5 July 2018]
For (not 1-ethylidene-2-methylidenetriphosphoxane)
read (not 1-ethylidene-5-methylidenetriphosphoxane)
Page 32, P-14.3.4.4, example 6.
For bromo(methyl)disulfane (PIN)
read (bromodisulfanyl)methane (PIN)
add [not bromo(methyl)disulfane see P-63.3.1; not 1-bromo-2-methyldisulfane]
Page 32, P-14.3.4.4, example 9.
For ...i.e., 1-propylidene-2-ethylidenedisilan-1-yl)
read ...i.e., 2-ethylidene-1-propylidenedisilan-1-yl)
Page 32, P-14.3.4.4, example 10.
Replace structure with:

Page 32, P-14.3.4.4, after examples add. [corrected 9 April 2025]
For As an exception the locant is not omitted from propan-2-one, butan-2-one, prop-2-enoic acid and prop-2-ynoic acid although unambiguous without a locant.
read As an exception the locant is not omitted from propa-1,2-diene, propan-2-one, butan-2-one, prop-2-enoic acid and prop-2-ynoic acid although unambiguous without a locant.
Page 33, P-14.3.4.6, example 2. [modified 16 June 2021]
For (not 4-chorotrioxetane)
read (not 4-chloro-1,2,3-trioxetane)
Page 35, P-14.4 (b) lines 2/3. [corrected 28 May 2024]
For ....accommodate a substituent suffix in accordance with structural feature (d)
read ....accommodate a substituent suffix in accordance with structural feature (c)
Page 37, P-14.4 (f), example.
For (the locants set ‘2,4,5,8’ is lower than ‘2,4,7,8’)
read (the locants set ‘4,5,8’ is lower than ‘4,7,8’)
Page 37, P-14.4 (g), example 1.
For 4-methyl-5-nitrooctanedioic acid
read 4-methyl-5-nitrooctanedioic acid (PIN)
Page 38, P-14.4 (h), example 1. [corrected 5 July 2018]
Replace structure with:
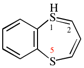
Page 38, P-14.4 (h), example 2.
Replace structure with:
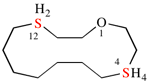
Page 38, P-14.4 (i), example 2.
For (2-14C,3,3-2H2)butane (PIN)
read (3-14C,2,2-2H2)butane (PIN)
for [not (3-14C,2,2-2H2)butane]
read [not (2-14C,3,3-2H2)butane]
Replace structure with:
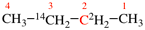
Page 39, P-14.4 (i), example 5 on this page.
For (2R)-[(1-131I)iodo]-3-iodo)propan-2-ol (PIN)
read (2R)-1-(131I)iodo-3-iodopropan-2-ol (PIN)
Page 40, P-14.4 (j), example 1 on this page.
Replace the structure with:
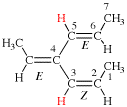
Page 41, P-14.4 (j), last example. [corrected 11 November 2020]
Replace the structure with:
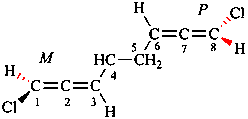
Page 41, P-14.5, paragraph 2, line 2. [corrected 31 December 2025]
For ...(not the detachable saturation prefixes, hydro and dehydro)...
read ...(also the separately cited dehydro and hydro prefixes)...
Page 42, P-14.5.1, example 4.
Replace structure with:
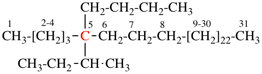
Page 44, P-14.5.4, example 1. [corrected 19 September 2018]
For 4-(2-methylbutyl)-N-(3-methylbutyl)benzeneamine
read 4-(2-methylbutyl)-N-(3-methylbutyl)benzenamine
Page 45, P-14.5.4, example 3 on this page.
Replace structure with:
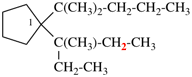
Page 46, P-14.6, example 2.
For 1-[4-(cyclohexylmethoxy)methyl]-4-{[4-(cyclohexylmethyl)cyclohexyl]methyl}cyclohexane (PIN)
read
1-[(cyclohexylmethoxy)methyl]-4-{[4-(cyclohexylmethyl)cyclohexyl]methyl}cyclohexane
for {not 1-({4-[(cyclohexylmethoxy)methyl]cyclohexyl}methyl)-4-(cyclohexylmethyl)cyclohexane [alphanumerical characters are identical; at the third character of the name, a square bracket is preferred to an open parenthesis}
read {not 1-({4-[(cyclohexylmethoxy)methyl]cyclohexyl}methyl)-4-(cyclohexylmethyl)cyclohexane; the alphabetic letters and the primary substituent locants are identical for both names; however, there is no locant for the first internal substituent in the PIN, but in the alternative name the locant for the first internal substituent is ‘4’ and no locant is preferred to ‘4’}
Page 46, P-14.6, example 3. [modified 28 April 2023]
For N-{2-[(acetyldimethylsilyl)methoxy]ethyl}-N′-[2-({2-[(2-aminoethyl)amino]ethyl}amino)ethyl]ethane-1,2-diamine
read N1-(2-{[acetyldi(methyl)silyl]methoxy}ethyl)-N2-[2-({2-[(2-aminoethyl)amino]ethyl}amino)ethyl]ethane-1,2-diamine
for N-[2-({2-[(acetyldimethylsilyl)methoxy]ethyl}amino)ethyl]-N′-{2-[(2-aminoethyl)amino]ethyl}ethane-1,2-diamine
read N1-{2-[(2-{[acetyldi(methyl)silyl]methoxy}ethyl)amino]ethyl}-N2-{2-[(2-aminoethyl)amino]ethyl}ethane-1,2-diamine
for ...nonalphanumerical ‘{’ is preferred to ‘(’]
read ...nonalphanumerical ‘{’ is preferred to ‘[’]
for N-(2-aminoethyl)-N′-(11,11-dimethyl-12-oxo-9-oxa-3,6-diaza-11-silatridecan-1-yl)ethane-1,2-diamine (PIN)
read 19-amino-3,3-dimethyl-5-oxa-8,11,14,17-tetraaza-3-silanonadecan-2-one (PIN)
Page 48, P-14.7.2, example 5 on this page. [corrected 10 October 2018]
Replace the structure with:
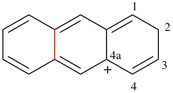
Page 49, P-14.8.1, line 9 on this page.
For ...by a double hyphen (--). The proportions...
read ...by a double hyphen (--) without spaces. The proportions...
Page 49, P-14.8.1, example 1,
Replace structure with:
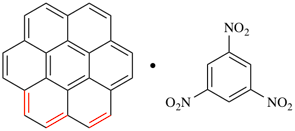
Page 50, P-14.8.1, example 1 on this page. [modified 11 December 2019]
For [(HOOC-CH2-NH)(CH2)2]2-N-CH2-COOH • HN[CH2-P(O)(OH)2]2 • H2N-(CH2)2-NH2
read (HOOC-CH2-NH-[CH2]2)2N-CH2-COOH • HN[CH2-P(O)(OH)2]2 • H2N-[CH2]2-NH2
for {[(carboxymethyl)azanediyl]bis(ethane-2,1-diylazanediyl)}diacetic acid—[iminobis(methylene)]bis(phosphonic acid—ethane-1,2-diamine (1/1/1)
read 2,2′-{[(carboxymethyl)azanediyl]bis(ethane-2,1-diylazanediyl)}diacetic acid—[azanediylbis(methylene)]bis(phosphonic acid—ethane-1,2-diamine (1/1/1)
for N,N-bis{2-[(carboxymethyl)amino]ethyl}glycine—[iminobis(methylene)]bis(phosphonic acid)—ethane-1,2-diamine (1/1/1)
read N-{[(carboxymethyl)azanediyl]ethyl}-N,N′-(ethane-2,1-diyl)diglycine—[azanediylbis(methylene)]bis(phosphonic acid)—ethane-1,2-diamine (1/1/1)
Page 50, P-14.8.1 example 2 on this page.
Replace structure with:
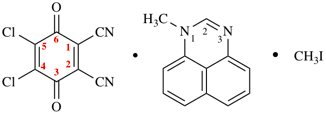
Page 51, P-14.8.1, example 2 on this page. [corrected 6 May 2020]
For ---2,2′-diylbis(diphenyl-λ5phosphanone) (1/1/1/1) (PIN)
read ---3,3′-diylbis(diphenyl-λ5phosphanone) (1/1/1/1) (PIN)
Page 51, P-14.8.1, example 3 on this page. [modified 18 September 2019]
For {piperazin-1-ium 115-[2-(2,4-dinitrophenyl)diazen-1-yl]-3,6,9,13,16,19-hexaoxa-...
read {piperazin-1-ium 115-[(2,4-dinitrophenyl)diazenyl]-3,6,9,13,16,19-hexaoxa-...
Page 53, P-14.8.2, last example.
For 4-[(4-aminomethyl)benzenesulfonyl]aniline—hydrogen chloride—water (1/1/1)
read 4-[4-(aminomethyl)benzene-1-sulfonyl]aniline—hydrogen chloride—water (1/1/1)
Page 54, P-15.0, line 17.
For oxydiaceticacid
read oxydiacetic acid
Page 55, P-15.0, line 1 on this page.
For α-aminoacids
read α-amino acids
Page 54, Table 1.1, column 2. [corrected 31 December 2025]
For Detachable saturation/unsaturation prefixes (hydro/dehydro)
read Saturation/unsaturation prefixes (hydro/dehydro)
Page 57, P-15.1.4, title.
For Position of the endings ‘‘ane’’, ‘ene’, and ‘yne’
read Position of the endings ‘ane’, ‘ene’, and ‘yne’
Page 59, P-15.1.7.1.3, text lines 2 and 4.
For (b)
read P-15.1.7.1.2
for (d)
read P-15.1.7.1.4
Page 61, P-15.1.7.2.3, line 2. [corrected 26 January 2022]
For ...For example, in a cyclic unsaturated alcohol having one substituent group...
read ...For example, in an unsaturated alcohol having one substituent group...
Page 64, P-15.1.8.2.1.2, example 2. [corrected 24 March 2021]
For N-phenylnitrous amide (PIN)
read phenylnitrous amide (PIN)
Page 64, P-15.1.8.2.1.2, example 2.. [corrected 10 June 2021]
For (not N-nitrosoaniline)
read N-nitrosoaniline
Page 65, P-15.1.8.3, example 2. [corrected 10 October 2018]
Replace the structure with:
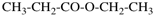
Page 67, P-15.2.1.2, example 3.
For ethyl methyl benzenedicarboxylate (PIN)
read ethyl methyl benzene-1,3-dicarboxylate (PIN)
Page 67, P-15.2.2, example 2.
For N,N-dimethylmethanamine oxide (PIN)
read N,N-dimethylmethanamine N-oxide (PIN)
for trimethylamine oxide
read trimethylamine N-oxide (traditional name, but cannot be substituted)
add trimethylazane N-oxide
Page 68, P-15.2.2, example 1 on this page. [corrected 24 July 2019]
For N-propylidenehydroxylamine (PIN)
read N-propylidenehydroxylamine
add N-hydroxypropan-1-imine (PIN)
Page 71, P-15.3.1.2.1.1, example 9.
Replace the structure with:
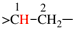
Page 72, P-15.3.1.2.1.2, example 5 on this page.
Replace the structure with:
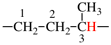
Page 73, P-15.3.1.2.2.1, example 1 on this page. [corrected 28 October 2016]
For [benzene-1,2,4-triyltris(oxy)] (preferred prefix)
read benzene-1,2,4-triyltris(oxy) (preferred prefix)
Page 73, P-15.3.1.2.2.2, example 2. [corrected 18 May 2023]
For ethane-1,2-diylidenebis(azanylylideneoxy)
read ethanediylidenebis(azanylylideneoxy)
Page 74, P-15.3.1.2.2.3, example 2.
For ethane-1,2- diylbis[azanylylidene(chloromethanylylidene)] (preferred prefix)
read ethane-1,2-diylbis[azanylylidene(chloromethanylylidene)] (preferred prefix) [removed space from name]
Page 74, P-15.3.1.2.2.4, example 3.
For peroxydi-4,1-phenylene (preferred prefix)
read peroxydi(4,1-phenylene) (preferred prefix)
for (not dioxydi-4,1-phenylene)
read [not dioxydi(4,1-phenylene)]
Page 75, P-15.3.1.2.2.4, example 2 on this page. [modified 31 July 2019]
For oxybis(1-chloroethane-2,1-diylphosphanylylideneethan-1-yl-2-ylidene)
read oxybis[(1-chloroethane-2,1-diyl)phosphanylylideneethan-1-yl-2-ylidene]
Replace the structure with:

Page 75, P-15.3.1.2.2.4, example 3 on this page. [corrected 31 July 2019]
Replace structure with:
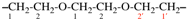
Page 75, P-15.3.1.2.2.4, example 4 on this page. [modified 24 March 2021]
For oxydipropan-1-yl-3-ylidene (preferred prefix)
read oxydi(propan-3-yl-1-ylidene) (preferred prefix)
replace structure with:
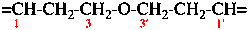
Page 75, P-15.3.1.2.2.4, example 1 on this page (example 4 of section). [corrected 9 April 2025]
For methylenebis(1,3,5-triazine-6,2,4-triyl) (preferred prefix)
read methylenedi(1,3,5-triazine-6,2,4-triyl) (preferred prefix)
Page 75, P-15.3.1.2.2.4, example 5 on this page. [modified 31 July 2019]
For hydrazinediylidenedipropan-1-yl-3-ylidene (preferred prefix;
read hydrazinediylidenedi(propan-1-yl-3-ylidene) (preferred prefix;
replace structure with:
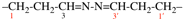
Page 77, P-15.3.2.1, example 1 on this page. [corrected 31 July 2019]
For 1,1′-[oxydi(tetradecane-14,1-diyl)]bis(silane) (PIN)
read [oxydi(tetradecane-14,1-diyl)]bis(silane) (PIN)
Page 77, P-15.3.2.1, example 6 on this page.
For 2,2′ -[oxybis(ethane-2,1-diyloxy)]diacetic acid (PIN)
read 2,2′-[oxybis(ethane-2,1-diyloxy)]diacetic acid (PIN) [Deleted space from name]
replace the structure with:

Page 78, P-15.3.2.1, example 4 on this page.
For [iminobis(methylene)]bis(phosphonic acid)
read [azanediylbis(methylene)]bis(phosphonic acid)
Page 78, P-15.3.2.1, example 5 on this page. [modified 25 August 2016]
For 1,1′,1′′,1′′′-[oxydi(pyridazine-4,3,5-triyl)]tetra(methan-1-ol) (PIN)
read [oxydi(pyridazine-4,3,5-triyl)]tetramethanol (PIN)
replace structure with:
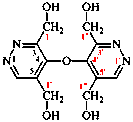
Page 78, P-15.3.2.1, example 6 on this page. [corrected 31 July 2019]
For 1,1′,1′′,1′′′-[oxydi(pyridazine-5,3,4-triyl)]tetra(methan-1-ol) (PIN)
read[oxydi(pyridazine-5,3,4-triyl)]tetramethanol (PIN)
delete [Note: The use of the locant ‘1’ here for methanol; see P-14.3.3]
replace structure with:

Page 79, P-15.3.2.1, example 3 on this page.
For 10,10′-[[1,1′-biphenyl]-4,4′-diylbis(oxy)]di(decanoic acid).
read 10,10′-[[1,1′-biphenyl]-4,4′-diylbis(oxy)]di(decanoic acid) (PIN)
Page 79, P-15.3.2.1, example 4 on this page. [corrected 31 July 2019]
For N,N′-ethane-1,2-diylbis[N-(carboxymethyl)glycine]
read N,N′-(ethane-1,2-diyl)bis[N-(carboxymethyl)glycine]
Page 80, P-15.3.2.1.1, example 1 on this page.
For 2,3′-oxydi(propanoic acid)
read 2,3′-oxydipropanoic acid
Page 80, P-15.3.2.2.1, example 1. [corrected 1 May 2019]
For N,N′-methylenedi(ethan-1-amine) (PIN)
read N,N′-methylenediethanamine (PIN)
Page 80, P-15.3.2.2.1, example 1. [corrected 3 December 2025]
For N,N′--methylenedi(ethan-1-amine) (PIN)
read N,N′--methylenediethanamine
Add N,N′-diethylmethanediamine (PIN)
Page 80, P-15.3.2.2.2, example 2.
Replace the structure with:
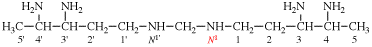
Page 81, P-15.3.2.3, example 1.
For 1,1′,1′′-(benzene-1,3,5-triyl)tris(silane) (PIN)
read (benzene-1,3,5-triyl)tris(silane) (PIN)
for (not benzene-1,3-5-triyltrisilane;...
read (not benzene-1,3,5-triyltrisilane;...
Page 81, P-15.3.2.3, example 2.
For 1,1′-[oxydi(tetradecane-14,1-diyl)]bis(silane) [preferred prefix; for...
read [oxydi(tetradecane-14,1-diyl)]bis(silane) (PIN) [for...
Page 82, P-15.3.2.4.1, example 3 on this page. [corrected 6 May 2020]
Delete tetramethyldiazoxane (see P-21.2.3.1)
Page 82, P-15.3.2.4.1, example 4 on this page.
Delete 1,1′-(2-ethenyl-1,2,3-trimethyldisilazane-1,3-diyl)bis(ethan-1-one)
Page 82, P-15.3.2.4.1, last example on this page.
For 1,1′,1′′′-( benzene-1,3-5-triyl)tris(trimethylsilane) (PIN)
read (benzene-1,3,5-triyl)tris(trimethylsilane) (PIN) [delete space from name, and change hyphen to comma]
Page 83, P-15.3.2.4.2, example 1.
For [not 3-[(5-chloro-5-cyanophenyl)methyl]benzonitrile; the PIN name has the greater number of substituents; see P-45.1.1]
read [not 3-[(5-chloro-2-cyanophenyl)methyl]benzonitrile; the PIN has the greater number of substituents; see P-45.2.1]
Page 83, P-15.3.2.4.2, example 2.
For 2-chloro-4-[2-(5-chloro-2-hydroxyphenyl)ethyl]phenol (PIN)
read [not 2-chloro-5-[2-(5-chloro-2-hydroxyphenyl)ethyl]phenol; the locant set for the substituents in the PIN, ‘2,4’, is lower than ‘2,5’; see P-14.4 (f); P-45.2.2]
for [not 4-chloro-2-[2-(4-chloro-3-hydroxyphenyl)ethyl]phenol; the locant set for the substituents in order of their occurence in the PIN, ‘2,4’, is lower than ‘4,2’; see P-45.2.2]
read 4-chloro-2-[2-(4-chloro-3-hydroxyphenyl)ethyl]phenol (PIN)
Page 84, P-15.3.2.4.2, example 1 on this page. [corrected 30 January 2019]
For [(2-chlorosilyl)ethyl]silane
read [2-(chlorosilyl)ethyl]silane
Page 84, P-15.3.2.4.2, example 2 on this page.
For 1,1,1-trimethyl-2-(disilanyldisulfanyl)disilane (PIN)
read 2-(disilanyldisulfanyl)-1,1,1-trimethyldisilane (PIN)
for [not [2-(trimethyldisilanyl)disulfanyl]disilane; the parent disilane in the PIN has more substituents; see P-45.1.1]
read [not [2-(2,2,2-trimethyldisilan-1-yl)disulfanyl]disilane; the parent disilane in the PIN has more substituents; see P-45.2.1]
Page 84, P-15.3.2.4.2, example 3 on this page.
For 2-chloro-4-(5-chloropyridin-2-yloxy)pyridine (PIN)
read 2-chloro-4-[(5-chloropyridin-2-yl)oxy]pyridine (PIN)
Page 84, P-15.3.2.4.3, example 1.
For 3-bromo-1-[(3-chlorophenyl)methyl]benzene (PIN)
read 1-bromo-3-[(3-chlorophenyl)methyl]benzene (PIN)
Page 85, P-15.3.2.4.3, last example.
For 5-bromo-2-(2-carboxy-4-fluorophenoxy)benzoic acid (PIN)
read 2-(4-bromo-2-carboxyphenoxy)-5-fluorobenzoic acid (PIN)
for [not 2-(4-bromo-2-carboxyphenoxy)-5-fluorobenzoic acid; ‘bromocarboxyfluoro’ is preferred alphanumerically to ‘bromocarboxyphenoxy’; see P-45.5]
read [not 5-bromo-2-(2-carboxy-4-fluorophenoxy)benzoic acid; ‘2,5’ is preferred to ‘5,2’; see P-45.2.3]
Page 85, P-15.3.3.1, Example 1.
For 1,1′-(propane-1,2-diyl)bis(trimethylsilane) (PIN)
read (propane-1,2-diyl)bis(trimethylsilane) (PIN)
Page 85, P-15.3.3.1, example 2.
For [not 1-bromo-4-[3-(4-bromophenyl)prop-3-en-1-yl]benzene;...
read [not 1-bromo-4-[3-(4-bromophenyl)prop-2-en-1-yl]benzene;...
Page 86, P-15.3.3.2.1, example 2. [corrected 30 January 2019]
For {(triphenylmethoxy)[(triphenylmethyl)sulfanyl]methanediyl}dibenzene
read 1,1′-{(triphenylmethoxy)[(triphenylmethyl)sulfanyl]methylene}dibenzene
for [not ({diphenyl[(triphenylmethyl)sulfanyl]methoxy}methanetriyl)tribenzene; ...
read [not 1,1′,1′′-({diphenyl[(triphenylmethyl)sulfanyl]methoxy}methanetriyl)tribenzene; ...
Page 86, P-15.3.3.2.2, example.
For 1,1′-[carbonylbis(diazene-2,1-diyl)]dibenzene
read 1,1′-[carbonylbis(diazenediyl)]dibenzene
Page 87, P-15.3.4.1.1, example (1). [modified 30 January 2019]
For 1-[(disilanylmethyl)peroxy]disilane (PIN)
read [(disilanylmethyl)peroxy]disilane (PIN)
for [not (disilanylperoxy)methyl]disilane;
read [not [(disilanylperoxy)methyl]disilane;
Page 87, P-15.3.4.1.1, example (2).
For 4-{[(4-hydroxyphenyl)amino]methyl}phenol (PIN)
read 4-[(4-hydroxyanilino)methyl]phenol (PIN)
for ‘hydroxyphenylaminomethyl’ precedes ‘hydroxyphenylmethylamino’ ...
read ‘hydroxyanilinomethyl’ precedes ‘hydroxyphenylmethylamino’ ...
Page 87, P-15.3.4.1.1 example (3). [modified 6 May 2020]
For 1-{[2-(disilan-1-yloxy)ethyl]sulfanyl}disilane
read {[2-(disilanyloxy)ethyl]sulfanyl}disilane
for [not 1-{[2-(disilan-1-ylsulfanyl)ethoxy}disilane;
read [not [2-(disilanylsulfanyl)ethoxy]disilane;
Page 88, P-15.3.4.2, example.
For 2-(ethylsulfanyl)-1,1-bis(propylsulfanyl)ethene (PIN...
read 1-{[2-(ethylsulfanyl)-1-(propylsulfanyl)ethen-1-yl]sulfanyl}propane (PIN...
Page 94, P-15.5.2, line 12. [corrected 6 May 2020]
For ...and 1-amido-2-imidodiphosphonic chloride (see P-67.2.3).
read ...and 3-amido-2-imidodiphosphonic chloride (see P-67.2.3).
Page 96, P-15.5.3.2, Table 1.6, line 1 on this page.
For isothiocyantido
read isothiocyanatido
Page 100, P-15.6.1.4, example 3. [corrected 25 August 2016]
For pyridine-2,3-bis(decanoic acid)
read pyridine-2,3-di(decanoic acid)
for 10,10′-(pyridine-2,3-diyl)bis(decanoic acid) (PIN)
read 10,10′-(pyridine-2,3-diyl)di(decanoic acid) (PIN)
Replace the structure with:
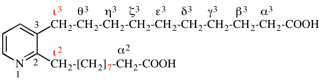
Page 102, P-15.6.2, last example. [modified 30 January 2019]
For β,5-dihydroxybenzene-1,3-diethanol
read β1,5-dihydroxybenzene-1,3-diethanol
for [5-hydroxy-3-(2-hydroxyethyl)phenyl]ethane-1,2-diol
read 1-[3-hydroxy-5-(2-hydroxyethyl)phenyl]ethane-1,2-diol
Page 104, P-15.6.3, example at top of page.
For cyclohexaneheptanal
read benzeneheptanal
Page 105, P-16.2.1 (b), example 1. [corrected 19 September 2018]
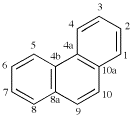
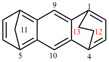
For dibenzo[c,g]anthracene [PIN, P-25.3.4.2.1 (c)]
read dibenzo[c,g]phenanthrene [PIN, P-25.3.4.2.1 (c)]
Page 106, P-16.2.5.
For P-16.2.5
read P-16.2.4.5
Page 106, P-16.2.4.5 (was P-16.2.5), example.
For carbon monoxide — methylborane (PIN...
read carbon monoxide—methylborane (PIN... [Deleted spaces from name]
Page 106, P-16.2.6.
For P-16.2.6
read P-16.2.5
Page 106, P-16.2.5 (was P-16.2.6) (b), example 2 on this page.
For 2-(carbonochloridothioyl)benzoyl cyanide
read 2-(carbonocyanidothioyl)benzoyl chloride
Page 107, P-16.2.5 (was P-16.2.6) (b), example 2 on this page. [corrected 19 September 2018]
For 1-(ethanesulfonyl)butane (PIN)
read 1-(ethanesulfinyl)butane (PIN)
Page 107, P-16.2.5 (was P-16.2.6) (c), example 2. [corrected 24 July 2019]
For (pentan-2-ylidene)hydroxylamine (PIN)
read (pentan-2-ylidene)hydroxylamine
add N-hydroxypentan-2-imine (PIN)
Page 107, P-16.2.5 (was P-16.2.6) (d), example 2.
For trimethyl-λ5-phosphanone (PIN, P-68.2.3.2.1, P-74.2.1.4)
read trimethyl-λ5-phosphanone (PIN, P-68.3.2.3.1, P-74.2.1.4)
Page 107, P-16.3.2 (a), line 3. [corrected 31 July 2019]
For ...ethyl or t-butyl...
read ...ethyl or tert-butyl...
Page 109, P-16.3.4 (d), example 3. [corrected 11 December 2019]
For tetra(decanoic acid) (preferred suffix, defines four...
read tetra(decanoic acid) (defines four...
Page 110, P-16.3.5 (b), example 1. [modified 11 December 2019]
For bis(thioic) acid [preferred suffix, describes two – C(O/S)H suffixes, see P-65.1.5.1; whereas dithioic acid describes –CSSH suffix, see P-65.1.5.1]
read bis(thioic acid) (multiple preferred suffix, describes two –C(O/S)H suffixes, see P-65.1.5.1) [delete space in structure]
Page 110, P-16.3.5 (b), example 2. [modified 11 December 2019]
For bis(dithioic) acid (preselected suffix, see P-65.1.5.1) not didithioic acid
read bis(dithioic acid) (multiple preferred suffix, describes two –CSSH suffixes, see P-65.1.5.1) not didithioic acid
Page 112, P-16.3.6(b), example 1 on this page. [corrected 1 May 2019]
For bis(azo) (preferred prefix, defines two...
read bis(azo) (defines two...
for 3,3′-[methylenebis(azo)]dipropanoic acid (PIN)
read 3,3′-[methylenebis(azo)]dipropanoic acid
Page 114, P-16.5.1.1, example 3. [corrected 28 October 2016]
For not 1,3,5-tris(decy)lcyclohexane
read not 1,3,5-tris(decyl)cyclohexane
Page 114, P-16.5.1.1, last example. [corrected 30 January 2019]
Delete 1,4-phenylenedi(tridecane).
Page 115, P-16.5.1.2, example 3. [corrected 8 July 2017)
For 1-(naphthalen-2-yl)-2-phenyldiazene (PIN...
read (naphthalen-2-yl)(phenyl)diazene (PIN...
Page 115, P-16.5.1.3, lines 1-4. [modified 6 December 2023]
For P-16.5.1.3 Parentheses are placed around prefixes denoting simple substituent groups in front of parent hydrides when no locants are necessary in order to avoid any misunderstanding that the substituent group is modified by a preceding one. When the simple substituent groups are accompanied by multiplicative prefixes such as ‘di’ and ‘tri’, the multiplicative prefixes are not included in the parentheses. Parentheses are placed also around prefixes denoting simple substituent groups qualified by locants. A minimum of parentheses must be used. Enclosing marks are never used around the names of the first cited simple substituent group.
read P-16.5.1.3 Parentheses for substituents of parent structures.
P-16.5.1.3.1 For mononuclear parent hydrides with two or more substituents the first cited substituent never has enclosing marks unless it is includes a locant. The second and further substituents are each enclosed with parentheses even for simple substituents. When the simple substituent groups are accompanied by multiplicative prefixes such as ‘di’ and ‘tri’, the multiplicative prefixes are not included in the parentheses.
Page 115, P-16.5.1.3 (now P-16.5.1.3.1), example 1.. [corrected 12 May 2021]
For butyl(ethyl)methyl(propyl)silane (PIN)
read butyl(ethyl)(methyl)(propyl)silane (PIN)
Page 115, P-16.5.1.3 (now P-16.5.1.3.1), example 3.. [corrected 12 May 2021]
For ethyl(methyl)propylphosphane (PIN)
read ethyl(methyl)(propyl)phosphane (PIN)
Page 116, P-16.5.1.3 (now P-16.5.1.3.1), example 1 on this page. [corrected 12 May 2021]
For ethyl(isopropyl)methylphosphane
read ethyl(isopropyl)(methyl)phosphane
Page 116, P-16.5.1.3 (now P-16.5.1.3.1), example 2 on this page. [corrected 8 July 2017)
For (chloromethyl)methylsilane (PIN; P-68.2.6.1)
read (chloromethyl)(methyl)silane (PIN; P-68.2.6.1)
Page 116, P-16.5.1.3 (now P-16.5.1.3.1), example 4 on this page. [corrected 20 February 2019]
For ethyl(dimethyl)phosphane (PIN)
read ethyldi(methyl)phosphane (PIN)
Page 116, P-16.5.1.3 (now P-16.5.1.3.1), example 5 on this page. [corrected 5 July 2018]
For ethyl[di(propan-2-yl)]propylsilane (PIN)
read ethyldi(propan-2-yl)silane (PIN)
add ethyldiisopropylsilane
Page 116, P-16.5.1.3 (now P-16.5.1.3.1), after example 5.. [corrected 12 May 2021]
Add ethyl(methyl)(propyl)silanecarboxylic acid

methyl(phenyl)phosphinic acid

cyclopropyl(phenyl)methanol

Page 116, P-16.5.1.3 (now P-16.5.1.3.1), after P-16.5.1.3.1. [corrected 12 May2021]
Add P-16.5.1.3.2 Simple substituents of a parent where only one possible position can be substituted do not require locants. Enclosing marks are used with second and subsequent simple substituents (see P-16.5.1.3.1). In some cases, such as acetic acid, alkyl substituents are not allowed as the resulting compound requires a different parent structure. Locants are required for related compounds where additional substitutable positions are available, for example acetamide.
bromo(nitro)(phenyl)acetic acid

diethyl ethyl(methyl)propanedioate

bromo(chloro)acetic acid

cyclopropyl(fluoro)acetonitrile

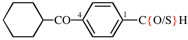
Page 116, P-16.5.1.4, example 1. [modified 7 November 2018]
For 4-(cyclohexanecarbonyl)benzenecarbothioic acid (PIN)
read 4-(cyclohexanecarbonyl)benzene-1-carbothioic acid (PIN)
replace the structure with:
Page 117, P-16.5.1.8, example.
For 4-ethanethioylbenzoic acid (PIN...
read 4-(ethanethioyl)benzoic acid (PIN...
Page 117, P-16.5.1.9, example 1. [modified 16 January 2019]
For {benzo[1,2-c:3,4-c′]bis([1,2,5]oxadiazole)}-5-carboxylic acid (PIN; P-65.1.2.2.2)
read [benzo[1,2-c:3,4-c′]bis([1,2,5]oxadiazole)]-4-carboxylic acid (PIN; P-65.1.2.2.2)
Page 118, P-16.5.1.11, example 3.
Replace structure with:
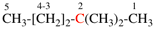
Page 119, P-16.5.3.1, example.
Replace the structure with:
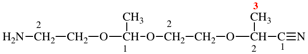
Page 119-120, P-16.5.4, Fig 1.3.
Replace the structure and text with:
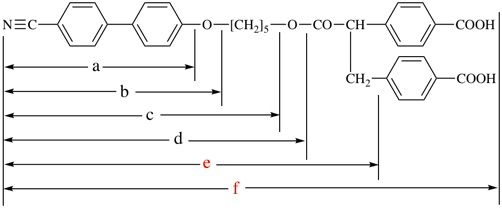
(a) = 4′-cyano[1,1′-biphenyl]-4-yl
(b) = (4′-cyano[1,1′-biphenyl]-4-yl)oxy
(c) = 5-[(4′-cyano[1,1′-biphenyl]-4-yl)oxy]pentyl
(d) = {5-[(4′-cyano[1,1′-biphenyl]-4-yl)oxy]pentyl}oxy
(e) = 3-({5-[(4′-cyano[1,1′-biphenyl]-4-yl)oxy]pentyl}oxy)-3-oxopropane-1,2-diyl
(f) = 4,4′-[3-({5-[(4′-cyano[1,1′-biphenyl]-4-yl)oxy]pentyl}oxy)-3-oxopropane-1,2-diyl]dibenzoic acid (PIN)
Page 120, P-16.5.4, example on this page. [corrected 28 October 2016]
For [N-(carboxymethyl)-N′-(2-hydroxyethyl)-N,N′-ethane-1,2-diyldiglycine
read N-(carboxymethyl)-N′-(2-hydroxyethyl)-N,N′-(ethane-1,2-diyl)diglycine
Page 120, P-16.5.4.1. [corrected 6 December 2023]
Replace section (retain box at end)
P-16.5.4.1 The presence of square brackets and/or parentheses that are an integral part of the name of a parent structure may affect the nesting order as described below.
P-16.5.4.1.1 In determining the nesting order in a name the parentheses of added indicated hydrogen are ignored.
Example:
Examples:
Examples:
Example:
Explanation: The order of insertion of stereochemistry for 2-{2-[(1-ethoxy-1-oxo-4-phenylbutan-2-yl)amino]propanoyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid is as shown below.
Stage 1 insert stereochemistry at the inner most position:2-{2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
The substituent (2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl requires square brackets to surround it hence subsequent enclosing marks need to be adjusted.
Stage 2 insert stereochemistry at the next position:
2-[(2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
The substituent (2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl has a parenthesis on the left and brace on the right so to make clear it requires square brackets around it.
Stage 3 insert stereochemistry at the third position:
(3S)-2-[(2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
Page 120, P-16.5.4.1.5 (revised text), Stage 1. [corrected 31 December 2025]
For 2-{2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
read 2-(2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
Page 121, P-16.6.1, example 2. [corrected 23 December 2020]
Delete this example.
Page 121, P-16.6.2, example 1.
For N,N-diethylethan-1-amine (PIN)
read N,N-diethylethanamine (PIN)
Page 122, P-16.7.1 (a), line 2.
For ...when followed by a suffix or 'en' ending beginning with 'a', 'e'....
read ...when followed by a suffix or ending beginning with 'a', 'e'....
Page 123, P-16.7.2 (d), example 2. [modified 31 July 2019]
For N,N′-ethane-1,2-diylbis[N-(carboxymethyl)glycine]
read N,N′-(ethane-1,2-diyl)bis[N-(carboxymethyl)glycine]
Page 124, P-16.8.2, example 1. [corrected 9 January 2019]
For (not but-1,2-diene)
read (not but-1,3-diene)
Page 124, P-16.9.1, example 1.
For N′-acetyl-N′-ethyl-N-methylpropanehydrazide
read N′-acetyl-N′-ethyl-N-methylbutanehydrazide
Page 126, P-16.9.3, example 2.
For N′′1-ethyl-N1,N1,-dimethylcyclohexane-1,1-dicarboximidamide (PIN, P-66.4.1.4.2)
read N′′1-ethyl-N1,N1-dimethylcyclohexane-1,1-dicarboximidamide (PIN, P-66.4.1.4.2)
for N′′′-ethyl-N,N,-dimethylcyclohexane-1,1-dicarboximidamide
read N′′-ethyl-N,N-dimethylcyclohexane-1,1-dicarboximidamide
Replace structure with:
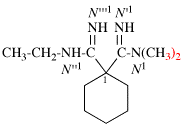
Page 127, P-16.9.3, example 5 on this page.
For N1,N′1-methylenedi(pentane-1,3,4-triamine) (PIN, P-62.25.2)
read N1,N1′-methylenedi(pentane-1,3,4-triamine) (PIN, P-62.25.2)
Page 127, P-16.9.3, last example on this page.
For N1-ethyl- N1′ ,N1′,N3-trimethylpropane-1,1,3-tricarboxamide (PIN, P-66.1.1.3.1.2)
read
N′1-ethyl-N1,N1,N3-trimethylpropane-1,1,3-tricarboxamide (PIN, P-66.1.1.3.1.2) [delete spaces from name]
Replace structure with:
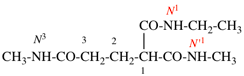
Page 128, P-16.9.3, example 1 on this page. [corrected 19 September 2018]
For N1, N1, N′3-triethyl-N′1, N3, N3-trimethylnapthalene-1,3-dicarboximidamide (PIN, P-66.4.1.4.2)
read N1,N1,N′3-triethyl-N′1,N3,N3-trimethylnaphthalene-1,3-dicarboximidamide (PIN, P-66.4.1.4.2) [deleted spaces from name]
Page 128, P-16.9.4 (b), example 2 on this page.
For 1H,1′H,1′′H,3′H-2,2′:7,2′′-dispiroter[naphthalene]
read 1H,1′H,1′′H,3′H-2,2′:7′,2′′-dispiroter[naphthalene]
Page 129, P-16.9.5 (a), example 1. [corrected 31 July 2019]
For pyrido[1′′,2′′:1,2′]imidazo[4′,5′:5,6]pyrazino[2,3-b]phenazine (PIN,
read pyrido[1′′,2′′:1′,2′]imidazo[4′,5′:5,6]pyrazino[2,3-b]phenazine (PIN,
Page 129, P-16.9.5 (a), example 2.
For difuro[3,2-b;2′,3′-e]pyridine (PIN,
read difuro[3,2-b:2′,3′-e]pyridine (PIN,
Page 129, add a new section:
P-16.9.6. Multiple primes
Long strings of primes may be needed with ring assemblies and polyspiro compound. It is difficult to count long streams of primes. For example, how many primes appear in this string: ′′′′′′′′ ? It is eight. In these recommendations, to simplify the counting process, long strings of primes are broken into groups of three as: ′′′ ′′′ ′′, which has the same number of primes as the string above. This is a change from the publication on spiro compounds (ref. 8) in which multiple primes were separated by a space after every four primes.
Page 130, P-20, lines 9/10. [modified 28.8.2019]
For ...for example, spiro[1,3-dioxolane-2,1′-[1H]indene], and there...
read ...for example, 1′H-spiro[[1,3]dioxolane-2,1′-indene], and there...
Page 131, P-21.1.1.1, line 6. [corrected 27 May 2020]
For ...tellane for H2Te, polonane for H2Po, and...
read ...tellane for H2Te, polane for H2Po, and...
Page 131, P-21.1.1.1, line 8.
For ...polane and bismane...
read ...polonane and bismane...
Page 132, P-21.1.1.1, example 4.
Change structure to:
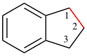
Page 135, P-21.2.3.1, example 5.
For N-silylsilan-1-amine
read N-silylsilanamine
Page 135, P-21.2.3.1, example 6. [corrected 11 November 2020]
Delete this example
Page 137, P-21.2.4.2, example 2. [corrected 28 April 2023]
For 2,5λ4,8,11-tetrathiadodecane
read 2,5λ4,8λ6,11-tetrathiadodecane
replace the structure with:
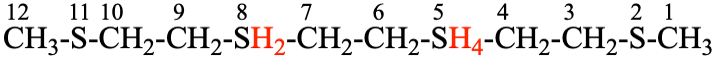
Page 140, P-22.2.1, Table 2.2, structure 2.
Replace the structure with:
Page 140, P-22.2.1, Table 2.2, structure 4.
For 1, 3-selenazole (PIN)
read 1,2-selenazole (PIN) [delete space in name]
for 1,3-tellurazole (PIN)
read 1,2-tellurazole (PIN)
Page 142, P-22.2.1, Table 2.3 structure 1.
For 1,3-thioxazolidine (PIN)
read 1,3-thiazolidine (PIN)
for 1,3-selenoxazolidine (PIN)
read 1,3-selenazolidine (PIN)
for 1,3-telluroxazolidine (PIN)
read 1,3-tellurazolidine (PIN)
Page 142, P-22.2.1, Table 2.3 structure 2.
For isothioxazolidine (S instead of O)
read isothiazolidine (S instead of O)
for 1,2-thioxazolidine (PIN)
read 1,2-thiazolidine (PIN)
for isoselenoxazolidine (Se instead of O)
read isoselenazolidine (Se instead of O)
for 1,2-selenoxazolidine (PIN)
read 1,2-selenazolidine (PIN)
for isotelluroxazolidine (Te instead of O)
read isotellurazolidine (Te instead of O)
for 1,2-telluroxazolidine (PIN)
read 1,2-tellurazolidine (PIN)
Page 146, P-22.2.2.1.5.1, lines 1/2. [corrected 9 January 2019]
For ...rings containing only nitrogen; otherwise...
read ...rings only containing nitrogen heterotoms; otherwise...
Page 149, P-22.2.3.2.1, example 2.
For 1H-1-azacyclotrideca-2,4,6,8,10,12-hexaene (PIN)
read 1-azacyclotrideca-2,4,6,8,10,12-hexaene (PIN)
Page 150, P-22.2.4, line 8.
For (see P-22.2.4)
read (see P-22.2.3)
Page 151, P-22.2.5, example 1. [modified 11 November 2020]
Delete pentazolane
Page 152, P-22.2.6, last line.
For ...heteromonocyclic hydrides, see P-52.1.7.
read ...heteromonocyclic hydrides, see P-52.1.5.2.
Page 152, P-22.2.6, example 2. [corrected 28 October 2016]
For 3,5,2,4,6-triphosphatriborinane
read 1,3,5,2,4,6-triphosphatriborinane
Page 160, P-23.2.6.2.3, right-hand structure of example 2.
For tetracyclo[5.3.2.12,4.06.13]tridecane
read tetracyclo[5.3.2.12,4.06,13]tridecane
Page 161, P-23.2.6.2.4, example 1.
Replace the structures with:
Page 161, P-23.2.6.2.4, example 2, left version. [modified 8 May 2019]
For tricyclo[5.5.1.03,11]tridecane (PIN)
read tricyclo[5.5.1.03,11]tridecane
replace the structure with:
Page 162, P-23.2.6.2.5, example 1, right structure. [corrected 30 January 2019]
Replace structure with:
Page 162, P-23.2.6.2.5, example 2, left-hand structure.
Replace the structure with:
Page 162, P-23.2.6.3, line 5.
For ... atom of the main bridge, then ...
read ... atom of the main ring, then ...
Page 167, P-23.5.1, example 1 on this page.
Replace the structure with:
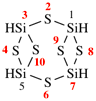
Page 167, P-23.5.2, example 2.
For (b) 2,4,6,8,9-pentaaza-1,3,5,7,10-pentasilatricyclo[3.1.1.12,4]decane
read (b) 2,4,6,8,9-pentaaza-1,3,5,7,10-pentasilatricyclo[3.3.1.12,4]decane
replace the structure with:
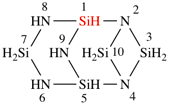
Page 168, P-23.5.3, line 8.
For Si2nH3nN3n
read Si2nH5nN3n
Page 168, P-23.6.2, line 4.
For phosphorus
read arsenic
Page 169, P-23.7, Table 2.6 structure 4.
Replace structure with:
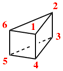
Page 175, P-24.2.3.1, example 1 on this page. [corrected 4 September 2019]
For tetraspiro[2.2.26.2.2.214.211.23]icosane (PIN)
read tetraspiro[2.2.26.2.2.214.211.23]icosane
Page 180, P-24.3.3, example 2.
For 4H-2,4′-spirobi[[1,3]dioxolo[4,5-c]pyran] (PIN)
read 2′H,4H-2,4′-spirobi[[1,3]dioxolo[4,5-c]pyran] (PIN)
Page 180, P-24.3.3, last example.
For 2,4′spirobi[1]benzopyran (PIN)
read 2,4′-spirobi[[1]benzopyran] (PIN)
Page 182, P-24.4, title.
For MONOSPIRO RING SYSTEMS ...
read DISPIRO RING SYSTEMS ...
Page 182, P-24.4.1, example 2.
Change structure to:
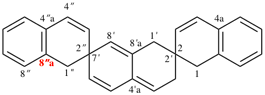
Page 184, P-24.4.3, example 1.
For correct 1′H,2H,3′′H,4a′H-3,7′:2′,7′′-dispiroter[quinoline]
read correct 1′H,2H,3′′H,4′aH-3,7′:2′,7′′-dispiroter[quinoline]
change correct structure to:
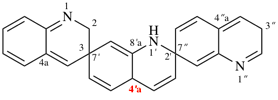
and
For incorrect 1′H, 2′′H,3H,4a′H-7,2′:7′,3′′-dispiroter[quinoline]
read incorrect 1′H,2′′H,3H,4′aH-7,2′:7′,3′′-dispiroter[quinoline] [delete space from name]
change incorrect structure to:
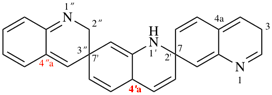
Page 184, P-24.4.3, example 1, Explanation. [corrected 23 January 2019]
For The locant set ‘1′,2,3′′...’ in the correct name, the PIN, is lower than the locant set ‘1′,2′′,3′′...’ in the incorrect name
read The locant set ‘2′,3,7′,7′′...’ in the correct name, the PIN, is lower than the locant set ‘2′,3′′,7,7′...’ in the incorrect name.
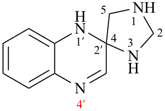
Page 184, P-24.4.3, last example.
For 6,6′,6′′,8,8′,8′′- hexaoxa[2,7′:2′,7′′]dispiroter[bicyclo[3.2.1]octane (PIN)
read 6,6′,6′′,8,8′,8′′-hexaoxa-2,7′:2′,7′′-dispiroter[bicyclo[3.2.1]octane] (PIN) [delete space in name]
Page 185, P-24.5.1, example 3. . (corrected 23 October 2019)
Replace the structure with:
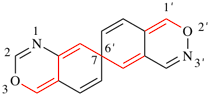
Page 186, P-25.5.3, line 4/8. [corrected 8 May 2019]
For Locants present in bicyclic fused benzo ring components are cited directly in front of the component and name when they correspond to the numbering of the spiro ring system; when they do not correspond to those of the spiro ring system, they are placed in brackets (see third and fourth examples below).
read All locants present in a bicyclic fused benzo ring component or Hantzsch-Widman named component are placed in brackets (without primes for the second component, see third and fourth examples below).
Page 186, P-24.5.2, example 1. [corrected 4 September 2019]
For [not 2-thiaspiro[bicyclo[2.2.2]octane-3,9′- fluorene] (II)]
read [not 2-thiaspiro[bicyclo[2.2.2]octane-3,9′-fluorene] (II)] [delete space in name]
Page 187, P-24.5.3, example 4 on this page. [modified 4 September 2019]
For (3,1-benzoxazine before 2,3,-benzoxazine)
read (3,1-benzoxazine before 2,3-benzoxazine)
Replace the structure with:
Page 188, P-24.6, line 7/8. [corrected 8 May 2019]
For ... and so on; accordingly, locants required by the names of the second and later ring components are enclosed in square brackets.
read ... and so on. In a complete name, all locants present in a bicyclic fused benzo ring component or Hantzsch-Widman named component are placed in brackets (without primes for the second and subsequent components, see fourth example below).
Page 188, P-24.5.4, example 1. [corrected 4 September 2019]
For [bicyclo[2.2.2]octane is cited before bicyclo[3.2.1]octane
read [bicyclo[2.2.2]octane is cited before bicyclo[3.2.1]octane]
Page 189, P-24.6, example 3.
For 7′-azadispiro[fluorene-9,2′-bicyclo[2.2.1]heptane-5′,1′′-indene]
read
7′-azadispiro[fluorene-9,2′-bicyclo[2.2.1]heptane-5′,1′′-indene] (PIN)
Page 189, P-24.6, example 4.
For 3,6-dioxadispiro[bicyclo[2.2.1]heptane-2,2′-[1,4]dioxane-5′,2′′-pyran (PIN)
read 3,6-dioxadispiro[bicyclo[2.2.1]heptane-2,2′-[1,4]dioxane-5′,2′′-pyran] (PIN)
Page 190, P-24.6.1, example. [modified 27 May 2020]
For dispiro[[1,3]dioxolane-2,3′-bicyclo[3.2.1]octane-6′,2′′-[1,3]dioxolane] (I)
read dispiro[[1,3]dioxolane-2,3′-bicyclo[3.2.1]octane-6′,2′′-[1,3]dioxolane] (I) (PIN)
For dispiro[bicyclo[3.2.1]octane-2,3′:6′,2′′-bis([1,3]dioxolane) (PIN, see P-24.7.1)
read [not dispiro[bicyclo[3.2.1]octane-3,2′:6,2′′-bis([1,3]dioxolane) (III) (see P-24.4.2)]
Replace structures with:
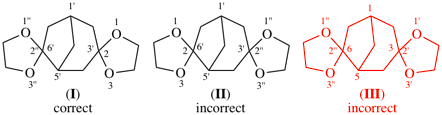
Page 190, P-24.7.1, example. [corrected 8 May 2019]
read trispiro[1,3,5-trithiane-2,2′:4,2′′:6,2′′′-tris(bicyclo[2.2.1]heptane)] (PIN)
read trispiro[[1,3,5]trithiane-2,2′:4,2′′:6,2′′′-tris(bicyclo[2.2.1]heptane)] (PIN)
Page 192, P-24.7.2, example (3). [modified 27 May 2020]
for trispiro[bis(cyclopentane)-1,1′:1,4′-cycloheptane-6′,2′′′-[1,4]-dioxolane] (I) (PIN)
for (not trispiro[bis(cyclopentane)-1,1′:1,5′-cycloheptane-3′,2′′′-[1,4]-dioxolane] (II)
Page 192, P-24.7.2, example (3), Explanation line 4.
Page 192, P-24.7.2, example (3). [corrected 11 November 2020]
Page 192, P-24.7.2, example (4).
Page 193, P-24.7.3, example. [modified 27 May 2020]
for trispiro[cyclohexane-1,2′-[1,5]dithiocane-6′,1′′-cyclopentane-4′,2′′′-indene] (I) (PIN)
for trispiro[cyclohexane-1,8′-[1,5]dithiocane-4′,1′′-cyclopentane-6′,2′′′-indene] (II)
for trispiro[cyclohexane-1,4′-[1,5]dithiocane-8′,1′′-cyclopentane-4′,2′′′-indene] (I)
for trispiro[cyclohexane-1,6′-[1,5]dithiocane-2′,1′′-cyclopentane-8′,2′′′-indene] (I)
Page 194, P-24.7.4.1, example 1. [modified 27 May 2020]
Page 194, P-24.7.4.1, example 2. [modified 27 May 2020]
for
octaspiro[2,4,6,8,9,10-hexathia-1,3,5,7-tetraphosphatricyclo[3.3.1.13,7]decane-1,2′λ5:3,2′′λ5:5,2′′′λ5:7,2′′′′λ5-tetrakis[1,3,2]oxathiaphosphetane- 4′,7′′′ ′′:4′′,7′′′ ′′′:=4′′′,7′′′ ′′′ ′:4′′′,7′′′ ′′′ ′′-tetrakis[7H]pyrano[2,3-c]acridine] (the CAS index name)
Page 195, P-24.7.4.1, example on this page. [modified 27 May 2020]
Page 196, P-24.8.1.2, example.
Page 196, P-24.8.1.3.1, line 3.
Page 198, P-24.8.2, example 1.
Page 199, P-24.8.2, last example.
Page 199, P-24.8.3, example.
Page 200, P-24.8.4.1, example 3.
Page 202, P-24.8.5, example.
Page 202, P-24.8.6, example 1. [modified 8 May 2019] replace structure with:
Page 206, P-25.1.1, table 2.7.
Page 211, P-25.2.1, Table 2.8 entry (3). replace structure with:
Page 211, P-25.2.1, Table 2.8 entry (4).
Page 212/3, P-25.2.1, Table 2.8 entries (13), (14) and (15). [modified 11 November 2020]
Page 214, Table 2.8, Structure (23), line 16.
Page 214, P-25.2.1, Table 2.8 footnote.
Page 214, P-25.2.1, Table 2.9. for quinoline (PIN), arsinoline (PIN), and phosphinoline (PIN) for quinolizine (PIN), arsinolizine (PIN), and phosphinolizine (PIN) add footnote † In CAS index nomenclature, quinolizine precedes quinoline and isoquinoline in seniority.
Page 216, P-25.2.2.2, example 1 in the left column. [corrected 30 January 2019]
Page 217, P-25.2.2.3, first example on page.
Page 217, P-25.2.2.4, lines 1/2. [corrected 22 April 2020]
Page 218, P-25.2.2.4, example 1. [corrected 26 January 2022]
Page 218, P-25.2.2.4, example 2. [corrected 26 January 2022]
Page 218, P-25.2.2.4, last example.
Page 221, P-25.3.1.3, example 1.
Page 222, P-25.3.1.3, example 1 on this page.
Page 222, P-25.3.1.3, left example at bottom of page.
Page 226, P-25.3.2.3.1, line 6. (corrected 1 December 2021)
Page 226, P-25.3.2.3.1, before the example add. [corrected 23 February 2022)
Page 226, P-25.3.2.3.2. (corrected 1 December 2021)
Page 226, insert new P-25.3.2.3.2 after P-25.3.2.3.1 (corrected 1 December 2021)
P-25.3.2.3.2 Distorted ring shapes
Page 229, P-25.3.2.3.2 (d), structure 3.
Page 231, P-25.3.2.4 (c), example 1. [corrected 24 October 2018]
Page 231, P-25.3.2.4 (e), example.
Page 232, P-25.3.2.4 (f), example 1. [corrected 24 October 2018]
Page 233, P-25.3.2.4 (j), example. (modified 23 October 2019) for indeno[1,7-kl]aceanthrylene
Page 234, P-25.3.2.5.2, line 2.
Page 234, P-25.3.2.5.2, example.
Page 235, P-25.3.3.1.1, example 1.
Page 237, P-25.3.3.1.2 (c) example 2. (corrected 23 October 2019)
Page 237, P-25.3.3.1.2 (d), example.. [corrected 12 May 2021]
add [1,3]diazeto[1,2-a:3,4-a′]bis([1,3]benzimidazole) (PIN)
Page 238, P-25.3.3.1.2 (f), example 2, right version. [corrected 10 October 2018]
Page 239, P-25.3.3.2.2, example.
Page 239, P-25.3.3.2.3, example
Page 240, P-25.3.3.3.1, example 3.
Page 242, P-25.3.4.1.2, example 1.
Page 246, P-25.3.4.2.2 (a) example. [corrected 19 September 2018] replace the structure with:
Page 248, P-25.3.4.2.3.1, example 3. replace the structure with:
Page 253, P-25.3.4.2.4(h) example.
Page 254, P-25.3.5.4, example 2. Replace the structure with:
Page 255, P-25.3.5.4, example 1 on this page. [corrected 30 January 2019]
Page 255, P-25.3.5.4, example on this page.
Page 255, P-25.3.6.1, example 3.
Page 256, P-25.3.6.1, last example.
Page 258, P-25.3.7.1, example 5.
Page 262, P-25.3.8.3, example. [corrected 14 November 2018] replace the structure with:
Page 262, P-25.3.8.4, example 3. [corrected 3 October 2018] replace the structure with:
Page 265, P-25.4.1.9, example.
Page 266, P-25.4.2.1.2, replace the structure of example 1. [corrected 26 January 2022]
Page 266, P-25.4.2.1.2, replace structure of example 2. [corrected 26 January 2022]
Page 267, P-25.4.2.1.3, replace structure of example 2 on this page. [corrected 26 January 2022]
Page 267, P-25.4.2.1.4, line 3 of box.
Page 271, P-25.4.2.3.2, example 4. Replace the diagram with:
Page 272, P-25.4.2.3.2, example 1 on this page. Replace the diagram with:
Page 273, P-25.4.3.2.1, Example 2. [corrected 30 January 2019]
Page 273, P-25.4.3.2.1, example 3. [modified 27 May 2020]
Page 274, P-25.4.3.2.2, example 1. [modified 11 December 2019] add (not 7H-3,5-(epoxymethano)furo[2,3-c]pyran) (II) [preferred ring system, see P-25.4.3.4.2 (d)]
Page 274, P-25.4.3.2.2, example 2. [modified 11 December 2019]
add (not 4H-6,1-(epoxymethano)furo[3,4-c]pyran) (II)
Page 277, P-25.4.3.4.2, (c)
Page 279, P-25.4.3.4.2 (h), example. [modified 27 May 2020] for [8,7-(azenoepiethanediylidene)cyclohepta[4,5]cycloocta[1,2-b]pyridine (II);
Page 280, P-25.4.3.4.2 (i), example. (modified 23 October 2019) for 5,14-(metheno)-2,3,4-(epiprop[2]ene[1,3]diyl[1]ylidene)dicyclopenta[f,f′]pentaleno[1,2-a:6,5-a′]dipentalene (PIN); for the single bond of the metheno group is given preference the lower locant of the ring system (see P-25.4.3.2.2)] for [not 5,14-(metheno)-2,4,3-(epiprop[2]ene[1,3]diyl[1]ylidene)dicyclopenta[f,f′]pentaleno[1,2-a:6,5-a′]dipentalene for (the locants ‘2,3,4’ for the independent bridge are lower than ‘2,4,3’)] for [not 14,5-(metheno)-2,3,4-(epiprop[2]ene[1,3]diyl[1]ylidene)dicyclopenta[f,f′]pentaleno[1,2-a:6,5-a′]dipentalene for (the locants '5,14' for the independent bridge are lower than '14,5')]
Page 282, P-25.4.4.1, example 2 on this page.
Page 283, P-25.4.5.1, example. [corrected 10 October 2018]
Page 283, P-25.4.5.2, example 1. (corrected 23 October 2019)
Page 285, P-25.5.1.1, example 1. [corrected 14 November 2018]
Page 287, P-25.5.3, example 1.
Page 288, P-25.6, example 1. [corrected 27 May 2020]
Page 290, P-25.7.1.1, example 3 on this page.
Page 294, P-25.8.1, structure. (corrected 23 October 2019)
Page 294, P-25.8.1, Note 3. [modified 11 August 2021]
Page 296, P-25.8.1, line 12. [corrected 30 January 2019]
Page 297, P-25.8.2, after line 6. [corrected 11 December 2019]
Page 298, P-25.8.2, line 7 on this page.
Page 298, P-26.0, line 3. [corrected 8 May 2019]
Page 299, P-26.1.1, line 1.
Page 299, P-26.1.1, lines 10/11.
Page 302, P-26.2.2.1, example 2. add furan (PIN) furana (preferred prefix)
Page 302, P-26.2.2.2.2 (a) (2). [corrected 8 May 2019]
Page 303, P-26.2.4.2, heading.
Page 304, P-26.3.2.1, Example. for [not 1(1,4),4(1,4)-dibenzenacycloheptaphane
Page 304, P-26.3.2.2, example. [corrected 8 May 2019]
Page 305, P-26.4.1.2, last example. for ...locant set ‘1,5,7’ in either of the other names. see P-26.4.2.1]
Page 307, P-26.4.1.4, example 2.
Page 308, P-26.4.1.4, example 2 on this page. for [not 3(5,2)-furana-1,7(1),5(1,4)-tribenzenaheptaphane; for for superatom 3 the attachment locant set ‘2,5’ for the PIN is lower than ‘5,2’]
Page 309, P-26.4.2.2, example 1. [corrected 27 May 2020]
Page 310, P-26.4.2.2, example 2 on this page.
Page 311, P-26.4.2.3, example 1.
Page 313, P-26.4.2.4, example 2. [corrected 19 September 2018]
Page 315, P-26.4.3.3, example 2 on this page. [corrected 13 February 2019]
for ...‘14,15,44,46 ’, is lower than ‘14,16,45,46 ’ (see P-26.4.3.3)]
Page 315, P-26.4.3.4, example.
Page 320, P-26.5.4.1, example 1 on this page. for [not 7(4)-pyridina-1(1),3,5(1,4)-tribenzenaheptaphane;
Page 321, P-26.5.4.2, example on this page. [corrected 30 March 2022]
Page 321, P-26.5.4.2, example on this page.
Page 322, P-27.0, lines 1/2.
Page 324, P-27.2.1, lines 4/5. [corrected 9 January 2019]
Page 324, P-27.2.2, lines 1/2. [corrected 18 September 2019]
Page 324, P-27.3, line 4. [corrected 3 October 2018]
Page 326, P-27.3 example 1 on this page. [corrected 19 September 2018]
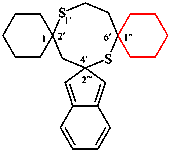
Replace the structures with:
read 3′′′H-trispiro[bis(cyclopentane)-1,1′:4′,1′′-cycloheptane-6′,2′′′-[1,4]benzodioxine] (I) (PIN)
read [not 3′′′H-trispiro[bis(cyclopentane)-1,1′:5′,1′′-cycloheptane-3′,2′′′-[1,4]benzodioxine] (II)]
For ...where 4′ is lower than 5′)
read ...where 4′ is lower than 5′.
Replace the structures with:

Replace the text by:
{not 3H-trispiro[[1,4]benzodioxine-2,1′-cycloheptane-4′,1′′:6′,1′′′-bis(cyclopentane)] (II)}
For trispiro[cyclopentane-1,1′-cyclohexane-3′,2′′- imidazole-5′,1′′′-indene
read trispiro[cyclopentane-1,1′-cyclohexane-3′,2′′-imidazole-5′,1′′′-indene [Deleted space from name]
Replace the structure of (I) with:
replace the structure of (II) with:
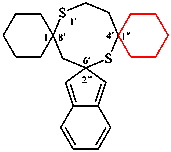
replace the structure of (III) with:
replace the structure of (IV) with:
read trispiro[bis(cyclohexane)-1,2′:6′,1′′-[1,5]dithiocane-4′,2′′′-indene] (I) (PIN)
read [not trispiro[bis(cyclohexane)-1,8′:4′,1′′-[1,5]dithiocane-6′,2′′′-indene] (II)
read nor trispiro[bis(cyclohexane)-1,4′:8′,1′′-[1,5]dithiocane-2′,2′′′-indene] (III);
read nor trispiro[bis(cyclohexane)-1,6′:2′,1′′-[1,5]dithiocane-8′,2′′′-indene] (IV)]
Delete the correction to the first name
For 1λ5,3λ5,5λ5,7λ5-tetraspiro[tetraspiro[2,4,6,8,9,10-hexathia-1,3,5,7-tetraphosphaadamantane-1,2′:3,2′′:5,2′′′:7,2′′′ ′-tetrakis([1,3,2]oxathiaphosphetane)]-4′,7′′′ ′′, 4′′,7′′′ ′′′,:4′′′,7′′′ ′′′ ′,4′′′ ′,7′′′ ′′′ ′′-tetrakis(pyrano[2,3-c]acridine)} [PIN, see SP-7.4(b)]
read 1λ5,3λ5,5λ5,7λ5-tetraspiro{tetraspiro[2,4,6,8,9,10-hexathia-1,3,5,7-tetraphosphaadamantane-1,2′:3,2′′:5,2′′′:7,2′′′ ′-tetrakis([1,3,2]oxathiaphosphetane)]-4′,7′′′ ′′:4′′,7′′′ ′′′: 4′′′,7′′′ ′′′ ′:4′′′ ′,7′′′ ′′′ ′′-tetrakis(pyrano[2,3-c]acridine)} [PIN, see SP-6.4(b), in ref 8]
read octaspiro[2,4,6,8,9,10-hexathia-1,3,5,7-tetraphosphatricyclo[3.3.1.13,7]decane-1,2′λ5:3,2′′λ5:5,2′′′λ5:7,2′′′′λ5-tetrakis[1,3,2]oxathiaphosphetane-4′,7′′′′′:4′′,7′′′′′′:4′′′,7′′′′′′′:4′′′′,7′′′′′′′′-tetrakis[7H]pyrano[2,3-c]acridine] (the CAS index name; note that multiple primes are not divided into groups of three) [delete spaces from name]
Delete the correction to this name
For 2λ6-thia-4λ4-thiaspiro[5.5]undecane
read 2λ6,4λ4-dithiaspiro[5.5]undecane
For ...an acyclic ring system...
read ...an alicyclic system...
For 2λ4-2,2′-spirobi[[1,3,2]benzodioxathiole] (PIN)
read 2λ4,2′-spirobi[[1,3,2]benzodioxathiole] (PIN)
For 1H-2λ5-2,2′-spirobi[[1,3,2]-benzodiazaphosphinine] (PIN)
read 1H-2λ5,2′-spirobi[[1,3,2]benzodiazaphosphinine] (PIN)
For 2λ6-2,2′,2′′-spiroter[[1,3,2]benzodioxathiole] (PIN)
read 2λ6,2′,2′′-spiroter[[1,3,2]benzodioxathiole] (PIN)
For 3H-2λ5-spiro[[1,3,2]benzoxazaphosphole-2,2′-[1,3,2,5]diazadiphosphinine] (PIN)
read 3H-2λ5,5′λ5-spiro[[1,3,2]benzoxazaphosphole-2,2′-[1,3,2,5]diazadiphosphinine] (PIN)
For 1′H,3′H -1λ4,1′′λ4-dispiro[thiane-1,2′-benzo[1,2-c:4,5-c′]dithiophene-6′,1′′-thiolane] (PIN)
read 1′H,3′H-1λ4,1′′λ4-dispiro[thiane-1,2′-benzo[1,2-c:4,5-c′]dithiophene-6′,1′′-thiolane] (PIN) [remove space from name]
For 1′′λ6-dispiro[bis([1,3,2]-benzodioxathiole)-2,1′′:2′,1′′-thiopyran-4′′,1′′′-cyclopentane] (PIN)
read 1′′λ6-dispiro[bis([1,3,2]benzodioxathiole)-2,1′′:2′,1′′-thiopyran-4′′,1′′′-cyclopentane] (PIN)
Add entries (11) and (12)
(11) anthracene (PIN)
(special numbering)(12) phenanthrene (PIN)
(special numbering)
Page 211, P-25.2.1, lines 7/8. [corrected 8.11.2023]
For Skeletal replacement (‘a’) nomenclature (see P-15.4), as described in Section P-25.5.4, is used...
read Skeletal replacement nomenclature, as described in Section P-15.5.2, is used...
For (1H-isomer shown)
read (1H-isomer shown; the PIN is 1H-perimidine)
For (4) acridine* (PIN)
read (4) acridine* (PIN)
(special numbering)
For *
read *†
For tellurochromene (2H-isomer shown)
read tellurochromene (Te instead of O) (2H-isomer shown)
For * In CAS index nomenclature, quinolizine precedes quinoline in seniority.
read † In CAS index nomenclature, quinolizine precedes quinoline and isoquinoline in seniority.
For isoquinoline (PIN), isoarsinoline (PIN), and isophosphinoline (PIN)
read isoquinoline† (PIN), isoarsinoline (PIN), and isophosphinoline (PIN)
read quinoline† (PIN), arsinoline (PIN), and phosphinoline (PIN)
read quinolizine† (PIN), arsinolizine (PIN), and phosphinolizine (PIN)
Delete dibenzo[1,4]dioxine)
For phenoazaphosphinine
read phenazaphosphinine
For Unless listed as a retained name in Table 2.8, for example quinoline and cinnoline, a benzene ring fused to a heteromonocycle of five or more members (a benzoheterocycle) is named...
read If the initial identified preferred components for naming a fused ring system include an isolated benzo component (i.e. not forming part of a component with a retained name such as quinoline or anthracene) ortho-fused to a heteromonocyclic component of five or more members, these two components are treated together as a one-component unit (a benzoheterocycle). See P-25.3.5 for the use of benzoheterocycles in fusion nomenclature and limitations on its use.. It is named....
Delete 3-benzooxepine
Delete 4H-3,1-benzooxazine
For benzo[j][1,5,8]cyclooxathiaazadodecine
read benzo[j][1,8,5]oxathiaazacyclododecine
Replace structure for azulene with:
Replace structure for azulene with:

Replace the structure with:
For ...horizontal rows. Such rows...
read ...horizontal rows. If the compound requires distorted rings not shown below see P-25.3.2.3.2. Such rows...
Add 
For P-25.3.2.3.2
read P-25.3.2.3.3
If a compound cannot be drawn only using the shapes shown in P-25.3.2.3.1 a distorted ring shape will be required. The distorted ring should be as small as possible.
not 
only preferred shapes used distorted five-membered ring 
not 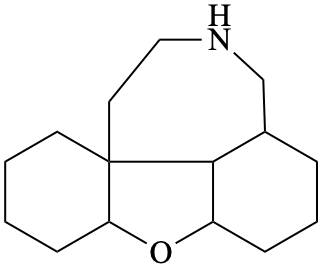
only preferred shapes used
(the separation to show the
seven-membered ring is not
fused to the left hand ring
does not count as a distortion) distorted seven-membered ring 
not 
distorted four-membered ring distorted six-membered ring
For ¾ ring in lower left
read ¾ ring in lower left quadrant
Replace the structure with:
Replace the structure with:
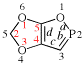
Replace the structure with:
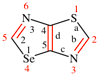
Replace the diagram with:
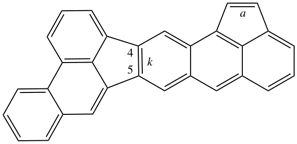
read acephenanthryleno[5,4-k]aceanthrylene
For ...convention (see P-14.1) The...
read ...convention (see P-14.1.3). The...
Replace the structure with:
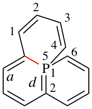
Replace the structure with:
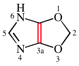
Replace the structures with:
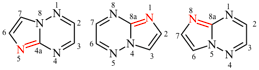
For [1,3]diazeto[1,2-a:3,4-a′]dibenzimidazole (PIN)
read [1,3]diazeto[1,2-a:3,4-a′]dibenzimidazole
Replace the structure with:
For pyrazino[2,1,6-cd:3,4,5-c′,d′]dipyrrolizine
read pyrazino[2,1,6-cd:3,4,5-c′d′]dipyrrolizine
Replace the structure with:
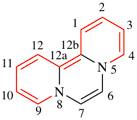
For 2H,6H-quinolizino[3,4,5,6,7-defg]acridine (PIN)
read 2H,6H-quinolizino[3,4,5,6,7-defg]acridine (PIN)
(recommended numbering)
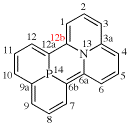
Replace the structure with:
For 8H-cyclopenta[3,4]napththo[1,2-d][1,3]oxazole (PIN)
read 8H-cyclopenta[3,4]naphtho[1,2-d][1,3]oxazole (PIN)
Delete Example:
Replace structure with:
For (not [1]benzofuro[5′,4′:3,4]cyclopenta[1,2-b]pyridine; pridine is senior to furan; indene...
read (not [1]benzofuro[5′,4′:3,4]cyclopenta[1,2-b]pyridine; pyridine is senior to furan; indene...
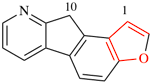
For 6H-benzo[b][2]benzopyran
read 6H-benzo[c][2]benzopyran
For dibenzo[b,d]pyran (PIN)
read 6H-dibenzo[b,d]pyran (PIN)
For dibenzo[g,p][1,3,6,9,12,15,18]heptaoxacycloicosine (PIN)
read 9H-dibenzo[g,p][1,3,6,9,12,15,18]heptaoxacycloicosine (PIN)
Replace the structure with:
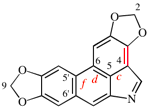
For cyclopenta[1,2-b:5,1-b′]bis([1,4]oxathiazine)
read cyclopenta[1,2-b:5,1-b′]bis([1,4]oxathiine)
For furo[3′,4′;5,6]pyrazino[2,3-c]pyridazine (PIN)
read furo[3′,4′:5,6]pyrazino[2,3-c]pyridazine (PIN)
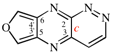
For naphtho[1,8a]azirine (PIN)
read naphtho[1,8a-b]azirine (PIN)
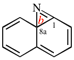
Replace the structure with:
For ...‘azano’, –NH–; –N=N–; epitriazano, –NH-NH-NH–; and –NH-N=N–, epitriaz[1]eno. ...
read ...‘azano’, –NH–; ‘diazeno’, –N=N–; ‘epitriazano’, –NH-NH-NH–; and –NH-N=N–, ‘epitriaz[1]eno’. ...
For epoxyethane[1,1,2]triyl (preferred prefix)
read (epoxyethane[1,1,2]triyl) (preferred prefix)
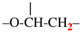
For epoxyethane[1,2,2]triyl (preferred prefix)
read (epoxyethane[1,2,2]triyl) (preferred prefix)
–O-CH2-CH<
For 1,4:6,9-dimethanodibenzo[b,e][1,4]dioxin
read 1,4:6,9-dimethanodibenzo[b,e][1,4]dioxine
For 6,9-epoxy-1,4-methanobenzocyclooctene (PIN)
read 6,9-epoxy-1,4-methanobenzo[8]annulene (PIN)
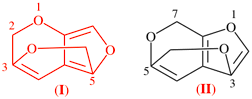
For 7H-3,5-(epoxymethano)furo[2,3-c]pyran (PIN)
read 2H-3,5-(epoxymethano)furo[3,4-b]pyran (PIN) (I)
replace the structure with:
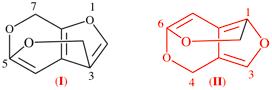
For 7H-5,3-(epoxymethano)furo[2,3-c]pyran (PIN)
read 7H-5,3-(epoxymethano)furo[2,3-c]pyran (PIN) (I)
[preferred ring system, see P-25.4.3.4.2 (d)]
replace the structure with:
Replace the example with:
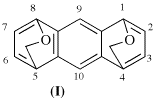
not 
1,4:8,5-bis(epoxymethano)anthracene (I) (PIN) [not 1H,3H-1,4:6,9-bisethenobenzo[1,2-c:5,4-c′]dipyran (II)]
Page 278, P-25.4.3.4.2 (e), example 2.. [corrected 12 May 2021]
For [not 7,5,13-(epoxyepimethanetriyl)benzo[4,5]cyclohepta[1,2-f]isochromene (II)
read [not 7,5,13-(epoxymethanetriyl)benzo[4,5]cyclohepta[1,2-f]isochromene (II)
For 8,7-(azenoetheno)cyclohepta[4.5]cycloocta[1,2-b]pyridine (I) (PIN)
read 8,7-(azenoepietheno)cyclohepta[4.5]cycloocta[1,2-b]pyridine (I) (PIN)
read [not 8,7-(azenoepiethanediylidene)cyclohepta[4,5]cycloocta[1,2-b]pyridine (II);
Replace the diagram with:
read 14,5-(metheno)-3,2,4-(epiprop[2]en[1,3]diyl[1]ylidene)dicyclopenta[f,f′]pentaleno[1,2-a:6,5-a′]dipentalene (PIN)
read the locants set for the independent bridge ‘3,2,4’ is the lowest; the locant for single bond attachment of metheno bridge is cited first.
read [not 5,14-(metheno)-3,4,2-(epipropan[1]yl[1,3]diylidene)dicyclopenta[f,f′]]pentaleno[1,2-a:6,5-a′]dipentalene;
read not 14,5-(metheno)-4,2,3-(epiprop[2]en[1,3]diyl[1]ylidene)dicyclopenta[f,f′]]pentaleno[1,2-a:6,5-a′]dipentalene;
read not 5,14-(metheno)-4,3,2-(epipropan[1]yl[1,3]diylidene)dicyclopenta[f,f′]]pentaleno[1,2-a:6,5-a′]dipentalene;
read (the locants ‘3,2,4’ for independent bridge is lower than ‘3,4,2’, ‘4,2,3’ and ‘4,3,2’]
Replace the structure with:
Replace the structure with:
Replace the structures with:
For 1,2,3,4,5,6-hexaazacyclopenta[c,d]pentalene (PIN)
read 1,2,3,4,5,6-hexaazacyclopenta[cd]pentalene (PIN)
Replace the left structure with:
For 7λ4-[1,2]dithiolo[1,5-b][1,2]dithiole (PIN)
read 7λ4-[1,2]dithiolo[5,1-e][1,2]dithiole (PIN)
For 2H-2λ5-2,6-(ethanylylidene)isophosphinoline (PIN)
read 2H-2λ5-2,6-(epiethanylylidene)isophosphinoline (PIN)
Add 10H-phenoxazine
For In the order of seniority used by CAS quinolizine precedes quinoline and the indacenes precede biphenylene.
read In the order of seniority used by CAS quinolizine precedes quinoline and isoquinoline; and indolizine preceeds indole and isoindole.
For selanthrene
read selenanthrene
Add (5) In the order of seniority used by CAS the indacenes precede biphenylene.
For fluoranthrene C6C6C5C5
read fluoranthrene C6C6C6C5
For ...recommendations contained therein.
read ...recommendations contained therein except a change in the usage of brackets for heterocyclic amplificants which require locants. When a phane system contains a heterocyclic amplificant with locants, they are enclosed in brackets (see P-26.4.2.2, example 1 and P-26.5.1, example 2).
For ...illustrated in Fig. 1. The...
read ...illustrated in Fig. 2.1. The...
For ...in Fig. 1 do not...
read ...in Fig. 2.1 do not...
For pyran (PIN) furana (preferred prefix)
read pyran (PIN) pyrana (preferred prefix)
For spiro[1,3-dioxolane-2,1′-indene]
read spiro[[1,3]dioxolane-2,1′-indene]
For P-26.2.4.2
read P-26.2.3.2
For 1,4(1,4)-dibenzenacycloheptaphane (PIN)
read 1,4(1,4)-dibenzenacyclohexaphane (PIN)
read [not 1(1,4),4(1,4)-dibenzenacyclohexaphane [change to justify the cross-reference]
For [not 1(1,3)-benzena-4(1,4)-benzenacycloheptaphane; see second example of P-26.4.2.2]
read [not 1(1,4),4(1,3)-dibenzenacycloheptaphane; see second example of P-26.4.2.2]
Replace simplified skeleton structure with:
read ...locant set ‘1,3,7’ or ‘1,5,7’ in either of the other names, see P-26.4.1.1]
Replace the structure with:
For 3(2,5)-furana-1,7(1)5(1,4)-tribenzenaheptaphane (PIN)
read 3(2,5)-furana-1,7(1),5(1,4)-tribenzenaheptaphane (PIN)
read [not 3(5,2)-furana-1,7(1),5(1,4)-tribenzenaheptaphane;
read for superatom 3 the lower attachment locant is not adjacent to the lower-numbered atom 2 of the simplified skeleton]
For {not 1,9(2,4)-diquinolina-3(5,3),7(2,4)-dipyridina-5(3,5)- [1,2]oxazolacyclotetradecaphane;
read {not 1(2,4),9(4,2)-diquinolina-3(5,3),7(2,4)-dipyridina-5(3,5)- [1,2]oxazolacyclotetradecaphane;
Replace the structure with:
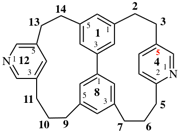
Replace the diagram with:
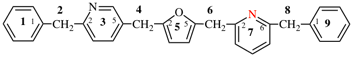
For [not 3(10,3)-phenanthrena-6(5,8,3,1)-naphthalena-8(1,3)-benzenaspiro[5.7]tridecane
read [not 3(10,3)-phenanthrena-6(5,8,3,1)-naphthalena-8(1,3)-benzenaspiro[5.7]tridecaphane
For [not 14,16,45,46-tetrachloro-1,4(1,3)-dibenzenacyclohexaphane; the...
read [not 14,16,44,45-tetrachloro-1,4(1,3)-dibenzenacyclohexaphane; the...
read ...‘14,15,44,46 ’, is lower than ‘14,16,44,45 ’]
For 14H-1(3,5)pyrana-4(1,3)-benzenacyclohexaphane (PIN)
read 14H-1(3,5)-pyrana-4(1,3)-benzenacyclohexaphane (PIN)
For 1(4)-pyridina-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
read
6-oxa-2-thia-4-selena-1(4)-pyridina-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
read [not 2-oxa-6-thia-4-selena-7(4)-pyridina-1(1),3,5(1,4)-tribenzenaheptaphane;
For Step 2: 14,2,4,514,6,12-hexaoxa-114,54-dithia-3(2,5)-furana-1,5(1,5)dicyclotetradecanacyclododecaphane (I) (PIN)
read Step 2: 14,2,4,514,6,12-hexaoxa-114,54-dithia-3(2,5)-furana-1,5(1,5)-dicyclotetradecanacyclododecaphane (I) (PIN)
For [not 114,2,4,54,6,12-hexaaoxa-14,514-dithia-3(2,5)-furana-1,5(1,5)-dicyclotetradecanacyclododecaphane (II);...
read [not 114,2,4,54,6,12-hexaoxa-14,514-dithia-3(2,5)-furana-1,5(1,5)-dicyclotetradecanacyclododecaphane (II);...
For This section is based on the publication ‘Phane nomencature, Part 1 Phane parent names’ (ref. 5) and the new edition 2012 contains...
read This section is based on the publication ‘Nomenclature for the C60-Ih and C70-D5h(6) Fullerenes’ (ref. 10) and the new edition 2013 contains...
For ...name (C70-I5h(5))[5,6]fullerene (PIN).
read ...name (C70-I5h(5))[5,6]fullerene (PIN).
For ...and [70-D5h(6)]fullerene in usage)...
read ...and [70]fullerene in usage)...
For ...(PIN) and (C70-D5h(6))-[5,6]fullerene (PIN)...
read ...(PIN) and (C70-D5h(6))[5,6]fullerene (PIN)...
For [60]fullerene (trivial numbering)
read [
Page 326, P-27.3 example 2 on this page. [corrected 3 October 2018]
For [C70-D5h]fullerene (trivial numbering)
read [C70-D5h]fullerene (trivial numbering)]
Page 335, P-27.6.1, example 1 on this page. [modified 8 May 2019]
For 3′H,3′′H,3′′′H-tricyclopropa[7,22:33,34:46,47](C70-Ih)[5,6]fullerene (PIN)
read 3′H,3′′H,3′′′H-tricyclopropa[7,22:33,34:46,47](C70-D5h(6))[5,6]fullerene (PIN)
for 7,22:33,34:46,47-tris(methylene)(C70-Ih)[5,6]fullerene (see P-27.6.2)
read 7,22:33,34:46,47-tris(methylene)(C70-D5h(6))[5,6]fullerene (see P-27.6.2)
Page 337, P-27.6.2 example 1 on this page. [corrected 9 January 2019]
For ...decanor(C60- Ih)[5,6]fullerene (PIN)
read ...decanor(C60-Ih)[5,6]fullerene (PIN) [delete space from name]
Page 337, P-27.6.2, example 2 on this page (example 4 of section). [modified 9 April 2025]
For 1,9:32,33-(ethane-1,2,2-triyloxymethylene-1,4-phenylenemethyleneoxyethane-1,2,2-triy)(C60-Ih)[5,6]fullerene
read 1,9:32,33-[ethane-1,1,2-triyloxymethylene(1,4-phenylene)methyleneoxyethane-1,2,2-triyl](C60-Ih)[5,6]fullerene
Page 338, P-27.6.3, example. [modified 24 October 2018]
For spiro[cyclohexane-1,1′a-[1(9a)H-1(9)a]-homo(C60-Ih)[5,6]fullerene] (PIN)
read spiro[cyclohexane-1,1′a-[1(9)a]homo(C60-Ih)[5,6]fullerene] (PIN)
replace the structure with:
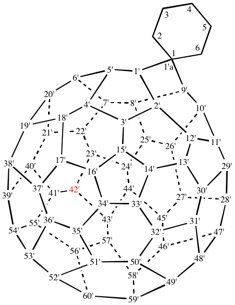
Page 340, P-28.2.1, example 4.
Replace the structure with:
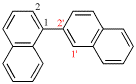
Page 341, P-28.2.2, example 2. [corrected 31 July 2019]
For 2,2′-bi(bicyclo[2.2.1]heptan-2-ylidene) (PIN)
read 2,2′-bi(bicyclo[2.2.1]heptanylidene) (PIN)
Page 341, P-28.2.3, example 1.
For 2H,2′H-6,6′-bipyran (PIN)
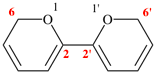
read 6H,6′H-2,2′-bipyran (PIN)
for
(not 6,6′-bi-2H-pyran)
read (not 2,2′-bi-6H-pyran)
replace the structure with:
Page 341, P-28.2.3, paragraph 3, lines 6-7. (corrected 1 December 2021)
For ...requiring a bivalent substituent group...
read ...requiring a divalent substituent group...
Page 342, P-28.2.3, example 4 from top.
For 1,2′-biindole
read 2′H-1,2′-biindole
delete the phrase (no indicated hydrogen needed)
Page 342, P-28.2.3, example 5 on this page.
Delete (not 4,4′-bi-1H-azepine)
Page 343, P-28.3.1, line 8. [Corrected 8 October 2025]
For ...usual way. Composite...
read ...usual way provided the lowest locant set is selected and, if a choice remains, in the order of citation in the name. Composite...
Page 343, P-28.3.1, Note, lines 5/6.
For ... two alternative names are 1,1′,2′,1′′-tercyclopropane and 11,21,22,31-tercyclopropane, where the ...
read ... two alternative names are 1,1′:2′,1′′-tercyclopropane and 11,21:22,31-tercyclopropane, where the ...
Page 344, P-28.3.1, example 1 on this page. [corrected 23 January 2019]
For (not 1,1′:3′,1′′:2′,1′′′-quatercyclobutane; ...
read (not 1,1′:3′,1′′:2′′,1′′′-quatercyclobutane; ...
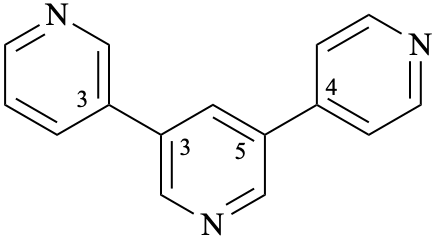
Page 344, P-28.3.1, example 3 on this page.
For 11H-13,23:23,33-terindole
read 11H,33H-13,23:23,33-terindole
for 1H-3,3′:3′,3′′-terindole
read 1H,3′′H-3,3′:3′,3′′-terindole
Page 344, P-28.3.1, last example.
For 2H,2′H,4′H,2′′H-1,1′3′,1′′-terpyrimidine
read 2H,2′H,2′′H,4′H-1,1′:3′,1′′-terpyrimidine
Page 344, P-28.3.1, add two new examples after example 4 on this page (example 5 of this section). [Corrected 8 October 2025]
Add
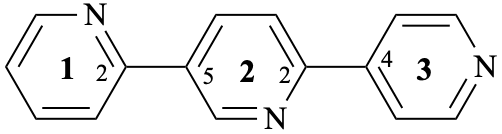 | not | 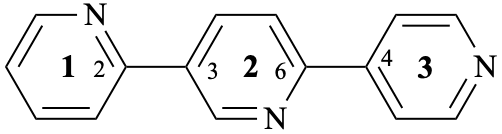 |
| 12,25:22,34-terpyridine (PIN)
(not 12,23:26,34-terpyridine); the locant set 12,22,25,34 is lower than the locant set 12,23,26,34) 2,5′:2′,4′′-terpyridine (not 2,3′:6′,4′′-terpyridine the locant set 2,2′,5′,4′′ is lower than the locant set 2,6′,3′,4′′) | ||
 | not | 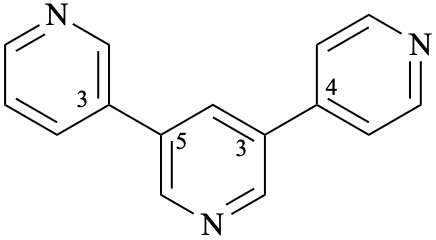 |
| 13,23:25,34-terpyridine (PIN)
(not 13,25:23,34-terpyridine); both locant sets are the same but 13,23:25,34 is lower than 13,25:23,34 in the order of citation. 3,3′:5′,4′′-terpyridine (not 3,5′:3′,4′′-terpyridine both locant sets are the same but 3,3′:5′,4′′ is lower than 3,5′:3′,4′′ in the order of citation. | ||
| Replace structure by |  |
Page 346, P-28.4.1, example 2 on this page. [corrected 19 September 2018]
For 2,2′-dithia-1,1′-bi(cyclododecan-2-ylidene) (PIN)
read 2,2′-dithia-1,1′-bi(cyclododecylidene) (PIN)
Page 347, P-28.5, example 2. [modified 30 January 2019]
For 1,8(2),2,3,4,5,6,7(2,5)-heptathiophenaoctaphane (PIN)
read 1,8(2),2,3,4,5,6,7(2,5)-octathiophenaoctaphane (PIN)
for 12,22:25,32:35,42:45,52:55,62:65,72,75,82-octithiophene
read 12,22:25,32:35,42:45,52:55,62:65,72:75,82-octithiophene
Page 347, P-28.5, example 3. [modified 30 March 2022]
For 11H,21H,31H,41H,51H,61H,71H-1,7(2),2,3,4,5,6(2,5)heptapyrrolaheptaphane (PIN)
read 11H,21H,31H,41H,51H,61H,71H-1,7(2),2,3,4,5,6(2,5)-heptapyrrolaheptaphane (PIN)
for 1H,1′H,1′′H,1′′′H,1′′′ ′H,1′′′ ′′,1′′′ ′′′H-2,2′:5′,2′′:5′′,2′′′:5′′′,2′′′ ′:5′′′ ′,2′′′ ′′:5′′′ ′′,2′′′ ′′′-septipyrrole
read 1H,1′H,1′′H,1′′′H,1′′′ ′H,1′′′ ′′H,1′′′ ′′′H-2,2′:5′,2′′:5′′,2′′′:5′′′,2′′′ ′:5′′′ ′,2′′′ ′′:5′′′ ′′,2′′′ ′′′-septipyrrole
Page 348, P-28.6, example 2.
For 3′′-([1,1′-biphenyl]-3-yl)-1,1′:3′1′′:3′′-1′′′:3′′′,1′′′′-quinquephenyl
read 5′′-([1,1′-biphenyl]-3-yl)-1,1′:3′,1′′:3′′,1′′′:3′′′,1′′′ ′-quinquephenyl
Page 351, P-29.1.2, example 2. [corrected 11 November 2020]
For chlorocarbonyl (preferred prefix)
read carbonochloridoyl (preferred prefix)
Page 356, P-29.3.2.2, example 7 on this page.
Replace the diagram with:
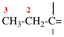
Page 356, P-29.3.2.2, example 10 on this page.
For AsH3-AsH2–
read AsH2-AsH–
Page 357, P-29.3.2.2, example 1 on this page.
For disilylamino (preferred prefix)
read disilylamino (preselected prefix)
Page 358, P-29.3.4.1, line 4.
For ‘added inducated hydrogen’
read ‘added indicated hydrogen’
Page 361, P-29.3.5, example 3 on the page.
For 1,1′:3′,1′′]-terphenyl]-4,4′-diyl
read [1,1′:3′,1′′-terphenyl]-4,4′-diyl
Page 362, P-29.3.6, example (I)
Replace the structure with:
Page 363, P-29.4.1, example 4 on this page.
Replace the structure with:
Page 364, P-29.4.2, example 1.
For silanediyldiethane-2,1-diyl (preferred prefix)
read silanediyldi(ethane-2,1-diyl) (preferred prefix)
Page 364, P-29.4.2, example 5. [corrected 23 January 2019]
For silanediylbis(1-fluoroethylene)
read [not silanediylbis(1-fluoroethylene)]
Page 366, P-29.6.1, example 3. [modified 18 September 2019]
For tert-butyl(dimethyl)phosphane (PIN)
read tert-butyldi(methyl)phosphane (PIN)
for dimethyl(1-methylethyl)phosphane
read (1,1-dimethylethyl)di(methyl)phosphane
Page 366, P-29.6.1, example 3. [modified 20 February 2019]
for dimethyl(1-methylethyl)phosphane
read (1,1-dimethylethyl)dimethylphosphane
Page 366, P-29.6.1, example 4.
For (2-chloro-2,2-dimethylethyl)silane
read (2-chloro-1,1-dimethylethyl)silane
Page 367, P-29.6.2.1, example 4.
For 3,4,5-trimethylbenzylidyne (preferred prefix)
read 3,4,5-trimethylbenzylidyne
for (3,4,5-trimethylphenyl)methylidyne
read (3,4,5-trimethylphenyl)methylidyne (preferred prefix)
Page 368, P-29.6.2.2, example 1.
Replace the structure with:
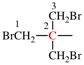
Page 368, P-29.6.2.2, example 4.
For trityl (no substitution allowed) (preferred prefix)
read trityl (no substitution allowed)
for triphenylmethyl
read triphenylmethyl (preferred prefix)
Page 368, P-29.6.2.2, example 5. [corrected 11 November 2020]
For (4-methylphenyl)diphenylmethyl (preferred prefix)
read (4-methylphenyl)di(phenyl)methyl (preferred prefix)
Page 369, P-29.6.2.3, example 2 on this page.
For anthacen-2-yl (preferred prefix)
read anthracen-2-yl (preferred prefix)
Page 369, P-29.6.2.3, example 4 on this page.
For 7-isoquinolyl (also 1-, 3-, 5-, 6-, 7-, and 8-isomers)
read 7-isoquinolyl (also 1-, 3-, 4-, 5-, 6- and 8-isomers)
for isoquinolin-7-yl (also 1-, 3-, 5-, 6-, 7-, and 8-isomers; preferred prefixes)
read isoquinolin-7-yl (also 1-, 3-, 4-, 5-, 6- and 8-isomers; preferred prefixes)
Page 369, P-29.6.2.3, example 5 on this page.
For 2-naphthyl (also 1- isomer
read 2-naphthyl (also 1-isomer)
Page 369, P-29.6.2.3, example 8 on this page.
For pyridin-2-yl (also 3- and 4-, preferred prefixes)
read pyridin-2-yl (also 3- and 4-isomers; preferred prefixes)
Page 370, P-29.6.2.3, example 2 on this page.
For 2-thienyl (also 3-isomer) (also 3-isomer; preferred prefixes)
read 2-thienyl (also 3-isomer)
for thiophen-2-yl (also 3-isomer)
read thiophen-2-yl (also 3-isomer; preferred prefixes)
Page 371, P-29-6.3, example 3 on this page.
For furan-2-ylmethyl (preferred prefix)
read (furan-2-yl)methyl (preferred prefix)
Page 371, P-29-6.3, example 4 on this page.
For thiophen-2-ylmethyl (preferred prefix)
read (thiophen-2-yl)methyl (preferred prefix)
Page 373, P-31, line 3. [corrected 31 December 2025]
For P-31.2 The prefixes ‘hydro’ and ‘dehydro’
read P-31.2 The parent hydrides modified by the prefixes ‘hydro’ and ‘dehydro’
Page 374, P-31.1.1.1, after text. [corrected 4 September 2024]
Add If the stereochemistry of a double bond is indicated in a structure it should be specified by E or Z with the appropriate locant. This includes double bonds in rings of eight or more members (see P-91.2.2).
Page 374, P-31.1.1.1, example 2. [corrected 19 September 2018]
For prop-1-yne (PIN)
read propyne (PIN)
Page 374, P-31.1.1.1, last example.
Replace the diagram with:
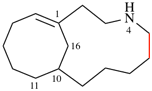
Page 375, P-31.1.2.2.1, structure 1.
Replace structure with:
Page 375, P-31.1.2.2.1, structure 3.
Replace structure with:
Page 377, P-31.1.3.1, example 2 on this page. [corrected 4 September 2024]
For cyclododeca-1,5-diene (PIN)
read (1Z,5E)-cyclododeca-1,5-diene (PIN)
Page 377, P-31.1.3.1, example 3 on this page. [corrected 4 September 2024]
Replace the structure with:
for cyclopentadec-1-en-4-yne (PIN)
read (1Z)-cyclopentadec-1-en-4-yne (PIN)
Page 377, P-31.1.3.2, example 1. [corrected 4 September 2024]
For1,4,7,10-tetraoxacyclododec-2-ene (PIN)
read (2Z)-1,4,7,10-tetraoxacyclododec-2-ene (PIN)
Page 377, P-31.1.3.2, example 2. [corrected 4 September 2024]
For 1-oxa-4-azacyclododec-3-ene (PIN)
read (3E)-1-oxa-4-azacyclododec-3-ene (PIN)
Page 377, P-31.1.3.2, example 3. [corrected 4 September 2024]
For 1,11-disilacycloicosa-5,7-dien-3-yne (PIN)
read (5Z,7E)-1,11-disilacycloicosa-5,7-dien-3-yne (PIN)
Page 378, P-31.1.3.2, example 1 on this page. [corrected 4 September 2024]
For 1,10-disilacycloicosa-12,14,16-trien-18-yne (PIN)
read (12Z,14E,16Z)-1,10-disilacycloicosa-12,14,16-trien-18-yne (PIN)
Page 378, P-31.1.3.3, examples.
Delete n = 11:
Replace the structures with:
Page 379, P-31.1.3.4, example 2.
For 1,1′-ethene-1,2-diyldibenzene (PIN)
read 1,1′-(ethene-1,2-diyl)dibenzene (PIN)
Page 380, P-31.1.4.2, example 2.
For [not bicyclo[6.5.1]tetradec-1(13)-decene]
read [not bicyclo[6.5.1]tetradec-1(13)-ene]
Page 381, P-31.1.4.2 (2), example 2. [modified 30 October 2019]
For tricyclo[9.3.1.14,8]hexadeca-1(15),4(16),5,7,11,13-hexaene (I) (PIN)
read tricyclo[9.3.1.14,8]hexadeca-1(15),4(16),5,7,11,13-hexaene (I)
for tricyclo[9.3.1.14,8]hexadeca-1(14),4(16)5,7,11(15),12-hexaene (IV
read tricyclo[9.3.1.14,8]hexadeca-1(14),4(16),5,7,11(15),12-hexaene (IV);
Page 382/383, P-31.1.4.3. [corrected 4 September 2024]
The order of the three criteria changed to make minimum number of compound locants first.
Page 382, P-31.1.4.3 (2) formerly (1), example. [corrected 4 September 2024]
For bicyclo[14.3.1]icosa-11,13,18-trien-2-yne (PIN)
read (11E,13E)-bicyclo[14.3.1]icosa-11,13,18-trien-2-yne (PIN)
Page 382, P-31.1.4.3 (3) formerly (2), example. [corrected 4 September 2024]
For bicyclo[11.3.1]heptadeca-2-en-11-yne (PIN)
read (2Z)-bicyclo[11.3.1]heptadeca-2-en-11-yne (PIN)
Page 383, P-31.1.4.3 (1) formerly (3), example. [corrected 4 September 2024]
For bicyclo[8.3.1]tetradeca-4,6,10-trien-2-yne (PIN)
read (4Z,6E)-bicyclo[8.3.1]tetradeca-4,6,10-trien-2-yne (PIN)
Page 384, P-31.1.5.1.2 (1), example. [corrected 4 September 2024]
For spiro[4.10]pentadec-10-en-8-yne (PIN)
read (10Z)-spiro[4.10]pentadec-10-en-8-yne (PIN)
Page 384, P-31.1.5.1.2 (2), example. [corrected 4 September 2024]
For spiro[4.10]pentadec-6-en-14-yne (PIN)
read (6E)-spiro[4.10]pentadec-6-en-14-yne (PIN)
Page 386, P-31.1.6.1, (1), (b) and (2). [changed 20 November 2024]
For (b) composite locants, neither of which is adjacent to a primary locant;
read (b) composite locants;
for (2) by a compound locant, when one locant is a composite locant adjacent to a primary locant.
read (2) by a compound locant, when two locants are not consecutive, or when one is a primary locant and the other is a composite locant.
Page 386, P-31.1.6.1 before examples. [added 20 November 2024]
Add in a box This is a change from PhII-5.3.2.
Page 386, P-31.1.5.2.1, example on this page. [corrected 27 May 2020]
For 2′′,7-dioxa-2,3′:7′,7′′-dispiroter[bicyclo[4.1.0]hexan]-4′′-ene (PIN)
read 2′′,7-dioxa-2,3′:7′,7′′-dispiroter[bicyclo[4.1.0]heptan]-4′′-ene (PIN)
for [not 5′′,7-dioxa-2,3′:7′,7′′-dispiroter[bicyclo[4.1.0]hexan]-2′′-ene;
read [not 5′′,7-dioxa-2,3′:7′,7′′-dispiroter[bicyclo[4.1.0]heptan]-2′′-ene;
Page 386, P-31.1.6.1, before examples on next page. [corrected 4 September 2024]
Add If there is a choice the criteria of P-31.1.4.3 are applied.
Page 387, P-31.1.6.1, example 2. [corrected 27 May 2020]
For 1(1,3)-benzena-9(1,3)-cyclohexanacyclohexadecaphane-91(96),93(94)-diene (PIN)
read 1(1,3)-benzena-9(1,3)-cyclohexanacyclohexadecaphane-91(96),93-diene (PIN)
Page 387, P-31.1.6.1, new example after example 3. [added 20 November 2024]
Add the structure:
(2E,8(91)Z)-1(1,3)-benzena-9(1,3)-cyclohexanacyclohexadecaphan-2,8(91),92-triene (PIN)
Page 388 , P-31.1.6.2, example 1 on this page. [corrected 4 September 2024]
For 1,7(1,3)dibenzenacyclotridecaphan-2-en-5-yne (PIN)
read (2E)-1,7(1,3)dibenzenacyclotridecaphan-2-en-5-yne (PIN)
Page 388, P-31.1.7.1, example 1.
For [1,1′-bicyclohexane]-1,2′-diene (PIN)
read [1,1′-bi(cyclohexane)]-1,2′-diene (PIN)
Page 388, P-31.1.7.1, example 2.
For [2,2′-bibicyclo[2.2.2]octane]-5,5′-diene (PIN)
read [2,2′-bi(bicyclo[2.2.2]octane)]-5,5′-diene (PIN)
Page 390, P-31.1.7.3, example.
For 12,23,33-trithia[11,21:24,31-terbicyclo-[2.2.2]octane]-15,25,35-triene (PIN)
read 12,23,33-trithia[11,21:24,31-terbicyclo[2.2.2]octane]-15,25,35-triene (PIN)
for 2,3′,3′′-trithia-[1,1′:4′,1′′-terbicyclo-2.2.2]octane]-5,5′,5′′-triene
read 2,3′,3′′-trithia[1,1′:4′,1′′-terbicyclo[2.2.2]octane]-5,5′,5′′-triene
Page 390, P-31.2, heading. [corrected 31 December 2025]
For P-31.2 THE PREFIXES ‘HYDRO’ AND ‘DEHYDRO’
read P-31.2
Page 390, P-31.2.1, after the first paragraph and before box. [corrected 31 December 2025]
Add If both hydro and dehydro prefixes are used in name, dehydro prefixes are cited before hydro prefixes with the order not affected by multipliers.
Page 390, P-31.2.1, after text. [corrected 31 December 2025, modified 22.1.2026]
Add Example.
1,2,7,8-tetradehydro-3,4-dihydro[12]annulene
(1Z,3Z,7Z)_cyclododeca-1,3,7-triene-5,11-diyne (PIN)
Page 392. P-31.2.3.2, after last example on this page.
Add:
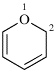 |  |
| 2H-pyran (PIN) | oxane (PIN) |
| tetrahydropyran |
Page 393, P-31.2.3.2, example 2 at top of page.
For 1,4-thiazepinane (PIN)
read 1,4-thiazepane (PIN)
Page 393, P-31.2.3.2, example 3 on this page. [modified 27 May 2020]
For pentazolidine
read pentazolidine (preselected name; see P-12.1; P-22.2.2.1.5.2, P-22.2.5)
delete pentazolane
Page 395, P-31.2.3.3.3, example 1.
Replace structure with:
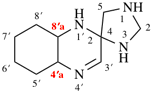
Page 395, P-31.2.3.3.3, example 2. [corrected 12 May 2021]
For 4,5-dihydro-3H-spiro[1-benzofuran-2,1′-cyclohexan]-2′-ene (PIN)
read 4,5-dihydro-3H-spiro[[1]benzofuran-2,1′-cyclohexan]-2′-ene (PIN)
Page 397, P-31.2.3.3.5.1 (a), example 1.
For 1-(cyclohexa-1,3-dien-1-yl)benzene
read (cyclohexa-1,3-dien-1-yl)benzene
Page 398, P-31.2.3.3.5.1 (b), example 1. [Corrected 8 October 2025]
For 11,16,24,25,32,35-hexahydro-12,23:26,34-terpyridine (PIN)
read 11,16,23,24,32,35-hexahydro-12,25:22,34-terpyridine (PIN)
for 1,2′′,4′,5′,5′′,6-hexahydro-2,3′:6′,4′′-terpyridine
read 1,2′′,3′,4′,5′′,6-hexahydro-2,5′:2′,4′′-terpyridine
Page 397, P-31.2.3.3.5.1, example 3.
13,16,22,23-tetrahydro-11,21:24,31-terphenyl (PIN)
read 12,15,22,23-tetrahydro-11,21:24,31-terphenyl (PIN)
for 2′,3,3′,6-tetrahydro-1,1′:4′,1′′-terphenyl
read 2,2′,3′,5-tetrahydro-1,1′:4′,1′′-terphenyl
replace structure with:

Page 397, P-31.2.3.3.5.1, example 4. [modified 27 May 2020]
For 11,12,13,14,15,16,21,22,23, 24, 25,26,41,42,43,44,45,46,51,52,53,54,55,56-tetracosahydro-11,21,24, 31,34,41,44,51,54,61-sexiphenyl (PIN)
read 11,12,13,14,15,16,21,22,23,24,25,26,41,42,43,44,45,46,51,52,53,54,55,56-tetracosahydro-11,21:24,31:34,41:44,51:54,61-sexiphenyl (PIN) [remove spaces from name]
For {not 1-(11,21,31,41,51,61, 21,22,23, 24, 25,26-dodecahydro[11,12:42,13-terphenyl]-41-yl)-4-[1,1′-bi(cyclohexan]-4-yl)benzene (substitutive name)}
read {not 1-(21,22,23,24,25,26,31,32,33,34,35,36-dodecahydro[11,21:24,31-terphenyl]-14-yl)-4-([1,1′-bi(cyclohexan)]-4-yl)benzene (substitutive name)} [remove spaces from names]
Page 398, P-31.2.3.3.5.1, example 1 on this page.
For 4-[4-(4′-phenyl[1,1′-bi(cyclohexan)]-4-yl)phenyl)-11,21:24,31-tercyclohexane substitutive name)
read 14-[4-(4′-phenyl[1,1′-bi(cyclohexan)]-4-yl)phenyl]-11,21:24,31-tercyclohexane (substitutive name)
for 4-[4-(4′-phenyl[1,1-bi(cyclohexan)]-4-yl)pheny]-1,1′:4′,1′′-tercyclohexane substitutive name)
read 4-[4-(4′-phenyl[1,1′-bi(cyclohexan)]-4-yl)phenyl]-1,1′:4′,1′′-tercyclohexane (substitutive name)
Page 398, P-31.2.3.3.5.1, example 3 on this page.
For 14,15,24,25,34,35-hexahydro-11H,21H,31H-12,22 :25,33- terazepine (PIN)
read 14,15,24,25,34,35-hexahydro-11H,21H,31H-12,22:25,33-terazepine (PIN) [delete space from name]
for 4,4′,4′′,5,5′,5′′-hexahydro-1H,1′H,1′′H-2,2′:5′ :3′′- tetrazepine
read 4,4′,4′′,5,5′,5′′-hexahydro-1H,1′H,1′′H-2,2′:5′,3′′-tetrazepine [remove space from name]
Page 399, P-31.2.3.3.5.2, example 2 on this page.
For 3a,3′a,4,4′,5,5′,6,6′,7,7′ 7a,7′a-dodecahydro-1H,1′H-2,2′-biindole (PIN)
read 3a,3′a,4,4′,5,5′,6,6′,7,7′,7a,7′a-dodecahydro-1H,1′H-2,2'-biindole (PIN) [replace space by a comma]
Page 400, P-31.2.4.3, example left. [corrected 4 September 2024]
For 2,3,4,5-tetrahydroazocine (PIN)
read (6Z,8Z)-2,3,4,5-tetrahydroazocine (PIN)
Page 400, P-32, line 5. [corrected 31 December 2025]
For ...by the prefix hydro
read ...by the prefixes ‘hydro’ and ‘dehydro’
Page 401, P-32.1.1, line 1. [corrected 8.11.2023]
For ...formation of preferred IUPAC names, see P-57.1.4.1.
read ...formation of preferred IUPAC names, see P-57.1.1.1.
Page 401, P-32.1.1, example 5. [corrected 6 February 2019]
For (2) 1-methyleth-1-en-1-yl
read (2) 1-methylethen-1-yl
Page 401, P-32.1.1, example 8.
Replace structure to:
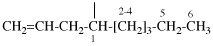
Page 402, P-32.1.1, example 1 on this page.
For (1) hept-1-en-6-yn- -yl (preferred prefix)
read (1) hept-1-en-6-yn-4-yl (preferred prefix)
Page 402, P-32.1.3, example 1. [corrected 11 November 2020]
Replace the structure with:
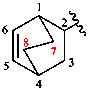
Page 403, P-32.2, heading, line1/2. [corrected 31 December 2025]
For ...BY THE PREFIX ‘HYDRO’
read ...BY THE PREFIXES ‘HYDRO’ AND ‘DEHYDRO’
Page 403, P-32.2.1, example 1. [corrected 19 September 2018]
For 1-azadodeca-1,5,7,9,11-pentaen-6-yl (preferred prefix)
read 1-azacyclododeca-1,5,7,9,11-pentaen-6-yl (preferred prefix)
Page 403, P-32.2.1, example 2. [corrected 19 September 2018]
For 12,13-dihydro-1-aza[13]annulen-4-yl
read 12,13-dihydro-1H-1-aza[13]annulen-4-yl
for 1-azatrideca-2,4,6,8,10-pentaen-4-yl (preferred prefix)
read 1-azacyclotrideca-2,4,6,8,10-pentaen-4-yl (preferred prefix)
Page 405, P-32.3, line 12.
For ...2-phenylethenyl’
read ...2-phenylethen-1-yl.
Page 406, P-32.4, Table 3.2, example 3. [corrected 9 April 2025]
For 2,3-dihydro-1H-isoindole-2-yl
read 1,3-dihydro-2H-isoindole-2-yl
Page 406, P-34.4, Table 3.2 structure 4 on this page.
Replace text with:
isochroman-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-isochromen-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-2-benzopyran-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers) (preferred prefixes)
isothiochroman-3-yl (S instead of O) (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-isothiochromen-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-2-benzothiopyran-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers; preferred prefixes)
isoselenochroman-3-yl (Se instead of O) (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-isoselenochromen-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-2-benzoisoselenopyran-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers; preferred prefixes)
isotellurochroman-3-yl (Te instead of O) (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-isotellurochromen-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers)
3,4-dihydro-2H-2-benzotelluropyran-3-yl (also 3-, 4-, 5-, 6-, 7- and 8-isomers; preferred prefixes) [remove spaces before isomer]
Page 408, P-33.1, example on this page, 2nd compound. (corrected 4 December 2019)
For ethyl (preferred prefix; the prefix ‘-yl describes the loss of one hydrogen atom)
read ethyl (PIN; the prefix ‘-yl’ describes the loss of one hydrogen atom)
Page 408, P-33.1, example on this page, 3rd compound. (corrected 4 December 2019)
For ethan-1-yl-2-ylidene (preferred prefix; the prefix ‘-ylidene’ describes loss the of two hydrogen atom)
read ethan-1-yl-2-ylidene (PIN; the prefix ‘-ylidene’ describes the loss of two hydrogen atoms)
Page 408, P-33.2.1, Table 3.3 (7). [corrected 26 September 2018]
For –CO-NNH2
read –CO-NHNH2
Page 408, P-33.2.1, Table 3.3 (8). [corrected 26 September 2018]
For –(C)O-NNH2
read –(C)O-NHNH2
Page 412, P-33.2.2 (7). [corrected 24 February 2021]
Replace by
Suffixes with –NH2 and =NH groups substituted by an –OH group are named in two ways:
(1) are named as N-hydroxy derivatives of amides or imidic acids;
(2) by modifying the ‘-oic acid’ or ‘-carboxylic acid’ suffix of a systematically named acid to ‘-hydroxamic acid’ or ‘-carbohydroxamic acid’ and ‘-hydroximic acid’ or ‘-carbohydroximic acid’, or the ‘-ic acid’ ending of the retained name of an acid to ‘-hydroxamic acid’ or ‘-hydroximic acid’. The letter ‘o’ is added for euphony between ‘h’ and a preceding consonant.
Method (1) is used for preferred IUPAC names.
Page 413, P-33.3, examples structure 6. [corrected 21 April 2021]
For methanamimiumyl (preferred prefix)
read methanaminiumyl (PIN)
replace structure with:

Page 413, P-33.3, example 8.
For naphthalene-3-yl-1(2H)-ylidene (PIN)
read naphthalen-3-yl-1(2H)-ylidene (PIN)
Page 415, P-34.1.1.2, example. [modified 11 March 2020]
Delete oxaldehyde (PIN, P-66.6.1.2)
for ethanedial
read ethanedial (PIN)
for glyoxal (see P-66.6.1.2; limited substitution allowed: see P-65.1.8.2)
read glyoxal
Page 416, P-34.1.1.5, example 2. [modified 6 February 2019]
For (hydrazinylidenemethyl)diazene
read (diazenylmethylidene)hydrazine
Page 416, P-34.1.1.5, example 3. [modified 24 October 2018]
For guanidine (special substitution; see P-66.4.1.2.1)
read guanidine (substitution allowed; see P-66.4.1.2.1)
replace the structure with:

Page 417, P-34.1.2, example 1 on this page, the name for the right version. [corrected 6 February 2019]
For (hydrazinylidenemethyl)diazene
read (diazenylmethylidene)hydrazine
Page 417, P-34.1.2, example 3 on this page.
For carbonimidoyl
read carbonimidic diamide
Page 417, P-34.1.2, example 5 on this page.
For glyoxal (see P-66.6.1.2; limited substitution allowed: see P-65.1.8.2)
read glyoxal (see P-66.6.1.2.1)
Page 417, P-34.1.2, name 5 on this page. [modified 7 August 2019]
Delete this entry
Page 417, P-34.1.2, example 9 on this page.
For benzenzenol
read benzenol
Page 418, P-34.2.1.1 example 2. [corrected 19 September 2018]
For phenylcarbonyl
read benzenecarbonyl
Page 418, P-34.2.1.1 example 3. [corrected 6 February 2019]
For aminocarbonimidoyl
read C-aminocarbonimidoyl
Page 419, P-34.2.1.3, examples 1 on this page. [corrected 26 September 2018]
Replace the structure with:
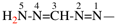
Page 419, P-34.2.1.3, example 2 on this page.
For fornazan-5-yl (preferred prefix) (full substitution; see P-8.3.1.3.5.2)
read formazan-5-yl (preferred prefix) (full substitution; see P-68.3.1.3.5.2)
Page 419, P-34.2.1.3, examples 5 on this page. [corrected 26 September 2018]
Replace the structure with:
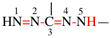
Page 419, P-34.2.1.3, examples 6 on this page. [corrected 10 October 2018]
Replace the structure with:
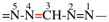
Page 420, P-34.2.2, example 2. [corrected 25 August 2016]
For anilino (no substitution allowed; see P-62.2.1.1.1)
read anilino (full substitution allowed; see P-62.2.1.1.1)
Page 420, P-34.2.2, example 4 right. [corrected 6 February 2019]
For aminocarbonimidoyl
read C-aminocarbonimidoyl
Page 420, P-34.2.2, example 6. [corrected 6 February 2019]
For carbonamidoyl
read aminocarbonyl
Page 420, P-34.2.2, example 15
For diazany(diazenylmethylidene)
read diazenyl(hydrazinylidene)methyl
Page 420, P-34.2.2, example 16
For diazenyl(hydrazinylidene)methyl
read (diazenylmethylidene)hydrazinyl
Page 421, P-35.0, line 7. [corrected 7 November 2018]
For ...example –S(O2)-OH, –Se(O2)-OH. These prefixes...
read ...example –S(O)2-OH, –Se(O)2-OH. These prefixes...
Page 424, P-35.2.2, example 12 on this page.
Delete this example (=NH). Incorrect and covered by P-35.2.1
Page 426, P-35.3.2, example 5. [corrected 31 October 2018]
For hydroxy(hydrazinylidene)methyl
read hydrazinylidene(hydroxy)methyl
Page 426, P-35.4.1, example 3.
For 4-(chlorophenyl)methoxy (preferred prefix)
read (4-chlorophenyl)methoxy (preferred prefix)
Page 431, P-41, example 2. [corrected 3 June 2020]
For (trimethylazaniumyl)acetate (PIN)
read (N,N-dimethylmethanaminiumyl)acetate (PIN)
Page 431, P-41, example 5. [corrected 3 June 2020]
For (trisulfanyl)silane (preselected name;
read trisulfanylsilane (preselected name;
Page 431, P-41, example 11.
For 1-(methanesulfonyl)-2-(methylsulfanyl)ethane
read 1-(methanesulfinyl)-2-(methylsulfanyl)ethane
for (C > sulfide and sulfone)
read C > sulfide and sulfoxide)
Page 432, P-42.1, example 1.
For –(C)(O)OH -oic acid
read –(C)O-OH -oic acid
Page 434, P-42.4, example 4. [corrected 7 November 2018]
For HO)2P(O)-O-P(O)(OH)-O-P(O)(OH)2
read (HO)2P(O)-O-P(O)(OH)-O-P(O)(OH)2
Page 434, P-42.4, example 17.
For orthosilicic acid
read silicic acid (formerly orthosilicic acid. See P-67.1.1.1, P-67.1.2.2 and Table IR-8.1, ref. 12.)
Page 434, P-42.4, line 3 from the bottom. [corrected 26 September 2018]
For (HO)SO2-O-[SO2(OH)-O-]nSO2(OH)
read (HO)SO2-O-[SO2-O-]nSO2(OH)
Page 434, P-42.4, line 2 from the bottom. [corrected 26 September 2018]
For (HO)SO-O-[SO(OH)-O-]nSO(OH)
read (HO)SO-O-[SO-O-]nSO(OH)
Page 435, P-42.4, line 1 on this page. [corrected 6 December 2023]
For disulfurous acid
read disulfurous acid (ambiguous see P-67.3.2) p>
Page 436, P-42.5, example 5 on this page.
For hypoiodous IOH
read hypoiodous acid IOH
Page 439, P-43.1, Table 4.3, line 10 on this page.
For sulfonimido(thioperoxoic) OO-acid
read sulfonimido(thioperoxoic) SO-acid
Page 439, P-43.1, Table 4.3, line 14 on this page. [corrected 19 September 2018]
For sulfoimidodithioic acid
read sulfonimidodithioic acid
Page 440, P-43.1, Table 4.4 (1), example 2.
For –(C)OOH peroxoic acid
read –(C)O-OOH peroxoic acid
Page 440, P-43.1, Table 4.4 (1), example 7.
For –CO-SeH selenoic Se-acid
read –(C)O-SeH selenoic Se-acid
Page 440, P-43.1, Table 4.4 (1), last example.
For –C(S)-SH
read –(C)S-SH
Page 441, P-43.1, Table 4.4, section 3, last two examples. [corrected 13 February 2019]
For –C(=NHNH2)-SH
read –C(=NNH2)-SH
for –(C)(=NHNH2)-SH
read –(C)(=NNH2)-SH
Page 442, P-43.1, Table 4.4 (7), line 2.
For Sulfonohydrazonoperoxoic
read Sulfonohydrazonoperoxoic acids
Page 442, P-43.1, Table 4.4 (9), line 4. [corrected 10 October 2018]
For –S(O)(S)-OOH
read –S(S)-OOH
Page 443, P-43.1, Table 4.4 (10), line 2.
For Sulfinoimidoperoxoic acids
read Sulfinimidoperoxoic acids
Page 443, P-43.1, Table 4.4 (10), line 3.
For Sulfinoimidoperoxoic acids modified by replacement with...
read Sulfinimidoperoxoic acids modified by replacement with...
Page 444, P-43.1, Table 4.4 (22), line 3. [corrected 10 October 2018]
For –S(S)(=NH)-NH2
read –S(S)(=NNH2)-NH2
Page 448, P-44.1.1, example 3.
For [not 7-chloro-3-(1-hydroxyethyl)heptan-1-ol; the locant set ‘1,4’ for the principal characteristic groups is lower than ‘2,5’]
read [not 7-chloro-3-(1-hydroxyethyl)heptan-1-ol; there are two of the principal characteristic groups in the parent of the PIN and only one in the other name]
add [not 3-(4-chlorobutyl)pentane-2,5-diol; the locant set ‘1,4’ for the principal characteristic groups is lower than ‘2,5’]
Page 448, P-44.1.1, example 7.
For 7,7-bis[(2-butoxyethoxy)methyl]-3,6,10,13-tetrathiapentadecanedioic acid (PIN)
read 7,7-bis[(2-butoxyethoxy)methyl]-3,6,10,13-tetrathiapentadecane-1,15-dioic acid (PIN)
for [not 9-[(2-butoxyethoxy)methyl]-9-({2-[(carboxymethyl)sulfanyl]ethyl}sulfanyl)-11,14-dioxa-3,6-dithiaoctadecanoic acid;
read
[not 9-[(2-butoxyethoxy)methyl]-9-({2-[(carboxymethyl)sulfanyl]ethyl}sulfanyl)-11,14-dioxa-3,6-dithiaoctadecan-1-oic acid;
for [nor 7-[(2-butoxyethoxy)methyl]-7-[2-({2-[(carboxymethyl)sulfanyl]ethyl}sulfanyl)ethyl]-9,12-dioxa-3,6-dithiahexadecanoic acid;
read
[nor 7-[(2-butoxyethoxy)methyl]-7-[2-({2-[(carboxymethyl)sulfanyl]ethyl}sulfanyl)ethyl]-9,12-dioxa-3,6-dithiahexadecan-1-oic acid;
for for ...two of the principal characteristic group in the PIN...
read ...two of the principal characteristic group in the parent of the PIN...
replace the structure with:
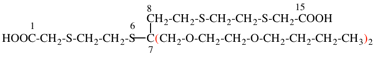
Page 448, P-44.1.1, example 8.
For (silanediyldiethane-2,1-diyl)bis(silane)
read [silanediyldi(ethane-2,1-diyl)]bis(silane)
Page 449, P-44.1.1, example 1 on this page. [modified 1 January 2020]
For 4-[4-(pyridin-4-ylmethyl)phenyl]-1,7(1),3(1,3),5(1,4)-tetrabenzenaheptaphane-14,74-dicarboxylic acid (PIN)
read 4-{4-[(pyridin-4-yl)methyl]phenyl}-1,7(1),3(1,3),5(1,4)-tetrabenzenaheptaphane-14,74-dicarboxylic acid (PIN)
for [not 4-{[3-(3-carboxyphenyl)methyl]phenyl}-1(4)-pyridina-7(1),3(1,4),5(1,3)-tribenzenaheptaphane-74-carboxylic acid;
there are two of the principal characteristic group in the PIN and only one in the other name]
read [not 4-{[4-(4-carboxyphenyl)methyl]phenyl}-1(4)-pyridina-3(1,4),5(1,3),7(1)-tribenzenaheptaphane-74-carboxylic acid;
nor 4-{[3-(4-carboxyphenyl)methyl]phenyl}-1(4)-pyridina-3,5(1,4),7(1)-tribenzenaheptaphane-74-carboxylic acid;
there are two of the principal characteristic group in the PIN and only one in the other names]
replace structure with:
Page 450, P-44.1.2.1, example 4 on this page. [modified 22 May 2019]
For tert-butyl(dimethyl)(oxiran-2-ylmethoxy)silane (PIN)
read tert-butyldi(methyl)(oxiranylmethoxy)silane (PIN)
replace the structure with:
Page 451, P-44.1.2.1, example 1 on this page.
For 2-(phosphinan-2-ylphosphanyl)furan (PIN)
read 2-[(phosphinin-2-yl)phosphanyl]furan (PIN)
Page 451, P-44.1.2.1, example 2 on this page.
For 1-(2H-pyran-3-yl)-2-silolan-2-yl)hydrazine (PIN)
read 1-(2H-pyran-3-yl)-2-(silolan-2-yl)hydrazine (PIN)
Page 451, P-44.1.2.1, example 5. [corrected 19 September 2018]
For 1-[ 5-(2,4,6,8-tetrasilanonan-1-yl)oxan-3-yl]-2,4,6,8-tetraoxaonane (PIN)
read 1-[5-(2,4,6,8-tetrasilanonan-1-yl)oxan-3-yl]-2,4,6,8-tetraoxanonane (PIN) [deleted space in name]
for [O-chain > Si-chain (see P-44.3); senior parent cannot be chosen by P-44.1.2)
read [O-chain > Si-chain (see P-44.3; senior parent cannot be chosen by P-44.1.2)]
replace the structure with:
Page 452, P-44.1.2.2, example 5. [corrected 5 July 2018]
For (2) 1-(pyridin-2-yl)hydrazine
read (2) (pyridin-2-yl)hydrazine
for 1-(pyridin-2-yl)diazane
read (pyridin-2-yl)diazane
Page 452, P-44.1.2.2, example 6. [corrected 3 June 2020]
For 2-hydrazino-4,5-dihydro-1H-imidazole
read 2-diazanyl-4,5-dihydro-1H-imidazole
Page 453, P-44.2.1 (g). [corrected 13 February 2019]
For ...the sequence as given in (c), above.
read ...the sequence: F > Cl > Br > I > O > S > Se > Te > N > P > As > Sb > Bi > Si > Ge > Sn > Pb > B > Al > Ga > In > Tl.
Page 454, P-44.2.1.1, example 1 on this page.
For 2-(naphthalen-2-ylmethyl)pyridine (PIN)
read 2-[(naphthalen-2-yl)methyl]pyridine (PIN)
Page 455, P-44.2.1.1, example on this page, explanation line 3. [corrected 18 May 2023]
For ...criterion (f) in P-44.2.1...
read ...criterion (g) in P-44.2.1...
Page 458, P-44.2.1.7 example (3).
For 1-oxadispiro[3.1.36.34]dodecane (PIN)
read 11-oxadispiro[3.1.36.34]dodecane (PIN)
Page 459, P-44.2.1.8 example (2).
For 2,6,8-dioxa-7-stannaspiro[3.5]nonane (PIN)
read 2,6,8-trioxa-7-stannaspiro[3.5]nonane (PIN)
Page 463, P-44.2.2.2.1.3 (b), example.
Replace diagram with:
for II dispiro[[1,3]dioxolane-2,2′-[1]azabicyclo[2.2.2]octane-5′,2′′-oxolane] (PIN)
read I 1′-azadispiro[[1,3]dioxolane-2,2′-bicyclo[2.2.2]octane-5′,2′′-oxolane] (PIN)
for I dispiro[[1]azabicyclo[2.2.2]octane-3,2′-oxolane-4′,2′′-dioxolane] (PIN)
read II 4-azadispiro[bicyclo[2.2.2]octane-2,2′-oxolane-3′,2′′-[1,3]dioxolane] (PIN)
for [first cited component azabicyclo[2.2.2]octane > dioxolane)]
read [first cited component dioxolane > bicyclo[2.2.2]octane]
Page 470, P-44.2.2.2.3.3, examples.
For 5H-[1,3]dioxolo[c][1,2]oxaphosphole (PIN)
read 5H-[1,3]dioxolo[4,5-c][1,2]oxaphosphole (PIN)
for 5H-[1,3]dioxolo[d][1,2]oxaphosphole (PIN)
read 5H-[1,3]dioxolo[4,5-d][1,2]oxaphosphole (PIN)
Page 472, P-44.2.2.2.4.2, example left. [corrected 3 January 2020]
For 1H-3,10a-methanophenanthrene (PIN)
read 1H-3,10a-methanophenanthrene (PIN)
Page 475, P-44.2.2.2.4.9, right example. [corrected 3 January 2020]
For (II) 6,16-methano-10,13-pentanonaphtha[2,3-c][1]benzazocine (PIN)
read (II) 13,18-methano-6,10-pentanonaphtha[2,3-c][1]benzazocine (PIN)
replace the structure with:
 >
>
Page 475, P-44.2.2.2.4.10, right example. [corrected 3 January 2020]
For (I) 6,17-methano-10,13-hexanonaphtho[2,3-c][1]benzazocine (PIN)
read (I) 13,18-methano-6,10-hexanonaphtho[2,3-c][1]benzazocine (PIN)
For (II) 6,16-methano-10,13-pentanonaphtho[2,3-c][1]benzazocine (PIN)
read (II) 13,18-ethano-6,10-heptanonaphtho[2,3-c][1]benzazocine (PIN)
for ...in (I) (atom 21) is fewer than two in (II) (atoms 20 and 21)
read ...in (I) (atom 22) is fewer than two in (II) (atoms 21 and 22)
replace the structure with:
Page 476, P-44.2.2.2.4.12, example. [corrected 3 January 2020]
For (I) 6,16-methano-9,13-pentanonaphtho[2,3-c][1]benzazocine (PIN)
read (I) 13,17-methano-6,10-pentanonaphtho[2,3-c][1]benzazocine (PIN)
for (II) 6,16-methano-10,13-pentanonaphtho[2,3-c][1]benzazocine (PIN)
read (II) 13,18-methano-6,10-pentanonaphtho[2,3-c][1]benzazocine (PIN)
for the locant set ‘9,13’ for the independent bridge in (I) is lower than the locant set ‘10,13’ in (II)
read the locant set ‘6,9’ for the independent bridge in (I) is lower than the locant set ‘6,10’ in (II)
replace the structure with:
Page 477, P-44.2.2.2.4.13, example. [modified 3 January 2020]
For (I) 6,15-ethano-10,13-pentanonaphtha[2,3-c][1]benzazocine (PIN)
read (I) 13,17-methano-6,10-pentanonaphtha[2,3-c][1]benzazocine (PIN)
For (II) 6,16-ethano-10,13-pentanonaphtha[2,3-c][1]benzazocine (PIN)
read (II) 13,18-methano-6,10-pentanonaphtha[2,3-c][1]benzazocine (PIN)
for the locant set ‘6,15’ for the independent bridge in (I) is lower than the locant set ‘6,16’ in (II)
read the locant set ‘13,17’ for the dependent bridge in (I) is lower than the locant set ‘13,18’ in (II)
replace the structure with:
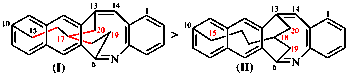
Page 477, P-44.2.2.2.5, line 3.
For ...through P-44.2.2.2.5.5.
read ...through P-44.2.2.2.5.3.
Page 478, P-44.2.2.2.5.2, example. [corrected 13 February 2019]
For [the bridge attachment locant set ‘1,4’ is lower than ‘1,5’]
read [the bridge attachment locant set ‘1,4’ is lower than ‘2,5’]
Page 480, P-44.2.2.2.6.1 (1), first structure. [corrected 31 October 2018]
For 1(4)-pyridina-7(1),3,5(1,4)-tribenzenaheptaphane (PIN)
read 1(4)-pyridina-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
Page 480, P-44.2.2.2.6.1 (1), second structure. [corrected 31 October 2018]
For 1(4)-silina-7(1),3,5(1,4)-tribenzenaheptaphane (PIN)
read 1(4)-silina-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
Page 481, P-44.2.2.2.6.1 (4), second structure. [corrected 24 October 2018]
Replace the structure with:
Page 482, P-44.2.2.2.6.2, example (1) second name.
For 1(4)-pyridina-7(2)-silina-3,5(1,4)dibenzenaheptaphane (PIN)
read 1(4)-pyridina-7(2)-silina-3,5(1,4)-dibenzenaheptaphane (PIN)
Page 482, P-44.2.2.2.6.2, example (2), second structure. [corrected 22 May 2019]
Replace the structure with:
Page 484, P-44.2.2.2.6.4, example 1, second compound
For 1(4),8(4,2)-dipyridina-3,5(1,4),10(1)-tribenzenadecaphane (PIN)
read 1(4),8(2,5)-dipyridina-3,5(1,4),10(1)-tribenzenadecaphane (PIN)
Page 484, P-44.2.2.2.6.5, example.
Replace first structure with:
Replace second structure with:
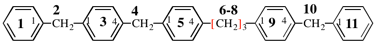
Page 485, P-44.2.2.2.6.6, example first name.
For 1(4),pyridina-7(2,5)-furana-9(2,5)-thiophena-3,5(1,4),11(1)-tribenzenaundecaphane (PIN)
read 1(4)-pyridina-7(2,5)-furana-9(2,5)-thiophena-3,5(1,4),11(1)-tribenzenaundecaphane (PIN)
Replace first structure with:
Replace second structure with:
for 1(4)-pyridina-9(2,5)-furana-7(2,5)-thiophena-1,4),11(1)-tribenzenaundecaphane (PIN)
read 1(4)-pyridina-9(2,5)-furana-7(2,5)-thiophena-3,5(1,4),11(1)-tribenzenaundecaphane (PIN)
Page 485, P-44.2.2.2.6.7, example.
For
1,7(1),3,5(1,3)-dibenzenaheptaphane (PIN)
read 1,7(1),3,5(1,3)-tetrabenzenaheptaphane (PIN)
for [the locant set ‘1,1,1,3,3’ for amplificant attachment locants in increaing numerical order is lower than ‘1,1,3,4’]
read [the locant set ‘1,1,1,1,3,3’ for amplificant attachment locants in increaing numerical order is lower than ‘1,1,1,1,3,4’]
Page 486, P-44.2.2.2.6.8, example. [corrected 13 February 2019]
Replace the example with:
 |
| 1(4)-pyridina-3(1,3),5(1,4),7(1)-tribenzenaheptaphane (PIN) |
| is senior to |
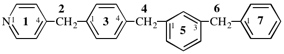 |
| 1(4)-pyridina-3(1,4),5(1,3),7(1)-tribenzenaheptaphane (PIN) |
| [the locant set ‘4,1,3,1,4,1’ for the amplificant locants in order of their citation in the name is lower than ‘4,1,4,1,3,1’] |
Page 487, P-44.2.2.2.6.10, example.
Replace first structure with:
Replace second structure with:
Page 487, P-44.2.2.2.7, example.
Replace structure with:
for 4-oxa-1(2),2,3(2,6)-tripyridina-5,6(1,4)7(1)-tribenzenaheptaphane (PIN)
read 4-oxa-1(2),2,3(2,6)-tripyridina-5,6(1,4),7(1)-tribenzenaheptaphane (PIN)
for 16-([11,21:24:31]terphenyl-14-yloxy)-12,22:26,32-terpyridine;
read 16-([11,21:24,31-terphenyl]-14-yloxy)-12,22:26,32-terpyridine;
Page 488, P-44.2.2.2.7, criterion (g).
For ... > Se > Te > P > As...
read ... > Se > Te > N > P > As...
Page 488, P-44.2.2.2.7.1, line 2. [corrected 19 September 2018]
For ... heterocycle [criterion (a) in P-44.2.2.2.7.
read ... heteroatom [criterion (a) in P-44.2.2.2.7.
Page 488, P-44.2.2.2.7.1, example, right structure.
For 1,1′:4,1′′-terphenyl
read 1,1′:4′,1′′-terphenyl
Page 489, P-44.2.2.2.7.4, example, right-hand side.
For 2,2′,2′′-terpyridine
read 2,2′:5′,2′′-terpyridine
Page 489, P-44.2.2.2.7.7, line 2.
For ... > Se > Te > P > As...
read ... > Se > Te > N > P > As...
Page 490, P-44.2.2.2.7.7, example 2.
For 2,2′-bi-3,1,5-benzooxadiarsinine (PIN)
read 2,2′-bi-3,1,5-benzoxadiarsepine (PIN)
Page 491, P-44.3.1, example (2), second structure.
Replace the structure with:
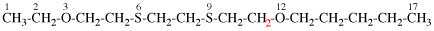
Page 491, P-44.3.2, example (3), first structure.
Replace the structure with:
Page 491, P-44.3.2, example (3), second structure.
Replace the structure with:
Page 492, P-44.3.2, example (4), second structure.
Replace the structure with:
Page 492, P-44.3.2, example (6).
Replace structure with:
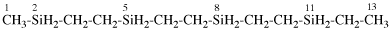
Page 492, P-44.3.2, example (7).
Replace structure with:
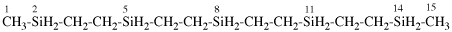
Page 494, P-44.4.1.1, example (2).
For [three multiple bonds greater than one]
read [two multiple bonds greater than one]
replace the structures with:
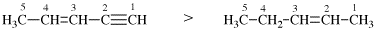
Page 494, P-44.4.1.1 (3), first structure. [corrected 26 September 2018]
Replace the structure with:
.gif)
Page 494, P-44.4.1.1 (3), second structure. [corrected 26 September 2018]
Replace the structure with:
a.gif)
Page 495, P-44.4.1.2 (1), example left. [corrected 4 September 2024]
For cycloicosene (PIN)
read (E)-cycloicosene (PIN)
Page 495, P-44.4.1.2 (2), example left. [corrected 4 September 2024]
For cycloicosa-1,8-diene (PIN)
read (1E,8E)-cycloicosa-1,8-diene (PIN)
Page 495, P-44.4.1.2 (2), example right. [corrected 4 September 2024]
For (1E)-cycloicos-1-en-3-yne (PIN)
read (1E)-cycloicos-1-en-3-yne (PIN)
Page 496, P-44.4.1.2 example (4), structure 1. [corrected 3 December 2025, modified 22.1.2026]
For 1,2,5,6-tetrasilacyclooct-3-en-7-yne (PIN)
read 1,2,5,6-tetrasilacyclooct-3-en-7-yne
add (7Z)-3,4-didehydro-1,2,5,6-tetrahydro-1,2,5,6-tetrasilocine (PIN)
Page 496, P-44.4.1.2 example (4), structure 2. [corrected 3 December 2025]
For 1,2,5,6-tetrasilacycloocta-3,7-diyne (PIN)
read 1,2,5,6-tetrasilacycloocta-3,7-diyne
add1,2,5,6-tetrahydro-3,4,7,8-tetradehydro-1,2,5,6-tetrasilocine (PIN)
Page 496, P-44.4.1.2 (5), left structure. [corrected 26 September 2018]
Replace the structure with:
.gif)
Page 496, P-44.4.1.2 (5), right structure. [corrected 26 September 2018]
Replace the structure with:
a.gif)
Page 497, P-44.4.1.3.1, example (1). [corrected 3 June 2020]
For 1,3λ6-thioxolane (PIN)
read 1,3λ6-oxathiolane (PIN)
for 1,3λ4-thioxolane (PIN)
read 1,3λ4-oxathiolane (PIN)
Page 500, P-44.4.1.5, example (1) second structure.
For 1,9-dioxa-4,6-dithia-5-stanna- cycloundecane (PIN)
read 1,9-dioxa-4,6-dithia-5-stannacycloundecane (PIN)
Page 500, P-44.4.1.5, example (3), first structure
For 2-oxa-5-sila-1,8(1),3,6(1,4)-tetrabenzenaoctaphane (PIN)
read 2-oxa-5-thia-1,8(1),3,6(1,4)-tetrabenzenaoctaphane (PIN) [example changed as with silicon P-44.2.2.2.6.10 decides seniority]
replace the structure with:
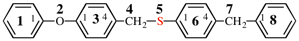
Page 500, P-44.4.1.5, example (3), second structure
For 2-oxa-7-thia-1,8(1),3,6(1,4)-tetrabenzenaoctaphane
read 2-oxa-7-thia-1,8(1),3,6(1,4)-tetrabenzenaoctaphane (PIN)
Page 502, P-44.4.1.6 (5), second compound. [corrected 31 October 2018]
For 2-oxa-5-selena-7-thia-1,8(1),3,6(1,4)-tetrabenzenaoctaphane (PIN)
read 2-oxa-7-thia-5-selena-1,8(1),3,6(1,4)-tetrabenzenaoctaphane (PIN)
Page 503, P-44.4.1.8, example 1. [corrected 25 August 2016]
For 2H-pyran-2-one (PIN)
read pyridin-2(1H)-one (PIN)
for 4H-pyran-4-one (PIN)
read pyridin-4(1H)-one (PIN) [examples changed as with pyran seniority is decided by P-44.4.1.4]
replace the structures with:
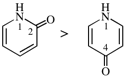
Page 504, P-44.4.1.8, example (2), second compound.
For octane-1,8-diol
read octane-1,8-diol (PIN)
Page 505, 44.4.1.10.1 (1), example left. [corrected 4 September 2024]
For cyclocosa-1,3-dien-5-yne (PIN)
read (1Z,3E)-cyclocosa-1,3-dien-5-yne (PIN)
Page 505, 44.4.1.10.1 (1), example right. [corrected 4 September 2024]
For cyclocosa-1,7-dien-3-yne (PIN)
read (1E,7Z)-cyclocosa-1,7-dien-3-yne (PIN)
Page 506, 44.4.1.10.1 (2), example left. [corrected 4 September 2024]
For cyclocosa-1,3-dien-5-yne (PIN)
read (1Z,3E)-cyclocosa-1,3-dien-5-yne (PIN)
Page 506, 44.4.1.10.1 (2), example right. [corrected 4 September 2024]
For cyclocosa-1,5-dien-3-yne (PIN)
read (1E,5E)-cyclocosa-1,5-dien-3-yne (PIN)
Page 506, P-44.4.1.10.1, example (5).
Replace first structure with:
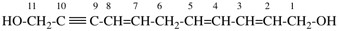
Replace second structure with:
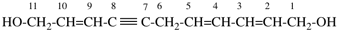
Page 507, P-44.4.1.10.1, example (8).
For 1,13(1),3,6,10(1,4)-pentabenzenatridecaphane-4,11-dien-8-yne (PIN)
read 1,13(1),3(1,2),6,10(1,4)-pentabenzenatridecaphane-4,11-dien-8-yne (PIN)
Page 508, P-44.4.1.10.2, example (2).
Replace first structure with:
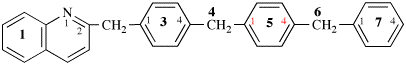
Replace second structure with:
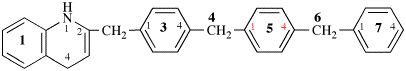
Page 510, P-44.4.1.11.2, example 2 right,
For [1,1-2H]pentane
read [1-2H1]pentane (PIN)
replace the structure with:
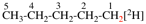
Page 510, P-44.4.1.11.3, example 2. [corrected 3 January 2020]
For [81Br]CH2-CH2-CH2-CH2-CH3
read [14C]H3-CH2-CH2-CH2-CH3
for 1-[81Br]bromopentane (PIN)
read [1-14C]pentane (PIN)
for [79Br]CH2-CH2-CH2-CH2-CH3 (PIN)
read [13C]H3-CH2-CH2-CH2-CH3 (PIN)
for 1-[79Br]bromopentane (PIN)
read [1-13C]pentane (PIN)
for [81Br is senior to 79Br]
read [14C is senior to 13C]
Page 511, P-44.4.1.11.5, example.
For [4-2H1,5-13C]pentanoic acid (PIN)
read [5-13C,4-2H1]pentanoic acid (PIN)
Page 512, P-44.4.1.12.1, example (2). [modified 3 June 2020]
For (2Z)-but-2-enoic acid
read (2Z)-2-methylbut-2-enoic acid (PIN)
for (2E)-but-2-enoic acid
read (2E)-2-methylbut-2-enoic acid (PIN)
Page 514, P-44.4.1.12.2, example (1).
For (1R)-5′H-spiro[indene-1,2′-(1,3)oxazole] (PIN)
read (1R)-5′H-spiro[indene-1,2′-[1,3]oxazole] (PIN)
for (1S)-5′H-spiro[indene-1,2′-(1,3)oxazole] (PIN)
read (1S)-5′H-spiro[indene-1,2′-[1,3]oxazole] (PIN)
Page 516, P-45.1.1, example on this page.
For 2-chloro-4-(2-chloro-4-carboxyphenoxy)benzoic acid (PIN, substitutive name)
read 4-(4-carboxy-2-chlorophenoxy)-2-chlorobenzoic acid (PIN, substitutive name)
replace structure on this page with:
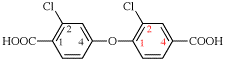
Page 516, P-45.1.2, example 2. [corrected 13 February 2019]
For N,N′-ethane-1,2-diylbis[N-(carboxymethyl)glycine]
read N,N′-(ethane-1,2-diyl)bis[N-(carboxymethyl)glycine]
Page 517, P-45.1.2, last example.
For 1,1′,1′′-({diphenyl[(diphenylmethyl)sulfanyl]methoxy}methanetriyl)tribenzene (PIN)
read 1,1′,1′′-({[(diphenylmethyl)sulfanyl]diphenylmethoxy}methanetriyl)tribenzene (PIN)
for (not 1,1′-({[diphenyl(triphenylmethoxy)methyl]sulfanyl}methanediyl)dibenzene
read (not 1,1′-({[diphenyl(triphenylmethoxy)methyl]sulfanyl}methylene)dibenzene
Page 518, P-45.2.1, example (2).
For [not 3-[(5-chloro-5-cyanophenyl)methyl]benzonitrile; ...
read [not 3-[(5-chloro-2-cyanophenyl)methyl]benzonitrile; ...
Page 519, P-45.2.1, example (5).
For 2-(naphthalen-2-yl)-13-chloro-1(2)-naphthalena-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
read 13-chloro-2-(naphthalen-2-yl)-1(2)-naphthalena-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
replace the structure with:
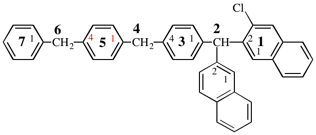
Page 519, P-45.2.1, example (6).
For 1,1-dimethyl-3-[(λ4-thian-3-ylsulfanyl)methyl]- λ4-thiane (PIN)
read 1,1-dimethyl-3-{[(1λ4-thian-3-yl)sulfanyl]methyl}-1λ4-thiane (PIN)
for [not 3-{[(1,1-dimethyl-λ4-thian-2-yl)methyl]sulfanyl}-λ4-thiane;
read [not 3-{[(1,1-dimethyl-1λ4-thian-3-yl)methyl]sulfanyl}-1λ4-thiane;
Page 519, P-45.2.1, example (6). [modified 3 June 2020]
For [not 3-{[(1,1,-dimethyl-λ4-thian-2-yl)methyl]sulfanyl}-λ4-thiane;
read [not 3-{[(1,1-dimethyl-1λ4-thian-3-yl)methyl]sulfanyl}-1λ4-thiane;
Page 519, P-45.2.1, example (7).
For [not 3-isopropylhexane or 3-propan-2-yl;
read [not 3-isopropylhexane or 3-(propan-2-yl)hexane;
Page 519, P-45.2.1, example (8), move to P-45.2.3 and adjust remaining example numbers. [corrected 3 March 2021]
For [not 3-ethyl-6-(2-methylbutyl)-5-propyldecane; the PIN parent chain has more substituents; four simple substituents vs. three (two simple and one compound) substituent]
read [not 6-butyl-3-ethyl-8-methyl-5-propyldecane; both have the set of locants '3,5,6,8' but the PIN has them in the order '5,8,3,6' which is lower than 6,3,8,5]
Page 520, P-45.2.1, example (10), move to P-45.2.2 and adjust the remaining example numbers. [modified 3 March 2021]
For [not 3-chloro-5-(4-hydroxypentan-2-yl)-4-methylnonane-2,8-diol; the PIN parent chain has more substituents; four (three simple and one compound) substituents vs. three (two simple and one compound)]
read [not 7-chloro-5-(3-hydroxybutyl)-4,6-dimethylnonane-2,8-diol: the locant set of the PIN is '3,4,5,6' which is lower than '4,5,6,7']
replace structure with:
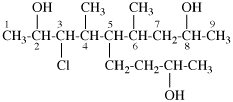
Page 520, P-45.2.1, example (12).
For 1-(disilanyldisulfanyl)-2,2,2-trimethyldisilane (PIN)
read 2-(disilanyldisulfanyl)-1,1,1-trimethyldisilane (PIN)
for [not [2-(trimethyldisilanyl)disulfanyl]disilane; the...
read [not [2-(2,2,2-trimethyldisilanyl)disulfan-1-yl]disilane; the...
replace structure with:
Page 521, P-45.2.1, example (14).
For 4-(4-carboxy-3λ6-sulfanylphenoxy)-2-phosphanyl-3λ6-sulfanylbenzoic acid (PIN)
read 4-[4-carboxy-3-(λ6-sulfanyl)phenoxy]-2-phosphanyl-3-(λ6-sulfanyl)benzoic acid (PIN)
for [not 4-(4-carboxy-3-phosphanyl-2λ6-sulfanylphenoxy)-2λ6-sulfanylbenzoic acid)
read [not 4-[4-carboxy-3-phosphanyl-2-(λ6-sulfanyl)phenoxy]-2-(λ6-sulfanyl)benzoic acid;
Page 522, P-45.2.2, example (4).
For 11-bromo-2-(4-chloronaphthalen-2-yl-1(2)-naphthalena-3,5(1,4)tribenzenaheptaphane (PIN)
read 11-bromo-2-(4-chloronaphthalen-2-yl)-1(2)-naphthalena-3,5(1,4)-tribenzenaheptaphane (PIN)
for [not 2-(1-bromonaphthalen-2-yl)-14-chloro-1(2)-naphthalena-3,5(1,4),7(1)tribenzenaheptaphane (PIN); ...
read [not 2-(1-bromonaphthalen-2-yl)-14-chloro-1(2)-naphthalena-3,5(1,4),7(1)-tribenzenaheptaphane (PIN); ...
replace the structure with:
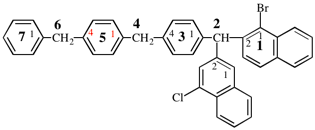
Page 523, P-45.2.2, example (5), move to P-45.2.3 and adjust the remaining example numbers. [corrected 3 March 2021]
For [not 1-ethyl-7-[(5-ethyl-8-propylnaphthalen-2-yl)selenyl]-4-propylnaphthalene; the locant set '1,4,6' in the PIN is lower than '1,4,7']
read [not 4-ethyl-6-[(5-ethyl-8-propylnaphthalen-2-yl)selanyl]-1-propylnaphthalene: both have the locant set '1,4,6' but the PIN set is in the order '1,6,4' which is lower than '4,6,1']
Page 523, P-45.2.2, example (6).
For [not 2-methyl-3-[4-methyl-3-(methylamino)-3-oxopropyl]propanamide; ...
read [not 2-methyl-3-{4-methyl-3-[3-(methylamino)-3-oxopropyl]phenyl}propanamide; ...
Page 524, P-45.2.2, example (9).
For [not 4-(buten-2-yl)-6-methylhepta-1,5-diene, ...
read [not 4-(but-2-en-2-yl)-6-methylhepta-1,5-diene, ...
Page 524, P-45.2.2, example (10), , move to P-45.2.3 and adjust the remaining example numbers. [corrected 3 March 2021]
For [not 1,6-dibromo-3-(1-bromo-2-chloroethyl)-6-chloro-2-iodohexane; the locant set '1,1,4,5,6' in the PIN is lower than '1,2,3,6,6']
read [not 1,6-dibromo-4-(1-bromo-2-chloroethyl)-1-chloro-5-iodohexane; both have the locant set '1,1,4,5,6' but the PIN set is in the order '1,5,4,1,6' which is lower than '1,6,4,1,5']
Page 524, P-45.2.2, example (11).
For 5-bromo-3-(3-nitro-1λ5-phosphanylpropyl)-4λ5-phosphanylhexanoic acid (PIN)
read 5-bromo-3-[3-nitro-1-(λ5-phosphanyl)propyl]-4-(λ5-phosphanyl)hexanoic acid (PIN)
for [not 3-(2-bromo-1λ5-phosphanylpropyl)-6-nitro-4λ5-phosphanylhexanoic acid,
read [not 3-[2-bromo-1-(λ5-phosphanyl)propyl]-6-nitro-4-(λ5-phosphanyl)hexanoic acid
Page 524, P-45.2.2, example (12).
For [not 4-[(4-carboxy-3-(λ5-phosphanyl)phenoxy]-3-phosphanylbenzoic acid;
read [not 4-[4-carboxy-3-(λ5-phosphanyl)phenoxy]-3-phosphanylbenzoic acid;
Page 526, P-45.2.3, example (3). [corrected 3 January 2020]
For not 2-ethyl-7-[(7-ethyl-8-propylnaphthalen-2-yl)oxy]-1-propylnaphthalene
read not 2-ethyl-7-[(8-ethyl-7-propylnaphthalen-2-yl)oxy]-1-propylnaphthalene
replace the structure with:
.gif)
Page 526-527, P-45.2.3, after example (6) add examples (7)-(12) which are transferred from P-45.5.1. For a PDF (348 KB) of the revised P-45.2.3 and P-45.5.1 click here
Page 527, P-45.2.3, example (5).
Replace structure with:
Page 528, P-45.3.1, line 2/3. [corrected 3 January 2020]
For ...cited as prefixes.
read ...cited as prefixes and directly connected to the parent structure.
Page 528, P-45.3.1, example 2. [modified 23 December 2020]
For 4-(2λ5-diphosphan-1-yl)-2-[2-(diphosphan-1-yl)ethyl]butanenitrile (PIN)
read 4-(2λ5-diphosphan-1-yl)-2-(2-diphosphanylethyl)butanenitrile (PIN)
for [not 4-(diphosphan-1-yl)-2-[2-(2λ5-diphosphan-1-yl)ethyl]butanenitrile;
read [not 4-diphosphanyl-2-[2-(2λ5-diphosphan-1-yl)ethyl]butanenitrile;
Page 529, P-45.3.2, example 1. [corrected 3 January 2020]
Delete as decided by P-45.2.2.
Page 529, P-45.3.2, example 2 and explanation. [corrected 3 January 2020]
Delete as decided by P-45.2.3.
Page 529, P-45.3.2, new example. [corrected 3 June 2020]
Add
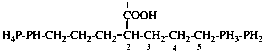
5-(1λ5-diphospan-1-yl)-2-[3-(2λ5-diphospan-1-yl)propyl]pentanoic acid (PIN)
[not 5-(2λ5-diphospan-1-yl)-2-[3-(1λ5-diphospan-1-yl)propyl]pentanoic acid]
The locant ‘1λ5’ for the nonstandard bonding number directly bonded to the parent structure is lower than ‘2λ5’.
Page 529, P-45.4, title. [corrected 3 January 2020]
For CRITERIA RELATED ONLY TO ISOTOPIC MODIFICATION, OTHER CRITERIA BEING EQUAL
read CRITERIA RELATED ONLY TO ISOTOPIC MODIFICATION OF SUBSTITUENTS
Page 529, P-45.4.1. [corrected 3 January 2020]
Delete as it is a restatement of P-44.4.1.11.1 and inappropriate in P-45.4.
Page 529, P-45.4.1 replacement structure. [modified 3 June 2020]
For 2-bromo-1-{[2-(81Br)bromopentyl]oxy}pentane
read 2-bromo-1-{[2-(81Br)bromopentyl]oxy}pentane (PIN)
for [not 2-(81)bromo-1-[(2-bromopentyl)oxy]pentane]
read [not 2-(81Br)bromo-1-[(2-bromopentyl)oxy]pentane]
Page 530, P-45.4.2. [corrected 3 January 2020]
Delete as it is a restatement of P-44.4.1.11.2 and inappropriate in P-45.4.
Page 530, P-45.4.3. [corrected 3 January 2020]
Delete as it is a restatement of P-44.4.1.11.3 and inappropriate in P-45.4.
Page 530, P-45.4.4, title. [corrected 3 January 2020]
For P-45.4.4
read P-45.4.1
Page 530, P-45.4.1 (formerly P-45.4.4), example. [corrected 3 January 2020]
Delete as decided by P-45.2.2.
Page 530, P-45.4.1 (formerly P-45.4.4) new example. [corrected 3 January 2020]
Add
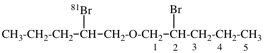
2-bromo-1-{[2-(81Br)bromopentyl]oxy}pentane (PIN)
[not 2-(81Br)bromo-1-[(2-bromopentyl)oxy]pentane]
The isotopically modified substituent for the PIN is attached at ‘1’ which is lower than ‘2’.
Page 531, P-45.4.5, title. [corrected 3 January 2020]
For P-45.4.5
read P-45.4.2
Page 531, P-45.4.2 (formerly P-45.4.5), example. [corrected 3 January 2020]
Delete as decided by P-45.2.2.
Page 531, P-45.4.2 (formerly P-45.4.5), new example. [modified 30 March 2022]
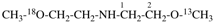
For 2-[(13C)methyloxy]-N-{2-[methyl(18O)oxy]ethyl}ethan-1-amine (PIN)
read 2-(13C)methoxy-N-[2-(18O)methoxyethyl]ethan-1-amine (PIN)
for [not 2-[methyl(18O)oxy]-N-{2-[(13C)methyloxy]ethyl}ethan-1-amine]
read [not 2-(18O)methoxy-N-[2-(13C)methoxyethyl]ethan-1-amine]
18O > 13C; the 18O isotopically modified substituent for the PIN is attached at ‘N’ which is lower than ‘2’.
Page 531, P-45.4.6, title. [corrected 3 January 2020]
For P-45.4.6
read P-45.4.3
Page 531, P-45.4.3 (formerly P-45.4.6), example. [corrected 3 January 2020]
Delete as decided by P-45.2.2.
Page 531, P-45.4.3 (formerly P-45.4.6), new example. [modified 30 March 2022]
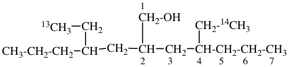
For 4-([2-13C]ethyl)-2-[2-([2-14C]ethyl)pentyl]heptan-1-ol (PIN)
read 4-[2-13C]ethyl-2-(2-[2-14C]ethylpentyl)heptan-1-ol (PIN)
for [not 4-([2-14C]ethyl)-2-[2-([2-13C]ethyl)pentyl]heptan-1-ol
read [not 4-[2-14C]ethyl-2-(2-[2-13C]ethylpentyl)heptan-1-ol
14C > 13C; the 14C isotopically modified substituent for the PIN is attached at ‘2’ which is lower than ‘4’
Page 531-535, P-45.5.1, examples (4), (5), (7)-(9) and (11) are transferred to P-45.2.3.
Page 531, P-45.5.1. title. [corrected 3 January 2020]
For P-45.5.1
read P-45.5
Page 532, P-45.5.1, example (1).
For but ‘bromo’. in the PIN is earlier alphabetically than ‘dibromo’.
read in both names the locant set is ‘1,2,4’, and the locants appear in the name in the same order set ‘1,4,2’ so
no decision can be made by P-45.2.2 or P-45.2.3; but but ‘bromo’. in the PIN is earlier alphabetically than ‘dibromo’.
Page 532, P-45.5.1, example (2).
For 4-bromo-N-(2,4-dibromophenyl)-2-nitroaniline] (PIN)
read 2-bromo-4-chloro-N-(2,4-dibromophenyl)aniline (PIN)
for [not 2,4-dibromo-N-(4-bromo-2-nitrophenyl)aniline;
read [not 2,4-dibromo-N-(2-bromo-4-chlorophenyl)aniline;
replace structure with:
Page 532, P-45.5.1, example (3).
For 14-bromo-13-chloro-2-(3,4-dibromonaphthalen-1-yl)-1(2)naphthalene-3,5(1,4),7(1)tribenzenaheptaphane (PIN)
read 13-bromo-14-chloro-2-(3,4-dibromonaphthalen-2-yl)-1(2)-naphthalena-3,5(1,4),7(1)-tribenzenaheptaphane (PIN)
for [not 13,14-dibromo- 2-(4-bromo-3-chloronaphthalen-2-yl)-1(2)naphthalene- 3,5(1,4),7(1)-tribenzenaheptaphane;
read [not 13,14-dibromo-2-(3-bromo-4-chloronaphthalen-2-yl)-1(2)-naphthalena-3,5(1,4),7(1)-tribenzenaheptaphane; [remove spaces from name]
replace structure with:
Page 533, P-45.5.1, example (4). Move to P-45.2.3 as example (7).
For 4-bromo-2-(2-chloroethyl)butan-1-ol (PIN)
read 4-bromo-2-(2-chloroethyl)butan-1-ol
for 2-(2-bromoethyl)-4-chlorobutan-1-ol
read 2-(2-bromoethyl)-4-chlorobutan-1-ol (PIN)
for (‘bromo-chloro’ in the PIN is earlier alphabetically than ‘bromo-ethyl’)
read (the locant sets are the same in both names, i.e., ‘2,4’ but in the order of appearance in the name, the locant set ‘2,4’ in the PIN is lower than ‘4,2’)
Page 533, P-45.5.1, example (5). Move to P-45.2.3 as example (8).
For (‘bromo’ in the PIN is earlier alphabetically than ‘dibromo’)
read (the locant sets are the same in both names, i.e., ‘1,1,5,6’ but in their order of appearance in the name, the locant set ‘1,5,1,6’ is lower than ‘1,6,1,5’)
Page 533, P-45.5.1, example (6).
Renumber as example (4)
Page 534, P-45.5.1, example (7). Move to P-45.2.3 as example (9).
For (‘ethyl....ethyl’ in the PIN name is earlier alphabetically than ‘ethyl....methyl’]
read (the locant sets are the same in both names, i.e. ‘6,7,8’ but in their order of appearance in the name, the locant set ‘7,6,8’ is lower than ‘8,7,6’)
Page 534, P-45.5.1, example (8). Move to P-45.2.3 as example (10).
For 3-{3-[3-(ethylamino)-3-oxopropyl]-4-methylphenyl}-N-methylpropanamide (PIN)
read N-methyl-3-{4-methyl-3-[3-oxo-3-(propylamino)propyl]phenyl}propanamide (PIN)
for [not N-ethyl-3-{2-methyl-5-[3-(methylamino)-3- oxopropyl]phenyl}propanamide;
oxopropyl]phenyl}propanamide; ‘ethylamino’ in the PIN is earlier alphabetically than ‘ethyl....methyl’]
read [not 3-{2-methyl-5-[3-(methylamino)-3-oxopropyl]phenyl}-N-propylpropanamide;
(the locant sets are the same in both names, i.e. ‘N,3’ but in their order of appearance in the name, the locant set ‘N,3’ in the PIN is lower than ‘3,N’)
replace structure with:
Page 534, P-45.5.1, example (9). Move to P-45.2.3 as example (11).
For 5-bromo-3-[2-chloro-1-(λ5-phosphanyl)propyl]-4-(λ5-phosphanyl)hexanoic acid (PIN)
read [not 5-bromo-3-[2-chloro-1-(λ5-phosphanyl)propyl]-4-(λ5-phosphanyl)hexanoic acid; [move to after corrected PIN]
for [not 3-[2-bromo-1-(λ5-phosphanyl)propyl]-5-chloro-4-(λ5-phosphanyl)hexanoic acid;
read
3-[2-bromo-1-(λ5-phosphanyl)propyl]-5-chloro-4-(λ5-phosphanyl)hexanoic acid (PIN)
for ‘bromo-chloro’ is lower alphanumerically than ‘bromo-phosphanyl’]
read the locant sets are the same in both names, i.e. ‘3,4,5’ but in their order of appearance in the name, the locant set ‘3,5,4’ in the PIN is lower than ‘5,3,4’]
retain structure:
Page 535, P-45.5.1, example (10).
Renumber as example (5).
Page 535, P-45.5.1, example (11). Move to P-45.2.3 as example (12).
For 4-(81Br)bromo-5-bromo-3-[1-(81Br)bromo-2-chloropropyl]hexanoic acid (PIN)
read [not 4-(81Br)bromo-5-bromo-3-[1-(81Br)bromo-2-chloropropyl]hexanoic acid; [move to after corrected PIN]
for [not 4-(81Br)bromo-3-[1-(81Br)bromo-2-bromopropyl]-5-chorohexanoic acid
read 4-(81Br)bromo-3-[1-(81Br)bromo-2-bromopropyl]-5-chlorohexanoic acid (PIN)
for ‘bromo....bromo....bromo....chloro’ is lower than ‘bromo’....bromo....bromo....propyl]
read the locant sets are the same in both names, i.e. ‘3,4,5’ but in their order of appearance in the name, the locant set ‘4,3,5’ in the PIN is lower than ‘4,5,3’]
Page 535, P-45.5.2. [corrected 3 January 2020]
Delete as decided by P-45.4.3 (formerly P-45.4.6).
Page 536, P-45.6.2, lines 2/3. [corrected 3 January 2020]
For ...and P-44.4.1.10 is not...
read ...and P-44.4.1.12) is not...
Page 536, P-45.6.2, line 5.
For The names with and without stereodescriptors are the same, i.e. butane.
read The names with and without stereodescriptors are the same, i.e. butene.
Page 536, P-45.6.2, example 1.
For (1E,1′E)-1,1′-sulfanediyldi[(1E)-cyclooct-1-ene] (PIN)
read (1E,1′E)-1,1′-sulfanediyldi(cyclooct-1-ene) (PIN)
Page 536, P-45.6.2, the paragraph 2, line 2.
For ... dependent as shown in P-47.1.2 and P-47.2.2 below. Two...
read ... dependent as shown below. Two...
Page 537, P-45.6.2, example (1) cont'd.
For (1Z,3S)-3-{2-[(1E,3R)-cyclooct-1-en-3-yl]ethyl}cyclooct-1-ene (PIN)
read (1Z,3S)-3-{2-[(1R,2E)-cyclooct-2-en-1-yl]ethyl}cyclooct-1-ene (PIN)
for (1-cis,3S)-3-{2-[(1-trans,3R)-cyclooct-1-en-3-yl]ethyl}cyclooct-1-ene
read (1-cis,3S)-3-{2-[(1R,2-trans)-cyclooct-2-en-1-yl]ethyl}cyclooct-1-ene
Page 537, P-45.6.2, example 2.
For 1-[(2R)-butan-2-yl-]-4-({4-[(2S)-butan-2-yl]phenyl}sulfanyl)benzene (PIN)
read
1-[(2R)-butan-2-yl]-4-({4-[(2S)-butan-2-yl]phenyl}sulfanyl)benzene (PIN)
for
1-[(2S)-butan-2-yl-]-4-({4-[(2R)-butan-2-yl]phenyl}sulfanyl)benzene
read
1-[(2S)-butan-2-yl]-4-({4-[(2R)-butan-2-yl]phenyl}sulfanyl)benzene
replace the second structure with:
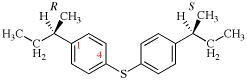
Page 537, P-45.6.2, example 3.
For 1,1′-oxybis[bis(3,4-(1-chloroethyl)benzene]
read 1,1′-oxybis[3,4-bis(1-chloroethyl)benzene]
Page 538, P-45.6.2, Example 3, second compound on this page. [modified 3 January 2020]
For 1,2-bis[(1S)-1-chloroethyl]-4-{4-[(1S)-1-chloroethyl]-2-[(1R)-1-chloroethyl]phenoxy}benzene (PIN)
read 1,2-bis[(1S)-1-chloroethyl]-4-{3-[(1R)-1-chloroethyl]-4-[(1S)-1-chloroethyl]phenoxy}benzene (PIN)
for ...; ‘bis.....chloroethyl....chloroethyl’ is lower alphabetically than ‘bis.....chloroethylphenoxy’]
read ...; ‘1,2,4’ is senior to ‘4,2,1’ (see P-45.2.3)]
Page 538, P-45.6.3, example.
For 1-[(R)-(1-bromoethyl)-1-[(S)-(1-bromoethyl)}cyclopentane
read 1-[(1R)-1-bromoethyl]-1-[(1S)-1-bromoethyl]cyclopentane
for 1-[(S)-(1-bromoethyl)-1-[(R)-(1-bromoethyl)}cyclopentane
read 1-[(1S)-1-bromoethyl]-1-[(1R)-1-bromoethyl]cyclopentane
Page 539, P-45.6.4. [corrected 3 January 2020]
Delete as decided by P-44.4.1.12.
Page 544, P-46.1.5, example 1.
For 5-{[(methoxymethyl)sulfanyl]]methyl}-4,9-dioxa-2,7λ4-dithiadecan-1-yl (preferred prefix)
read 5-{[(methoxymethyl)sulfanyl]methyl}-4,9-dioxa-2,7λ4-dithiadecan-1-yl (preferred prefix)
Page 544, P-46.1.5, example 2.
For 5-({[(methylsulfanyl)methyl]-λ6-sulfanyl]}methyl)-4-oxa-2λ4,7λ4,9λ4-trithiadecan-1-yl (preferred prefix)
read 5-({[(methylsulfanyl)methyl]-λ6-sulfanyl}methyl)-4-oxa-2λ4,7λ4,9λ4-trithiadecan-1-yl (preferred prefix)
for [not 5-({[(methyl-λ4-sulfanyl)methyl]-λ4-sulfanyl]}methyl)-4-oxa-2λ4,7λ6,9-trithiadecan-1-yl;
read [not 5-({[(methyl-λ4-sulfanyl)methyl]-λ4-sulfanyl}methyl)-4-oxa-2λ4,7λ6,9-trithiadecan-1-yl;
Page 544, P-46.1.5, example 3.
For 5-({[(methyl-λ6-sulfanyl)methyl]-λ4-sulfanyl]}methyl)-4-oxa-2λ4,7λ6 ,9λ6 -trithiadecan-1-yl (preferred prefix)
read 5-({[(methyl-λ6-sulfanyl)methyl]-λ4-sulfanyl}methyl)-4-oxa-2λ4,7λ6,9λ6-trithiadecan-1-yl (preferred prefix) [deleted spaces in name]
for [not 5-({[(methyl-λ6-sulfanyl)methyl]-λ6-sulfanyl]}methyl)-4-oxa-2λ4,7λ4,9λ6-trithiadecan-1-yl;
read [not 5-({[(methyl-λ6-sulfanyl)methyl]-λ6-sulfanyl}methyl)-4-oxa-2λ4,7λ4,9λ6-trithiadecan-1-yl;
Page 546, P-46.1.10, example 1.
For 5-({[2-(methyl-λ4-sulfanyl)ethyl]-λ4-sulfanyl}methyl)-4-oxa-2λ4,7λ4,9λ6-trithiaundecan-1-yl (preferred prefix)
read 5-({[2-(methyl-λ6-sulfanyl)ethyl]sulfanyl}methyl)-4-oxa-2λ4,7λ4,10-trithiaundecan-1-yl (preferred prefix)
for [not 5-({[(ethyl-λ6-sulfanyl)methyl]-λ4-sulfanyl}methyl)-4-oxa-2λ4,7λ4,10λ4-trithiadecan-1-yl; the locant set for the λ4 atoms ‘2,7,9’ is lower than ‘2,7,10’]
read [not 5-({[(methylsulfanyl)ethyl]-λ4-sulfanyl}methyl)-4-oxa-2λ4,7,10λ6-trithiaundecan-1-yl; the locant set for the nonstandard bonding atoms ‘2,7’ is lower than ‘2,10’]
Replace the structure with:
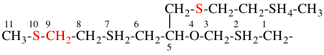
Page 547, P-46.1.11, example 1, right version.
For [not-(hydroxymethyl)ethyl]
read [not 1-(hydroxymethyl)ethyl]
Page 547, P-46.1.11, example 2, left version.
For [not 7-chloro-5-(1,2-dichloropropyl)]octan-2-yl; a complex substituent group)
read [not 7-chloro-5-(1,2-dichloropropyl)octan-2-yl; a complex substituent group]
replace structure with:
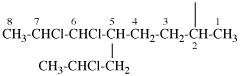
Page 547, P-46.1.11, example 3 left version.
For 2λ5,6-bis(phosphanyl)heptan-4-yl
read (2) 2-(λ5-phosphanyl)-6-phosphanylheptan-4-yl
Page 547, P-46.1.11, example 3 right version.
For 3-(λ5-phosphanyl)-1-(2-phosphanylpropyl)butyl
read (1) 3-(λ5-phosphanyl)-1-(2-phosphanylpropyl)butyl
replace structure with:
Page 547, P-46.1.11, example 4 left version.
For 2,3,5λ5-tris(phosphanyl)heptan-4-yl
read (2) 5-(λ5-phosphanyl)-2,3-bis(phosphanyl)heptan-4-yl
Page 547, P-46.1.11, example 4 right version.
For 2,3-bis(phosphanyl)-1-[1-(λ5-phosphanyl)propyl]butyl
read (1) 2,3-bis(phosphanyl)-1-[1-(λ5-phosphanyl)propyl]butyl
replace the structure with:
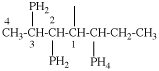
Page 549, P-46.1.12, left example on this page. [corrected 3 January 2020]
Replace the structure with:
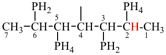
Page 549, P-46.1.12, left example on this page method (2).
For (2) 2λ5,3,5λ5,6-tetakis(phosphanyl)heptan-4-yl
read (2) 2,5-bis(λ5-phosphanyl)-3,6-bis(phosphanyl)heptan-4-yl
for [not 2,5-bis(phosphanyl)-3,6-bis(λ5-phosphanyl)heptan-4-yl;
read [not 3,6-bis(λ5-phosphanyl)-2,5-bis(phosphanyl)heptan-4-yl;
Page 549, P-46.1.12, right example on this page. [corrected 3 January 2020]
Replace the structure with:
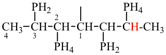 >
>
Page 549, P-46.1.12, right example on this page method (1).
For (1) 2λ5,3-bis(phosphanyl)-1-[1,2λ5-bis(phosphanyl)propyl]butyl
read (1) 2-(λ5-phosphanyl)-3-phosphanyl-1-[2-(λ5-phosphanyl)-1-phosphanylpropyl]butyl
Page 549, P-46.1.13, example 1 right. [corrected 3 January 2020]
For [not 1-(bromoethyl)-2-chloropropyl;
read [not 1-(1-bromoethyl)-2-chloropropyl;
Page 549, P-46.2.1, example 1.
For (1) 3-methyl-4-[2H]butyl (preferred prefix)
read (1) 3-methyl[4-2H1]butyl (preferred prefix)
Page 550, P-46.2.1, example 1 on this page.
For (2) 1-(1-18O)hydroxy-3-hydroxypropan-2-yl (preferred prefix)
read (2) 1-(18O)hydroxy-3-hydroxypropan-2-yl (preferred prefix)
Page 550, P-46.2.2, example.
For (1) 3-[2H]methyl-4-[14C]butyl (preferred prefix)
read (1) 3-[2H1]methyl[4-14C]butyl (preferred prefix)
Page 551, P-46.3.1, example on this page. [modified 22 January 2020]
Add (2) [(2Z,4R,5E)-4-methylhepta-2,5-dien-4-yl]siline (PIN)
(1) {(1R,2Z)-1-[(1E)-prop-1-en-1-yl]-1-methylbut-2-en-1-yl}siline
Replace the structures with:
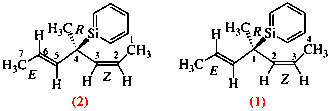
Page 551, P-46.3.2, example 1 method (1).
For (4R)-4-chloro-1-[(3S)-3-chorobutyl]-1-methylpentyl
read (4R)-4-chloro-1-[(3S)-3-chlorobutyl]-1-methylpentyl
Page 551, P-46.3.2, Note lines 4/5. [corrected 3 January 2020]
For ...and a ‘s’ configuration...
read ...and an ‘s’ configuration...
Page 551, P-46.3.2, example 2. [modified 22 January 2020]
Add (2) [(2R,5s,8S)-2,8-dichloro-5-methylnonan-5-yl]trimethylsilane (PIN)
(1) {(1s,4R)-4-chloro-1-[(3S)-3-chlorobutyl]-1-methylpentyl}trimethylsilane
Replace the structures with:
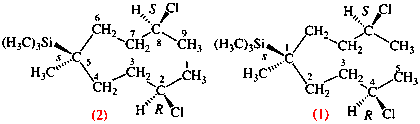
Page 553, P-51.1.1, example. [corrected 3 June 2020]
For 1,2-di(propan-2-ylidene)hydrazine (PIN)
read di(propan-2-ylidene)hydrazine (PIN)
Page 554, p-51.1.5, example. [corrected 3 June 2020]
For 2-[2-(carboxymethoxy)ethoxy]acetic acid (substitutive name)
read [2-(carboxymethoxy)ethoxy]acetic acid (substitutive name)
Page 555, P-51.2.1, example 1.
For N,N-dimethylmethanamine oxide (PIN...
read N,N-dimethylmethanamine N-oxide (PIN...
Page 555, P-51.2.1, example 1. [corrected 3 June 2020]
Add (N,N-dimethylmethanaminiumyl)oxidanide
Page 555, P-51.2.1, example 2. [modified 11 November 2020]
For N-chloromethanimine oxide (PIN...
read N-chloromethanimine N-oxide (PIN...
Add (N-chloromethaniminiumyl)oxidanide
Page 555, P-51.2.1, example 2. [corrected 3 June 2020]
Add (N,N-dimethylmethaniminiumyl)oxidanide
Page 555, P-51.2.1, example 3. [corrected 8.11.2023]
For acetyl chloride (PIN; P-65.5.1.1)
read acetyl chloride (PIN; P-65.5.1)
Page 555, P-51.2.1, example 5.
Replace structure with:
CH3-CH2-CO-CN
Page 556, P-51.2.2, example 3. [corrected 3 June 2020]
For 1,2-di(propan-2-ylidene)hydrazine (PIN)
read di(propan-2-ylidene)hydrazine (PIN)
Page 556, P-51.2.2, example 4. [corrected 24 July 2019]
For N-propylidenehydroxylamine (PIN)
read N-propylidenehydroxylamine
add N-hydroxypropan-1-imine (PIN)
Page 558, P-51.3.1, example 1 on this page. [corrected 29 January 2020]
Replace the structures with:
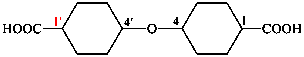
Page 558, P-51.3.1, example 2 on this page.
For 3,3′-[oxybis[(1-chloroethane-2,1-diyl)]oxy]di(propan-1-ol) (PIN...
read 3,3′-{oxybis[(1-chloroethane-2,1-diyl)oxy]}di(propan-1-ol) (PIN...
Page 558, P-51.3.1, example 6 on this page (example 7 of section). [corrected 9 April 2025]
For 2,2′-{ethane-1,2-diylbis[(oxyethane-2,1-diyl)oxy]diacetic acid (a multiplicative name)
read 2,2′-[ethane-1,2-diylbis(oxyethane-2,1-diyloxy)]diacetic acid (a multiplicative name)
Page 559, P-51.3.1, last example. [corrected 7 November 2018]
For 2,2′-{[ethane-1,2-diylbis(oxyethane-2,1-diyloxyethane-2,1-diyl)}dibenzoic acid
read 2,2′-[ethane-1,2-diylbis(oxyethane-2,1-diyloxyethane-2,1-diyl)]dibenzoic acid
Page 560, P-51.3.2.1, example 1.
For 4,4′,4′′-ethane-1,1,2-triyltribenzoic acid (PIN)
read 4,4′,4′′-(ethane-1,1,2-triyl)tribenzoic acid (PIN)
Page 561, P-51.3.2.1, example 1 on this page. [corrected 24 October 2018]
For [not {(triphenylmethoxy)[(triphenylmethyl)sulfanyl]methanediyl}dibenzene];
read [not 1,1′-{(triphenylmethoxy)[(triphenylmethyl)sulfanyl]methylene}dibenzene];
for [not ({diphenyl[(triphenylmethyl)sulfanyl]methoxy}methanetriyl)tribenzene;
read [not 1,1′,1′′-({diphenyl[(triphenylmethyl)sulfanyl]methoxy}methanetriyl)tribenzene;
for PIN is lower alphanumerically (‘diphenyltriphenylmethyl’) is lower...
read PIN is lower alphabetically (‘diphenyltriphenylmethoxy’) is lower...
replace the structure with:
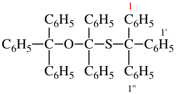
Page 561, P-51.3.2.1, last example.
Replace structure by:
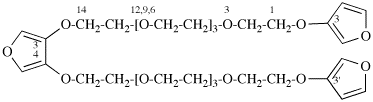
Page 562, P-51.3.2.2, example 1. [modified 29 January 2020]
For 1,1′-oxybis(3,1-phenoxybenzene) (multiplicative name)
read 1,1′-[oxybis(3,1-phenyleneoxy)]dibenzene (multiplicative name)
Delete [not 1,1′-oxybis(3,1-phenyleneoxy)dibenzene (multiplicative name)
Page 562, P-51.3.2.2, example 3. [modified 7 November 2018]
For [not 3,3′-[furan-3,4-diylbis(oxyethane-2,1-diyloxyfuran-4,3-diyloxyethane-2,1-diyloxy]difuran (a multiplicative name)]
read [not 3,3′-[furan-3,4-diylbis(oxyethane-2,1-diyloxyfuran-4,3-diyloxyethane-2,1-diyloxy)]difuran (a multiplicative name)]
for 2,5,7,10,12,15,17,20-octaoxa-1,21(3),6,11,16,21(3,4)-pentafuranahenicosaphane (PIN, a phane name see P-51.4)
read 2,5,7,10,12,15,17,20-octaoxa-1,21(3),6,11,16(3,4)-pentafuranahenicosaphane (PIN, a phane name see P-51.4, P-52.2.5)
Page 563, P-51.3.2.3, example.
For ...; not 1,1′-carbonylbis(diazene-2,1-diyl)]dibenzene; ...
read ...; not 1,1′-[carbonylbis(diazenediyl)]dibenzene; ...
Page 563, P-51.3.3, line 1.
For ...as defined in P-53.3.1, above,...
read ...as defined in P-51.3.1, above,...
Page 563, P-51.3.3, example 2.
For [not (disilanylperoxy)methyl]disilane;
read [not [(disilanylperoxy)methyl]disilane;
Page 564, P-51.4.1.2, example 3. [corrected 29 January 2020]
For 3,6,9,12-tetraoxatetradecane-1,14-dioic acid [PIN,
read 3,6,9,12-tetraoxatetradecanedioic acid [PIN,
for ...a multiplicative name]
read ...(a multiplicative name)
for ...a substitutive name]
read ...(a substitutive name)
Page 565, P-51.4.1.2, last example on this page.
For (not 3,4,6-trioxa-5-silanonanan-7-one;...
read (not 2,2,5,5-tetramethyl-3,4,6-trioxa-5-silanonan-7-one;...
Page 568, P-51.4.2.1, example 1.
Replace structure with:
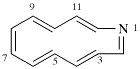
Page 568, P-51.4.2.1, example 2. [modified 29 January 2020]
Delete this correction. These are organic substitutive names and chloro is the substituent name (RB page 138). The error is on page 1044, P-69.4, example 1
Page 569, P-51.4.2.4, example. [modified 29 January 2020]
Replace the structures with:
for 3a1-azaphenalene (PIN)
read 1,3a1,4 -triazaphenalene (PIN)
for 9b-azaphenalene (PIN)
read [not 1,4,9b-triazaphenalene; see P-25.3.3.3]
Page 569, P-51.4.2.4, example replaced printed compound. [corrected 3 December 2025]
For 1,3a1,4-triazaphenylene (PIN)
read 1,3a1,4-triazaphenalene (PIN)
For [not 1,4,9b-triazaphenylene; see P-25.3.3.3]
read [not 1,4,9b-triazaphenalene; see P-25.3.3.3]
Page 569, P-51.4.2.6, example 1.
For 13,23,34-trioxa-11,21,26,31-tercycloundecane (PIN;...
read 13,23,34-trioxa-11,21:26,31-tercycloundecane (PIN;...
for 3,3′,4′-trioxa-1,1′;6′,1′′-tercycloundecane
read 3,3′,4′′-trioxa-1,1′:6′,1′′-tercycloundecane
for 13,23,28,33-ter-1-oxacycloundecane
read 13,23:29,34-ter-1-oxacycloundecane
Page 570, P-51.4.2.6, example on this page.
For 23-thia-12,22,26,32-terbicyclo[2.2.1]heptane (PIN;
read 23-thia-12,22:26,32-terbicyclo[2.2.1]heptane (PIN;
for 3′-thia-1,1′;6′,1′′-terbicyclo[2.2.1]heptane
read 3′-thia-2,2′:6′,2′′-terbicyclo[2.2.1]heptane
Page 570, P-51.5, example 1.
For 2,2′-naphthalene-2,3-diyldiacetic acid (PIN)
read 2,2′-(naphthalene-2,3-diyl)diacetic acid (PIN)
for naphthalene-1,3-diacetic acid
read naphthalene-2,3-diacetic acid
Page 571, P-51.5, example 1 on this page.
For benzene-1,3,5-triyltriacetic acid (PIN)
read 2,2′,2′′-(benzene-1,3,5-triyl)triacetic acid (PIN)
Page 572, P-52.1.3, lines 2/3. [corrected 3 June 2020]
For ...except where 'b' is carbon or nitrogen, are described...
read ...except carbon or halogen, are described...
Page 572, P-52.1.3, example 1. [corrected 20 February 2019]
For [not (distannyloxy)stannane]
read [not bis(stannyloxy)stannane]
Page 572, P-52.1.3, example 4.
For (not tristannazane)
read (not trisilazane)
Page 572, P-52.1.4, example 4.
For SH5
read SH6
Page 573, P-52.1.5.1, example 1. [corrected 27 May 2020]
Delete pentazolane
Page 573, P-52.1.5.1, example 3. [corrected 11 November 2020]
Replace the structure with:
 >
>
Page 574, P-52.1.5.2, line 2 on this page. [corrected 10 October 2018]
For ...alternating heteroatoms, i.e., [aba]n, are...
read ...alternating heteroatoms, i.e., [ab]n, are...
Page 575, P-52.1.6.2, example 2 . [corrected 19 September 2018]
For 2,4,6,8,9,10-hexaoxa-1,3,5,7-tetrasilabicyclo[3.3.1.13,7]decane
read 2,4,6,8,9,10-hexaoxa-1,3,5,7-tetrasilatricyclo[3.3.1.13,7]decane
Page 576, P-52.1.7, example 1 on this page. [modified 11 November 2020]
For 1H,5H-pentarsolopentarsole (preselected name)
read 1H,4H-pentarsolopentarsole (preselected name)
for 1H,5H-octaarsapentalene
read 1H,4H-octaarsapentalene (numbering shown)
replace the structure with:

Page 576, P-52.1.7, example 2. [modified 11 November 2020]
For [1,3,5,2,4,6]triazatriborino[1,2-a][1,3,5,2,4,6]triazatriborine (preselected name)
read [1,3,5,2,4,6]triazatriborinino[1,2-a][1,3,5,2,4,6]triazatriborinine (preselected name)
replace the structure with:
Page 578, P-52.2.4.1, lines 1/2. [corrected 29 January 2020]
For ...having at least two five-membered rings. This...
read ...having at least two rings of at least five or more members. This...
Page 579, P-52.2.4.2, example 1. [corrected 20 February 2019]
For dibenzo[g, p] [1,3,6,9,12,15,18]heptaoxacycloicosine (PIN; see P-25.3.6.1)
read 9H-dibenzo[g, p] [1,3,6,9,12,15,18]heptaoxacycloicosine (PIN; see P-25.3.6.1)
Page 581, P-52.2.4.4.2, last line.
For ...here in P-52.2.4.4.
read ...here in P-52.2.4.4.2.1.
Page 582, P-52.2.4.4.2.2, example 2. [corrected 20 February 2019]
For 4H-9,2b-(epoxymethano)-2-oxacycloocta[cd]pentalene
read 4H-9,2a1-(epoxymethano)-2-oxacycloocta[cd]pentalene
Page 583, P-52.2.4.4.2.2, example 1 on this page. [corrected 20 February 2019]
For 2H-4,7,12-trioxa-1-thia-5,9b-[1,2]epicyclopentacyclopenta[2′,3′:6,7]cyclohepta[cd]pentalene (PIN)
read 2H-4,7,12-trioxa-1-thia-5,9b-[1,2]epicyclopentadicyclopenta[cd,h]azulene (PIN)
for 2H-5,9b-[2,3]furano-4,7-dioxa-1-thiacyclopenta[2′,3′:6,7]cyclohepta[cd]pentalene
read 2H-5,9b-[2,3]furano-4,7-dioxa-1-thiadicyclopenta[cd,h]azulene
Page 584, P-52.2.5.1 (1). [corrected 1 November 2023]
For (1) cyclophanes are cyclic phane structures containing one or more rings or ring systems, at least one ring or ring system of which must be a mancude system attached to adjacent atoms or chains at nonadjacent ring positions;
read
(1) cyclophanes are cyclic phane structures with at least six nodes including two or more rings or ring systems not ortho or ortho and peri-fused to the cyclophane ring, and with at least one ring or ring system of which must be a mancude system;
Page 584, P-52.2.5.2.1, example 2. [corrected 1 November 2023]
For 1(1,3)-benzenacyclopeptadecaphane (PIN; a phane name)
read 1(1,3)-benzenacyclopeptadecaphane (a phane name)
for bicyclo[16.3.1]docosa-1(22),18,20-triene (a von Baeyer name)
read bicyclo[16.3.1]docosa-1(22),18,20-triene (PIN; a von Baeyer name)
add Explanation: Only one ring system, see P-52.2.5.1 (1)
Page 586, P-52.2.5.2.1, example 1 on this page, right version. [corrected 29 May 2019]
For bicyclo[12.4.0]octadeca-(14),15,17-triene
read bicyclo[12.4.0]octadeca-1(14),15,17-triene
Page 586, P-52.2.5.2.1, example 2 on this page, name for (II).
For 5,7,14,16-tetrahydro-1,17:8,10-diethanodibenzo[c,j][1,8]dithiacyclotetradecine
read 5,7,14,16-tetrahydro-1,17:8,10-diethenodibenzo[c,j][1,8]dithiacyclotetradecine
Page 586, P-52.2.5.2.1, structure 3 on this page.
For (I) 3,6-dithia-1(1,7),5(7,1)-dinaphthalenacyclooctaphane (PIN;
read (I) 3,7-dithia-1(1,7),5(7,1)-dinaphthalenacyclooctaphane (PIN;
for (II) 5,7,14,16-tetrahydro-1,17:8,10-diethanodibenzo[c,j][1,8]dithiacyclotetradecine
read (II) 5,7,14,16-tetrahydro-1,17:8,10-diethenodibenzo[c,j][1,8]dithiacyclotetradecine
Page 587, P-52.2.5.3, example 1, second compound. [modified 20 February 2019]
For 3,5-bis([1,1′-biphenyl]-3-yl)pyridine (PIN, substitutive name)
read 3,5-di([1,1′-biphenyl]-3-yl)pyridine (PIN, substitutive name)
Page 587, P-52.2.5.3, example 1.
For 3,5-bis([11,21 :23,31-terphenyl]-13-yl)pyridine (a substitutive name, see P- 28.3.1)
read 3,5-di([11,21:23,31-terphenyl]-13-yl)pyridine (a substitutive name, see P-28.3.1) [delete space in name]
for 3,5-bis([1,1′ :3′,1′′-terphenyl]-3-yl)pyridine (a substitutive name)
read 3,5-di([1,1′:3′,1′′-terphenyl]-3-yl)pyridine (a substitutive name) [delete space in name]
Page 587, P-52.2.5.3, example 2. [modified 20 February 2019]
for (not diphenyl ether)
read diphenyl ether (a functional class name)
Page 588, P-52.2.5.3, example 3. [corrected 7 November 2018]
For 3,3′-[furan-3,4-diylbis(sulfanediylethane-2,1-diylsulfanediyl]difuran
read 3,3′-[furan-3,4-diylbis(sulfanediylethane-2,1-diylsulfanediyl)]difuran
Page 589, P-52.2.6.1, line 9 on this page. [corrected 29 January 2020]
For ...or a C70-D5h(6))[5,6]fullerene derived...
read ...or a (C70-D5h(6))[5,6]fullerene derived...p>
Page 589, P-52.2.6.1 (1) lines 3/4. [corrected 29 January 2020]
For ...and C70-D5h(6))[5,6]fullerene.
read ...and (C70-D5h(6))[5,6]fullerene.
Page 589, P-52.2.6.1 (2) line 4. [corrected 29 January 2020]
For ...and C70-D5h(6))[5,6]fullerene.
read ...and (C70-D5h(6))[5,6]fullerene.
Page 589, P-52.2.6.2, Example 1, structure (II). [corrected 19 September 2018]
For ...33,34,37,38-tricontanor(C60-Ih)[5,6]fullerene (II)
read ...33,34,37,38-triacontanor(C60-Ih)[5,6]fullerene (II)
Page 590, P-52.2.6.2, Example 2. [corrected 29 January 2020]
For [not bis(benzo[1,8]-as-indaceno[3,4,5,6-fghij:3′4′5′6′-lmnoa])cyclopenta[cd]fluoranthene (II)]
read [not bis(benzo[1,8]-as-indaceno[3,4,5,6-fghij:3′,4′,5′,6′-lmnoa])cyclopenta[cd]fluoranthene (II)]
Page 591, P-52.2.6.2, Example 4. [modified 29 January 2020] replace the structures with:
Page 593, P-52.2.6.4, Example 2. [corrected 29 January 2020]
Page 594, P-52.2.6.4, Example 3. [corrected 29 January 2020] for 2,15:3,14-dimethenobenzo[h]cyclododeca[1,2-a:6,7-a′]difluorene (I)(PIN) replace the structure for (I) [previously (II)] with:
Page 595, P-52.2.7.1, example 2 on page. [modified 3 June 2020]
Page 595, P-52.2.7.2, example 1, left hand structure.
Page 596, P-52.2.7.3, example on this page. [corrected 19 September 2018]
Page 596, P-52.2.8, last example. for [not 1,2-phenylenedi(tridecane);
Page 597, P-54.2, example 2. [modified 4 September 2024]
Page 598, P-54.3, example 2. [modified 6 December 2023]
Page 598, P-54.3, example 3. [modified 29 January 2020]
Page 600, P-54.4.2, example 6 on this page.
Page 600, P-54.4.2, example 7 on this page. [corrected 27 May 2020]
Page 601, P-54.4.3.1 box, line 3. [corrected 8.11.2023]
Page 601, P-54.4.3.1, last structure on this page. [corrected 14 November 2018]
Page 603, P-55, paragraph 5 lines 2/3. (corrected 4 December 2019)
Page 604, P-56.2, lines 3/4. [modified 20 February 2019]
Page 604, P-56.2, box line 2.
Page 605, P-56.4, line 3 on this page.
Page 605, P-56.4, last example.
Page 606, P-57.1.1.1, example 4. [corrected 31 October 2018]
Page 607, P-57.1.3, line 3.
Page 607, P-57.1.4, line 2.
Page 608, P-57.1.5.3, example 2. [corrected 19 September 2018]
Page 608, P-57.1.5.3, example 4. for isoquinol-7-yl (also 1-, 3-, 5-, 6-, 7-, and 8- isomers, preferred prefixes)
Page 609, P-57.1.5.3, example 1 on this page.
Page 613, P-57.4, example 1 Explanation lines 3 and 4.
Page 613, P-57.4, example 3. [modified 3 June 2020]
Page 614, P-57.4, example 1 on this page. [corrected 3 June 2020]
Page 614, P-57.4, example 1 on this page, explanation, lines 4/5. [corrected 3 June 2020]
Page 614, P-57.4, example 2 on this page. [corrected 7 November 2018]
Page 615, P-58.1, example 1, Explanation line 1. [corrected 21 August 2019]
Page 615, P-58.1, example 2.
Page 616, P-58.2.1.2, example 4. [corrected 26 May 2021]
Page 617, P-58.2.1.2, example 1 on this page. [corrected 25 August 2016]
Page 619, P-58.2.2.3, example 3. [corrected 19 September 2018]
Page 620, P-58.2.3.1.1, example 1. [corrected 21 November 2018]
Page 620, P-58.2.3.1.1, example 2. [corrected 20 February 2019]
Page 621, P-58.2.3.1.1, example 1 on this page. [corrected 19 September 2018] add [not 1,2,3,4,4a,5,7,11b-octahydro-6H-dibenzo[a,c]cycloheptene-6,6-dicarboxylic acid]
Page 622, P-58.2.3.1.2, example 1. [modified 27 February 2019] for (not 1H,3H,6H-3a,6a-methanocyclopenta[c]furan-1,3-dione; ...
Page 622, P-58.2.3.1.2, example 2. [corrected 29 January 2020]
for 2H,6H’ is lower than ‘2H,8H’)
Page 624, P-58.2.3.1.4, example 2. [corrected 14 November 2018]
Page 625, P-58.2.3.1.4, example 1 on this page, Explanation, lines 2/3. [corrected 7 November 2018]
Page 625, P-58.2.4, example 1.
Page 627, P-58.3.2, example 2. [modified 3 June 2020]
for (not phenyl(tetraazan-1-yl)methanone; nor 1-benzoyltetraazane)
Page 627, P-58.3.2, example 3. [modified 3 June 2020] for (not triazan-1-ylcarbamic acid; the carboxylic acid is senior to the carbonic acid derivative)
Page 627, P-58.3.2, example 7.
Page 627, P-58.3.2, example 8.
Page 627, P-58.3.2, example 9. [corrected 24 March 2021]
Page 628, P-58.3.2, example 1 on this page. [corrected 19 September 2018]
Page 630, P-59.1.9, Table 5.1, column 3, line 12. [corrected 7 November 2018]
Page 630, Table 5.1, Footnote 5. [corrected 20 February 2019]
Page 631, P-59.1.9, paragraph 4, line 9 on this page. [corrected 29 January 2020]
Page 633, P-59.2.1.2, Analysis, line 5. [corrected 26 September 2018]
Page 634, P-59.2.1.3, Example 1 Functionalized parent hydride. [corrected 29 January 2020]
Page 635, P-59.2.1.3, example 3, line 4.
Page 637, P-59.2.1.6, first structure on this page.
Page 639, P-59.2.1.8, third structure on this page.
Page 644, p-59.2.3.3, Example 3. Together with other rules, this analysis leads to the preferred IUPAC name: 3,3a-dihydro-1H-indene-1,4(2H)-dione
Page 645, P-59.2.3.3, example 4 on this page, last line.[corrected 20 February 2019]
Page 654, P-61.2.3, example 3 on this page.
Page 654, P-61.2.3, example 6 on this page.
Page 654, P-61.2.3, example 8 on this page.
Page 655, P-61.2.3, example 1 on this page. [modified 27 February 2019] Delete 1,2,4-tris(3-p-tolylpropyl)benzene
Page 655, P-61.2.3, last example on this page. [corrected 27 February 2019]
Page 656, P-61.2.4, example 2. add 2,6-di(tetraphen-1-yl)pyridine (PIN)
Page 657, P-61.3.1, example 5.
Page 658, P-61.3.1, example 6 on this page. for 1-(4-bromo-1-methylbut-1-en-1-yl)cyclopropane
Page 659, P-61.3.1, examples 2 and 3, I and II. [modified 5 June 2019]. replace the structure of I with: Replace the structure of II with:
Page 660, P-61.3.2.1, example 1. [corrected 27 February 2019]
Page 661, P-61.3.2.2, example 2. [corrected 7 November 2018]
Page 661, P-61.3.2.2, example 1. [corrected 21 April 2021]
Page 662, P-61.4, example 3.
Page 664, P-61.6, example 2.
Page 665, P-61.8, example 3.
Page 665, P-61.9, example 1. [corrected 26 September 2018]
Page 666, P-61.10, example 3. [corrected 24 July 2019] add N-hydroxy-λ2-methanimine (PIN)
Page 670, P-62.2.1.2, example 4. [corrected 19 September 2018]
Page 670, P-62.2.1.2, example 6.
Page 671, P-62.2.1.3, example 2.
Page 671, P-62.2.2.1, paragraph 4.
Page 671, P-62.2.2.1, example 2. for triethlazane
Page 672, P-62.2.2.1, example 4 on this page. [modified 18 September 2019]
Page 673, P-62.2.2.2, example 4 on this page. [corrected 24 June 2020]
Page 673, P-62.2.2.2, example 7 on this page. [modified 26 January 2022]
For N-butylcyclopropan-1-amine (PIN)
Page 674, P-62.2.2.2, example on this page. [corrected 24 June 2020]
Page 675, P-62.2.3, example 2 on this page. (modified 4 March 2020)
Page 675, P-62.2.4.1.1, example 2. [corrected 7 November 2018]
Page 676, P-62.2.4.1.2, example 1. [corrected 23 February 2022)
Page 677, P-62.2.4.1.3, example 2. [corrected 24 October 2018]
Page 678, P-62.2.4.1.3, example 1 on this page. [modified 27 February 2019] delete N1-(2-aminoethyl)-N2-{2-[(aminomethyl)amino]ethyl}ethane-1,2-diamine
Page 678, P-62.2.4.1.3, example 3 on this page. [corrected 27 February 2019]
Page 678, P-62.2.4.1.3, example 4 on this page.
Page 679, P-62.2.4.1.3, example on this page. [corrected 27 February 2019] Add N4-[4-(4-aminoanilino)phenyl]-N1,N1-bis(4-aminophenyl)benzene-1,4-diamine
Page 679, P-62.2.4.1.3, example on this page [corrected 9 April 2025]
Page 679, P-62.2.5.1, line 3.
Page 679, P-62.2.5.1, example 2.
Page 679, P-62.2.5.1, example 1. [corrected 27 February 2019]
Page 680, P-62.2.5.2 (1), example. [modified 5 June 2019] for N,N′-methylenedi(ethan-1-amine)
replace structure with:
Page 681, P-62.2.5.3, example.
Page 682, P-62.2.6.2, example 2 on this page. for (5,6,7,8-tetrahydronaphthalen-2-y l)amine
Page 682, P-62.2.6.2, example 4 on this page. [modified 27 February 2019] for 2,4a-dihydronaphthalen-4a-amine add naphthalene-2,4a(2H)-bis(azane)
Page 683, P-62.3.1.1, example 5 on this page. [modified 27 February 2019] Add N1,N4-dimethylnaphthalene-1,4-diimine (PIN; see also P-16.9.2) for [not N,N′-naphthalene-1,4-lidenebis(methanamine)] for [not N,N′naphthalene-1,4-diylidenebis(methylamine)] for dimethyl(napththalene-1,4-diylidene)bis(amine)] replace the structure with:
Page 684, P-62.3.1.3, example 1.
Page 685, P-62.4, box, lines 3-5. [corrected 24 July 2019]
Page 685, P-62.4, example 1.
Page 685, P-62.4, example 2. add N-hydroxyethanamine (PIN)
Page 685, P-62.4, example 3. [corrected 7 April 2021]
Page 685, P-62.4, example 3. [corrected 5 June 2019]
Page 685, P-62.4, example 4. [modified 21 April 2021]
Page 685, P-62.4, example 5. [modified 21 April 2021]
for (not N,N-dinitromethanamine)
Page 685, P-62.4, example 6. [corrected 21 April 2021]
for (not N-bromosylmethanamine)
Page 697, P-63.1.5, line 9. [corrected 8.11.2023]
Page 687, P-62.5, example 1 on this page. [modified 27 February 2019]
Page 687, P-62.5, example 3 on this page. [modified 7 November 2018] for 2-{3-[(dimethylamino)methyl]phenyl}-N,N-dimethylethane-1-amine N1,N3-dioxide for (3) 2-(3-{[dimethyl(oxido)azaniumyl]methyl}phenyl)-N,N-dimethyl-ethan-1-amine N-oxide (PIN; see also P-74.2.1.2) Replace the structure with:
Page 687, P-62.5, last example.
Page 688, P-62.6.1, example 3. [modified 11 November 2020]
delete (3) ethyldimethylammonium iodide
Page 688, P-62.6.1, example 5. add N-methylanilinium bromide (PIN)
Page 688, P-62.6.1, example 7.
Page 688, P-62.6.1, last example.
Page 689, P-62.6.2, example 2 on this page. [corrected 26 September 2018]
Page 689, P-63.0, lines 5/6. [corrected 27 February 2019]
Page 690, P-63.1, line 12.
Page 692, P-63.1.2, example 4 on this page. [corrected 26 September 2018]
Page 694, P-63.1.2, example 1 on this page. [modified 27 February 2019] For 1,9-dihydro(C60-Ih)[5,6] fulleren-1-ol replace the structure with:
Page 694, P-63.1.2, example 3 on this page.
Page 696, P-63.1.3, example 1. [corrected 27 February 2019]
Page 697, P-63.1.5, line 9. [corrected 8.11.2023]
Page 700, P-63.2.2.1.1, example 2. [corrected 5 June 2019]
Page 700, P-63.2.2.1.1, example 3. [corrected 5 June 2019]
Page 700, P-63.2.2.1.1, example 4. [corrected 5 June 2019]
Page 700, P-63.2.2.1.1, example 5. [corrected 5 June 2019]
Page 701, P-63.2.2.2, last example on this page. [corrected 5 June 2019]
Page 702, P-63.2.2.2, example 1 on this page. [corrected 5 June 2019]
Page 702, P-63.2.3, example 3. [corrected 27 February 2019]
Page 704, P-63.2.4.2, example 3.
Page 705, P-63.2.4.2, example 2 on this page.
Page 706, P-63.2.5.1, example 5. for 1-[(penta-1,4-dien-3-yl)thio)]cyclobutane
Page 706, P-63.2.5.1, example 6.
Page 707, P-63.2.5.1, example 1 on this page. for 1-(propan-2-ylseleno)-2-(propylseleno)propane
Page 707, P-63.2.5.1, example 2 on this page. [corrected 5 July 2018]
for 3-[3-(phenoxyphenyl)sulfanyl]-1-(phenylselanyl)benzene (substitutive name) for (the first substitutive name would be preferred because phenoxy-phenylsulfanyl is lower alphabetically than phenoxyphenyl-sulfanyl)
Page 708, P-63.3.1, example 4. [corrected 24 June 2020]
Page 708, P-63.3.1, last example on this page. [corrected 19 September 2018]
Page 709, P-63.3.1, example 1 on this page. [corrected 7 November 2018]
Page 709, P-63.3.1, example 3 on this page. [corrected 27 February 2019]
Page 709, P-63.3.1, example 4 on this page. [corrected 27 February 2019]
Page 710, P-63.3.2, example 3 on this page. [corrected 7 November 2018]
Page 710, P-63.3.2, example 4 on this page. [corrected 27 February 2019] for [not (methylsufanyl)methyl 2-{[2-(methylsulfanyl)ethyl]sulfanyl}ethanesufenothioate
Page 710, P-63.3.2, example 5. [modified 24 June 2020] for (not 3-(phenylsulfanyl)phenyl 3-(phenyltelluranyl)benzenesulfenothioate)
Page 711, P-63.4.1, example 3.
Page 712, P-63.4.2.1, Table 6.1. for -TeSe-elenotelluroperoxol
Page 713, P-63.4.2.2, example 1.
Page 716, P-63.6, example 1 on page.
Page 716, P-63.6, example 2 on page.
Page 716, P-63.6, last example.
Page 717, P-63.7, example 8 on this page. [modified 7 November 2018] for (not [2-(methoxyethyl)peroxy]ethane;... replace structure with:
Page 717, P-63.7, example 9 on this page. [modified 5 June 2019] replace structure with:
Page 718, P-63.7, example 4 on this page. [corrected 26 September 2018]
Page 719, P-63.8.1, paragraph 3. [modified 1 November 2023]
Page 719, P-63.8.1, example 1. for sodium methoxide
Page 719, P-63.8.1, example 2. for sodium propoxide
Page 719, P-63.8.1, example 4. [modified 9 January 2017] for lithium phenoxide
Page 719, P-63.8.2, example.
Page 720, P-63.8.3, example 1. (corrected 4 March 2020)
Page 722, P-64.1.2.1, example 2 on this page. [corrected 5 July 2018]
Page 722, P-64.1.2.2, example 1. (corrected 4 March 2020)
Page 723, P-64.2.1.1, last example
Page 723, P-64.2.1.2, lines 1/2. [corrected 11 March 2020]
Page 723, P-64.2.1.2 last example on this page. for acetophenone (PIN)
Page 724, P-64.2.1.2, example 1 on this page. [corrected 11 March 2020]
Page 724, P-64.2.1.2, example 2 on this page. [corrected 11 March 2020]
Page 724, P-64.2.1.2, example 3 on this page. [corrected 11 March 2020]
Page 724, P-64.2.1.2, example 5 on this page. [corrected 11 March 2020]
Page 724, P-64.2.1.3, example 1. [corrected 11 March 2020]
Page 724, P-64.2.1.3, example 2. [corrected 11 March 2020]
Page 725, P-64.2.1.3, example 3 on this page. [modified 26 January 2022]
For
Page 725, P-64.2.1.3, example 2 on this page. [corrected 11 March 2020]
Page 725, P-64.2.1.3, example 3 on this page. [modified 11 March 2020] for benzal
Page 725, P-64.2.1.3, example 4 on this page. [modified 11 March 2020]
Page 725, P-64.2.1.3, example 5 on this page. [modified 11 March 2020]
Page 726, P-64.2.2.1, example 2 on this page.
Page 726, P-64.2.2.1, last example on this page.
Page 728, P-64.2.2.2.2, last example. for 1,2,3,6,7,8-hexahydrochrysene-1,3,6,8-tetrone
Page 728, P-64.2.2.2.3, lines1/2. [corrected 11 March 2020]
Page 730, P-64.2.2.3, example 8. [corrected 4 September 2024]
Page 732, P-64.2.2.4, example 3. (modified 4 March 2020)
Page 733, P-64.3.1, example 1 on this page.
Page 733, P-64.3.1, example 4 on page. add 1,3-diazinane-2,4,6-trione (PIN)
Page 733, P-64.3.1, example 5 on page. add 1,3,5-triazinane-2,4,6-trione (PIN)
Page 733, P-64.3.1, last example on page.
Page 734, P-64.3.1, last example.
Page 734, P-64.3.2, example 1.
Page 734, P-64.3.2, example 4.. [corrected 12 May 2021]
Page 736, P-64.4.2, example 1 on this page. for 1-oxo-1λ4-thiophene
Page 736, P-64.4.2, example 2 on this page. for 5,5-dioxo-5λ6-thianthrene
Page 737, P-64.5.1, example 1.
Page 738, P-64.5.2.1, example 1.
Page 738, P-64.5.2.1, example 2.
Page 738, P-64.5.2.1, example 4.
Page 741, P-64.6.2, example 2 on this page. [corrected 7 November 2018] for 1,1′-thiocarbonyldi[pyridin-2(1H)-one]
Page 746, P-65.1.1.2.1, example 1.
Page 747, P-65.1.1.2.2, example 3 on this page.
Page 747, P-65.1.1.2.2, end of list. [corrected 18 September 2019]
Page 748, P-65.1.1.2.4, example 1. [modified 11 March 2020] for but-2-ynoic acid (PIN)
Page 748, P-65.1.1.2.4, example 2. [modified 11 March 2020] for 3-methylpropanoic acid (PIN)
Page 748, P-65.1.1.2.4, example 3. [corrected 11 March 2020]
Page 748, P-65.1.1.2.4, example 4. [corrected 11 March 2020]
Page 748, P-65.1.1.2.4, example 5. [modified 11 March 2020]
for hydroxy(diphenyl)acetic acid (PIN)
Page 748, P-65.1.1.2.4, example 6. Move this example to P-65.1.1.2.2. [modified 3 March 2021]
Add 2,2′,2′′,2′′′-(ethane-1,2-diyldinitrilo)tetraacetic acid
for (HOOC-CH2)2-N-CH2-CH2-N-(CH2-COOH)2
Page 748, P-65.1.1.2.4, example 7. [corrected 11 March 2020]
Page 748, P-65.1.1.2.4, example 8. [corrected 11 March 2020]
Page 749, P-65.1.1.2.4, examples 1-3 on this page. [corrected 7 August 2019]
Page 749, P-65.1.2.1, example 3. [corrected 24 October 2018]
Page 749, P-65.1.2.1
Page 751, P-65.1.2.2.2. example 3 on this page. [corrected 24 October 2018] replace the structure with:
Page 751, P-65.1.2.2.3, example 1.
Page 752, P-65.1.2.2.3, example 1 on this page. [corrected 19 September 2018]
Page 753, P-65.1.2.4, example 6.
Page 754, P-65.1.2.4, example 1 on this page.
Page 754, P-65.1.2.4, example 4 on this page. [corrected 26 September 2018]
Page 754, P-65.1.2.4, example 5. [corrected 6 March 2019]
Page 755, P-65.1.2.4, example 1 on this page. [corrected 6 March 2019]
Page 757 P-65.1.3.1.1, example on this page. [corrected 25 August 2016]
Page 757, P-65.1.3.2.1, example 1. [corrected 10 October 2018]
Page 760, P-65.1.3.4, example 1. [deleted 24 March 2021]
Page 760, P-65.1.3.4, example 2. [deleted 24 March 2021]
Page 761, P-65.1.4.1, example 1.
Page 761, P-65.1.4.1, example 2.
Page 761, P-65.1.4.1, example 4.
Page 763, P-65.1.5.1, example 4. [corrected 9 January 2019]
Page 763, P-65.1.5.1, example 10.
Page 764, P-65.1.5.1, example 2 from top of page.
Page 764, P-65.1.5.1, example 3 from top of page.
Page 764, P-65.1.5.1, example 8 from top of page.
Page 764, P-65.1.5.1, last example.
Page 765, P-65.1.5.2, example 4. [modified 5 June 2019] for H2N-CO-CO-C(S/O)-OH
Page 765, P-65.1.5.2, example 5.
Page 765, P-65.1.5.2, example 8. [corrected 19 September 2018]
Page 765, P-65.1.5.2, example 10.
Page 765, P-65.1.5.2, example 11. [corrected 6 March 2019]
Page 766, P-65.1.5.2, example on this page.
Page 766, P-65.1.5.3, example 5.
Page 767, P-65.1.5.3, example 1 on this page. [corrected 26 September 2018]
Page 767, P-65.1.5.3, example 2 on this page.
Page 767, P-65.1.5.3, example 3 on this page.
Page 767, P-65.1.5.3, last example.
Page 768, P-65.1.6.2, example 1.
Page 768, P-65.1.6.3, example 3. [corrected 18 September 2019]
Page 769, P-65.1.7.1, paragraph 2, line 3.
Page 770, P-65.1.7.2.2, example 3. [corrected 7 November 2018]
Page 770, P-65.1.7.2.2, example 4. [corrected 6 March 2019]
Page 771, P-65.1.7.2.2, example 2 on this page. [corrected 28 October 2016]
Page 771, P-65.1.7.2.3, last example. [corrected 5 June 2019]
Page 772, P-65.1.7.2.4, example 1.
Page 772, P-65.1.7.3.1, line 2. [corrected 25 August 2016]
Page 774, P-65.1.7.3.3, example 3.
Page 774, P-65.1.7.4.1, example 2. [corrected 6 March 2019]
Page 775, P-65.1.7.4.1, example 1 on this page. [corrected 9 January 2019]
Page 775, P-65.1.7.4.2, example 3. replace the structure with:
Page 775, P-65.1.7.4.2, example 4. for 1-methylcyclopentyl(hydrazinylidene)methyl
Page 775, P-65.1.7.4.2, example 6. [corrected 19 September 2018] replace the structure with:
Page 776, P-65.1.7.4.3, example 2. (modified 27 November 2019) for 1,4-phenylenebis(thioxomethylene)
Page 778, P-65.2, example. [corrected 6 March 2019]
Page 778, P-65.2.1.1, example 2.
Page 778, P-65.2.1.1, example 3.
Page 782, P-65.2.1.5, example 4 on this page. [corrected 6 March 2019]
Page 783, P-65.2.1.7, example 4. [corrected 6 March 2019]
Page 784, P-65.2.2, example 1. [corrected 19 September 2018]
Page 785, P-65.2.2, example 1 on this page. [corrected 18 September 2019]
Page 785, P-65.2.2, example 2 on this page. [corrected 6 March 2019]
Page 787, P-65.2.3.1.2.3, example 1.
Page 787, P-65.2.3.1.2.3, example 2. [modified 31 October 2018]
Page 787, P-65.2.3.1.3, example 2. [modified 31 October 2018]
Page 788, P-65.2.3.1.5, example 2. [corrected 7 November 2018]
Page 788, P-65.3.0, example 4. [corrected 19 September 2018]
Page 790, P-65.3.1.3, example 1. [modified 24 June 2020]
Page 790, P-65.3.1.4, line 2. [corrected 10 October 2018]
Page 791, P-65.3.1.4, example 4 on this page. [corrected 26 September 2018]
Page 791, P-65.3.1.5, example 2.
Page 792, P-65.3.2.1, example 4 on this page.
Page 792, P-65.3.2.1, example 5 on this page. [modified 6 March 2019] for sulfanylsulfonodithioyl
Page 797, P-65.5.1.1, example 3.
Page 797, P-65.5.1.1, example 4.
Page 797, P-65.5.1.1, example 6.
Page 798, P-65.5.2.1, example 3. [corrected 31 October 2018]
Page 799, P-65.5.3.2, example 3.
Page 799, P-65.5.4 (2), line 2. (corrected 1 December 2021)
Page 800, P-65.5.4, example 2. [corrected 24 June 2020]
for (2) 2-chlorocarbonylbenzoic acid
Page 800, P-65.5.4, example 4. [modified 24 June 2020]
read (2) 2-(cyanocarbothioyl)benzoyl chloride
replace structure with:
Page 800, P-65.5.4, example 5. [modified 24 June 2020] add [not 4-(2-isocyanato-2-oxoethyl)benzenecarbothioyl cyanide, acetyl is senior to carbothioyl] replace the structure with:
Page 800, P-65.5.4, example 6. [corrected 24 June 2020]
Page 800, P-65.5.4, example 7. [corrected 28 October 2016]
Page 800, P-65.5.4, example 8. [corrected 24 June 2020]
Page 801, P-65.6.2.1, example 2.
Page 802, P-65.6.2.2, example.
Page 802, P-65.6.2.3.1, example 1. [corrected 28 October 2016]
Page 803, P-65.6.2.3.1, example 2 on this page. [corrected 28 October 2016] for (2) antimony tris(hydrogen butanoate)
Page 804, P-65.6.3.2.1, example 2. [corrected 28 October 2016]
Page 804, P-65.6.3.2.2.
Page 805, P-65.6.3.2.2, example 1 on this page. [corrected 11 December 2023]
Page 805, P-65.6.3.2.3, lines 4/5. [corrected 25 August 2016]
Page 805, P-65.6.3.2.3, example 1. (modified 4 March 2020)
Page 805, P-65.6.3.2.3, example 2. [corrected 25 August 2016]
Page 805, P-65.6.3.2.3, first example.
Page 806, P-65.6.3.2.3, last example. for 3-[(cyclohexanesulfinyl)sulfanyl]propanenitrile (PIN) for 3-[(cyclohexylsulfinyl)sulfanyl]propanenitrile; see P-65.4.1 for naming acyl groups derived from acids]
Page 807 P-65.6.3.3.1, example 2.
Page 808, P-65.6.3.3.2.1, last example. [corrected 25 August 2016]
Page 809, P-65.3.3.2.2.1 (2), lines 2/3. [corrected 28 May 2024]
Page 809, P-65.6.3.3.2.2.1, example 1. [corrected 28 May 2024]
Page 809, P-65.6.3.3.2.2.1, example 3. [modified 18 December 2023]
Page 809, P-65.6.3.3.2.2.1, example 3. [corrected 28 May 2024]
Page 809, P-65.6.3.3.2.2.1, example 3. [corrected 7 November 2018]
Page 809, P-65.6.3.3.2.2.1, example 3. [modified 9 October 2024]
Page 809, P-65.6.3.3.2.2.1, example 3. [corrected 7 November 2018]
Page 810, P-65.6.3.3.2.2.2, example. Replace the structure with:
Page 810, P-65.3.3.3.1, example 4. [modified 24 June 2020]
Page 811, P-65.6.3.3.3.2, example 3.
Page 811, P-65.6.3.3.3.2, last example. [corrected 28 October 2016] for (2S)-2-(acetyloxy)-3-(hexadecanoyloxy)propyl (9Z)-octadec-9-enoate replace structure with:
Page 812, P-65.6.3.3.4.1, example. [corrected 18 December 2023]
Page 812, P-65.6.3.3.4.1, example. [corrected 28 May 2024]
Page 812, P-65.6.3.3.4.2, example 1. [corrected 6 March 2019]
Page 812, P-65.6.3.3.4.2, example 2.
Page 813, P-65.6.3.3.4.2, example 1 on this page.
Page 813, P-65.6.3.3.4.2, example 2 on this page. (modified 4 March 2020)
Page 813, P-65.6.3.3.4.2, example 3 on this page. [corrected 6 March 2019]
Page 813, P-65.6.3.3.4.2, example 4 on this page. [corrected 5 July 2018]
Page 813, P-65.6.3.3.4.2, example 5 on this page.
Page 813, P-65.6.3.3.4.2, replace structure of example 5 on this page. [corrected 26 January 2022]
Page 814, P-65.6.3.3.4.2, example 1 on this page (example 10 of this section). [corrected 9 April 2025]
Page 814, P-65.6.3.3.4.2, example 2 on this page. [corrected 26 September 2018]
Page 814, P-65.6.3.3.4.2, example 4 on this page. [corrected 19 September 2018]
Page 814, P-65.6.3.3.4.2, example 5 on this page. [corrected 28 October 2016]
Page 814, P-65.6.3.3.4.2, example 6 on this page. [corrected 28 October 2016]
Page 815, P-65.6.3.3.4.3, example 2. [corrected 28 October 2016]
Page 815, P-65.6.3.3.4.3, example 3. [corrected 28 October 2016] For dimethyl propanedioylbis(oxyethane-2,1-diyloxymethylene dipropanedioate) add dimethyl 3,10,12,19-tetraoxo-4,6,9,13,16,18-hexaoxahenicosane-1,21-dioate (PIN) replace the structure with:
Page 815, P-65.6.3.3.4.3, example 4. [corrected 28 October 2016] add dimethyl 3,7,9,13,16,21-hexaoxo-4,6,10,12,17,20-hexaoxatricosane-1,23-dioate (PIN) replace the structure with:
Page 816, P-65.6.3.3.4.4, example 1.
Page 816, P-65.6.3.3.5, example 2. [corrected 24 October 2018]
Page 817, P-65.6.3.3.5, example 1 on this page. (modified 4 March 2020) for (2) potassium 1-ethyl hydrogen 2-hydroxypropane-1,2,3-tricarboxylate for (2) potassium 5-ethyl hydrogen citrate
add (the anionic –OOC– group is preferred to the ester group CH3-CH2-O-CO–)
Page 817, P-65.6.3.3.5, example 2 on this page. replace the structure with:
Page 817, P-65.6.3.3.5, example 3 on this page.
Page 819, P-65.6.3.3.7.1, example 6 on this page. [corrected 24 June 2020]
Page 819, P-65.6.3.3.7.1, example 8 on this page. [corrected 24 October 2018]
Page 819, P-65.6.3.3.7.1, example 9 on this page. [corrected 24 October 2018]
Page 819, P-65.6.3.3.7.1, last example on page.
Page 820, P-65.6.3.3.7.1, example on this page. [corrected 28 October 2016] for [not methyl 2-{4-[(2-methoxy-2-sulfanylideneacetyl)oxy]phenoxy}-2-sulfanylideneacetate; the PIN is lower in alphanumerical order) Add 4-{[methoxy(oxo)ethanethioyl]oxy}phenyl methoxy(sulfanylidene)acetate (PIN)
Page 820, P-65.6.3.3.7.2.1, example 1.
Page 820, P-65.6.3.3.7.2.2, example 1.
Page 820, P-65.6.3.3.7.2.2, example 2.
Page 821, P-65.6.3.4.1, example 5.
Page 821, P-65.6.3.4.1, example 7. [corrected 24 July 2019] add 1-[(dimethylamino)oxy]ethan-1-one (PIN)
Page 821, P-65.6.3.4.2, example. for (not S-methylperoxyl propanethioate
Page 822, P-65.6.3.5.1, example 2. for dodecano-12-lactone
Page 823, P-65.6.3.5.1, example 2 on this page. [corrected 6 March 2019]
Page 824, P-65.6.3.5.2, example 1.
Page 824, P-65.6.3.5.2, example 3.
Page 824, P-65.6.3.5.2, example 3. [corrected 9 January 2019]
Page 825, P-65.6.3.5.4, example 3. [corrected 24 June 2020]
Page 825, P-65.6.3.5.4, example 4.
Page 826, P-65.6.3.5.4, example on this page.
Page 827, P-65.7.1, example 7 (return to how printed). [Corrected 8 October 2025]
Page 827, P-65.7.1, example 8. [modified 9 January 2019] for 2-chloroethane-1-sulfinic anhydride (PIN)
add bis(2-chloroethane-1-sulfinic) anhydride (PIN)
Page 828, P-65.7.1, example 8 (return to how printed). [Corrected 8 October 2025]
Page 828, P-65.7.3, example 3 (return to how printed). [Corrected 8 October 2025]
Page 829, P-65.7.4, example 2.
Page 830, P-65.7.5.1, example 1. [modified 6 March 2019] Replace the structure with:
Page 830, P-65.7.5.1, example 3. [corrected 6 March 2019]
Page 830, P-65.7.5.1, example 4.
Page 831, P-65.7.6.2, last example on this page.
Page 832, P-65.7.6.3, example 2. [corrected 31 October 2018]
Page 832, P-65.7.6.3, example 3. [corrected 16 June 2021]
Page 833, P-65.7.6.3, example 1 on this page. [corrected 31 October 2018]
Page 833, P-65.7.6.4.2, example 1.
Page 834, P-65.7.6.4.3, example 3.
Page 834, P-65.7.6.4.3, example 4.
Page 837, P-65.7.7.3 (2), line 1/2. [corrected 6 March 2019]
Page 837, P-65.7.7.3, example 1. [corrected 6 March 2019] for hexahydro-1H-benzo[c]thiophene-1,3(4H)-dione
Page 838, P-65.7.7.3, example 1 on this page. [corrected 19 September 2018]
Page 838, P-65.7.7.3, example 3 on this page. [corrected 6 March 2019] for (2) 2-benzothiophene-5,6-dicarboxylic anhydride. for isobenzothiofuran-5,6-dicarboxylic anhydride
Page 838, P-65.7.7.3, example 4 on this page. (modified 4 March 2020)
Page 838-839, P-65.7.8. [Corrected 8 October 2025]
Page 840, P-66.1.0, lines 3-5. [corrected 26 January 2022]
Page 841, P-66.1.1.1.1.3, example 3.
Page 843, P-66.1.1.3.1.1, line 6. [corrected 13 March 2019]
Page 844, P-66.1.1.3.1.1, example 1. [corrected 26 January 2022]
Delete dimethylformamide
Page 844, P-66.1.1.3.1.1, example 2. [corrected 10 October 2018]
Page 844, P-66.1.1.3.1.1, example 3. [corrected 26 September 2018]
Page 844, P-66.1.1.3.1.1, example 5. [corrected 19 September 2018]
Page 845, P-66.1.1.3.2, lines 2/3. [corrected 24 February 2021]
Page 845, P-66.1.1.3.1.2, example 2.
Page 845, P-66.1.1.3.2, line 3. [modified 24 March 2021]
Page 845, P-66.1.1.3.3, example 1.
Page 847, P-66.1.1.3.5, example 2. [corrected 26 September 2018]
Page 847, P-66.1.1.3.5, last example.
Page 850, P-66.1.1.4.2, examples 1 and 4.
Page 851, P-66.1.1.4.3, example 4.
Page 851, P-66.1.1.4.3, example 5.
Page 851, P-66.1.1.4.3, example 6. for (2) [N-(cyclopropylmethyl)sulfonamido]acetic acid
Page 853, P-66.1.1.4.5.1, example. [corrected 13 March 2019]
Page 853, P-66.1.1.4.5.2, example. for 2,2′-[oxalylbis(azanediyl)]diacetonitrile (PIN) for 2,2′-[ethanedioylbis(azanediyl)]diacetonitrile
Page 854, P-66.1.2.1, example 2. [corrected 26 September 2018]
Page 854, P-66.1.2.1, example 5.
Page 855, P-66.1.3, example 2. for (not 1-propionyl-1,2,3,4-tetrahydroquinoline)
Page 857, P-66.1.4.2, example 1. for N-thioacetylthioacetamide
Page 857, P-66.1.4.2, example 2. for N-cyclohexyl-N-thioacetylthioacetamide
Page 857, P-66.1.4.2, example 3.
Page 857, P-66.1.4.3, example. for not 1-ethanethioylpyrrolidine
Page 858, P-66.1.4.4, example 1 on this page. for 4-(ethanethioylamino)benzamide
Page 858, P-66.1.4.4, example 2 on this page.
Page 858, P-66.1.4.4, last example.
Page 859, P-66.1.5.2.1, example 1.
Page 860, P-66.1.6.1.1.1, line 1. [corrected 26 January 2022]
Page 861, P-66.1.6.1.1.2, example 1. [corrected 24 October 2018]
Page 862, P-66.1.6.1.1.3, example 4 on this page. for N-(aminocarbonyl)benzene-1-sulfonamide
Page 863, P-66.1.6.1.1.5, example 2. [corrected 13 March 2019]
Page 864, P-66.1.6.1.2.2, line 1.
Page 864, P-66.1.6.1.3.1, structures 1 and 2. [modified 7 August 2019] for thiourea replace structures with:
Page 864, P-66.1.6.1.3.1, example.
Page 865, P-66.1.6.1.3.2, example 2 (third structure on page).
Page 865, P-66.1.6.1.3.2, example 4.
Page 865, P-66.1.6.1.3.3, example bottom of page.
Page 866, P-66.1.6.1.4, example 3. [corrected 13 March 2019]
Page 867, P-66.1.6.1.4, example 1 on this page.
Page 867, P-66.1.6.3, example 1. [corrected 23 February 2022)
Page 868, P-66.1.7, example 2. [modified 23 December 2020]
for N-{[({[(acetylamino)methyl]nitroamino}methyl)nitroamino]methyl}prop-2-enamide
add N-[({[(acetamidomethyl)nitramido]methyl}nitramido)methyl]prop-2-enamide (PIN)
Page 869, P-66.2.1, example 4. add hexahydro-1H-isoindole-1,3(2H)-dione (PIN)
Page 869, P-66.2.1, example 5. add 2-phenyl-1H-isoindole-1,3(2H)-dione (PIN)
Page 870, P-66.2.2, example 1 on this page. [corrected 19 September 2018]
Page 871, P-66.3.1.1, box, line 4. [corrected 26 September 2018]
Page 872, P-66.3.1.1, example on this page.
Page 875, P-66.3.2.2, example 1. [corrected 24 October 2018]
Page 875, P-66.3.2.3 (1), line 3. [corrected 13 March 2019]
Page 875, P-66.3.2.3, example 1. [corrected 7 August 2019]
Page 877, P-66.3.3.2, example 2. [modified 11 March 2020]
Page 878, P-66.3.4, example 2.
Page 879, P-66.3.5.2, example 1. [corrected 7 November 2018]
Page 879, P-66.3.5.2, example 2. [corrected 7 November 2018]
Page 879, P-66.3.5.3, example 2.
Page 879, P-66.3.5.3, example 3. for {[2-(hydrazinylcarbonyl)hydrazine-1-yl}acetic acid
Page 879, P-66.3.5.3, example 4.
Page 880, P-66.4.1, structure 1.
Page 881, P-66.4.1.1, example 1. [corrected 13 March 2019]
Page 883, P-66.4.1.1, example on this page. replace structure with:
Page 883, P-66.4.1.2.1.2, example 2. [corrected 24 October 2018]
Page 884, P-66.4.1.2.1.3, example 1.
Page 884, P-66.4.1.2.1.3, example 5. [corrected 13 March 2019]
Page 884, P-66.4.1.2.1.3, example 6. [corrected 24 October 2018]
Page 885, P-66.4.1.2.2, structure 2. [corrected 24 October 2018]
Page 885, P-66.4.1.2.2, example 3. Replace structure with:
Page 885, P-66.4.1.2.2, example 4.
Page 888, P-66.4.1.4.1, example 1 on this page.
Page 888, P-66.4.1.4.1, example 2 on this page. for (not N′-ethyl-N -methylbenzene-1-carboxamidine)
Page 888, P-66.4.1.4.1, example 3 on this page. for (not N-phenylbenzene-1-carboxamidine;
Page 889, P-66.4.1.4.2, example 1.
Page 889, P-66.4.1.5, example 1. [modified 13 March 2019] for ...; not N1-ethyl-N2-methyl-α,α′-dithiobisformamidine...
Page 889, P-66.4.1.4.2, example 2. Replace structure with:
Page 891, P-66.4.2.1, example 7 on this page.
Page 891, P-66.4.2.1, example 8 on this page.
Page 892, P-66.4.2.1, example 1 on this page.
Page 893, P-66.4.2.2, example 1 on this page. for (carbamohydrazonoyloxy)methanimidohydrazide for (hydrazinecarboximidoyloxy)formohydrazonamide
Page 893, P-66.4.2.3.1, example 1. for hydrazinecarboximidoylacetic acid
Page 893, P-66.4.2.3.1, example 2.
Page 894, P-66.4.2.3.3, lines 2/3. [corrected 1 July 2020]
Page 894, P-66.4.2.3.3, example 2. for 3-(methanehydrazonoylamino)propanenitrile for 3-[(hydrazinylidenemethyl)amino]propanenitrile
Page 894, P-66.4.2.3.4, lines 1-2. [corrected 13 March 2019]
Page 894, P-66.4.2.3.4, line 3. [corrected 13 March 2019]
Page 894, P-66.4.2.3.4, line 5. [corrected 1 July 2020]
Page 894, P-66.4.2.3.4, example. [modified 13 March 2019] replace the structure with:
Page 894, P-66.4.2.3.5, example 1. for
3-(ethanehydrazonoylamino)propanoic acid
Page 895, P-66.4.2.3.5, example on this page. for 4-(benzenesulfinohydrazonoylamino)benzoic acid
Page 895, P-66.4.2.3.6, example 1. for 3-(2-methanimidoylhydrazin-1-yl)propanoic acid
Page 895, P-66.4.2.3.6, example 2. [corrected 10 October 2018] for methyl (2-ethanimidoylhydrazin-1-yl)acetate replace the structure with:
Page 895, P-66.4.2.3.6, example 3.
Page 896, P-66.4.3.1, example 5. [corrected 26 September 2018]
Page 897, P-66.4.3.1, example 1 on this page.
Page 897, P-66.4.3.1, example 3 on this page.
Page 898, P-66.4.3.3, example 1. [modified 3 March 2021]
Page 898, P-66.4.3.4.1, example 1. for [C-(hydrazinylcarbonohydrazonoyl)]acetic acid
Page 898, P-66.4.3.4.1, example 2. for 3-[hydrazinyl(hydrazanylidene)methyl]benzoic acid
Page 898, P-66.4.3.4.1, example 3. for 4-{2-[(carbamimidoyloxy)methanimidoyl]hydrazin-1-yl}benzoic acid
Page 899, P-66.4.3.4.2, example. [modified 1 July 2020]
for [(hydrazinylmethylidene)hydrazinyl]acetic acid
Page 900, P-66.5.0, lines 6-8. [corrected 26 January 2022]
Page 900, P-66.5.0, line 6.
Page 902, P-66.5.1.2.1, example 3, second line.
Page 904, P-66.5.2, example 1. [corrected 5 July 2018]
Page 904, P-66.5.2 example 6.
Page 905, P-66.5.3.1, example 1 and 2.
Page 905, P-66.5.3.1, last example.
Page 906, P-66.5.4.2, example. add (not methyl 4-[(oxo-λ5-azanylidyne)methyl]benzoate) replace structure with:
Page 906, P-66.5.4.2, new example. add structure:
Page 907, P-66.6.1.1.2, example.
Page 908, P-66.6.1.2.1, example 3.
Page 908, P-66.6.1.2.1, example 4. [corrected 7 August 2019]
Page 909, P-66.6.1.3, example 1 on his page.
Page 909, P-66.6.1.3, example 2 on this page.
Page 909, P-66.6.2, example 1. [corrected 26 January 2022]
Page 909, P-66.6.2, example 2. [modified 26 January 2022]
Page 910, P-66.6.3, example 5.
Page 913, P-66.6.5.1.2, example on this page. [corrected 30 March 2022]
Page 913, P-66.6.5.3, example 1.
Page 917, P-67.1.1.1, line 1 on this page.
Page 917, P-67.1.1.1, line 12 on ths page.
Page 919, P-67.1.1.2, example 1 on this page.
Page 919, P-67.1.2.1, example 4.
Page 919, P-67.1.2.1, example 5. [corrected 25 August 2016]
Page 921, P-67.1.2.3.1, example (3). [corrected 25 August 2016]
Page 922, P-67.1.2.3.2. [corrected 1 November 2023]
Page 922, P-67.1.2.3.4. [corrected 1 November 2023]
Page 923, P-67.1.2.4.1.1, example 5. [corrected 9 April 2025]
Page 923, P-67.1.2.4.1.1, example 9.
Page 923, P-67.1.2.4.1.1, example 9. (corrected 4 December 2019)
Page 924, P-67.1.2.4.1.1, example 1 on this page. [modified 25 November 2020]
Page 924, P-67.1.2.4.1.1, example 2 on this page,
Page 924, P-67.1.2.4.1.1, example 7 on this page. [corrected 25 August 2016]
Page 924, P-67.1.2.4.1.1, example 12 on this page. [modified 20 March 2019]
Page 924, P-67.1.2.4.1.1, example 17 on this page. [modified 20 March 2019] For HO-SO-NCS
Page 925, P-67.1.2.4.1.3, example 1. [corrected 25 August 2016, modified 10 October 2018] for CH3-P(OCN)OH
Page 925, P-67.1.2.4.2, line 1.
Page 925, P-67.1.2.4.2, lines 4-6.
Page 926, P-67.1.2.5.1, example 11. Correction deleted [11 November 2020]
Page 927, P-67.1.2.5.1, example 1 on this page. [corrected 26 September 2018]
Page 927, P-67.1.2.5.2, example 1. [corrected 30 March 2022]
Page 927, P-67.1.2.5.2, example 2.
Page 927, P-67.1.2.5.2, example 3, (with last two names under example 2). add structure: SiCl4
Page 927, P-67.1.2.6.1 (b), box.
Page 928, P-67.1.2.6.1, example 5.
Page 929, P-67.1.2.6.1, example 1 on this page. [corrected 25 August 2016]
Page 929, P-67.1.2.6.2, example 3.
Page 929, P-67.1.2.6.2, example 5.
Page 929, P-67.1.2.6.2, last example.
Page 930, P-67.1.2.6.3, example 1. [corrected 24 March 2021]
Page 930, P-67.1.2.6.3, example 2. [corrected 24 March 2021]
Page 930, P-67.1.2.6.3, example 3. [corrected 24 March 2021]
Page 930, P-67.1.2.6.3, example 4. [corrected 24 March 2021]
Page 932, P-67.1.3.2, example 1 on this page.
Page 932, P-67.1.3.2, example 4 on this page. [corrected 16 August 2016]
Page 932, P-67.1.3.2, example 15 on this page. [corrected 7 November 2018]
Page 933, P-67.1.3.2, example 1 on this page.
Page 933, P-67.1.3.2, example 2 on this page.
Page 935, P-67.1.4.1.1.2, examples 5-7. for –NH2(O)< azinoyl for –PH(O)< phosphonoyl
Page 936, P-67.1.4.1.1.2, examples 1-5 on this page. for –AsH(O)< arsonoyl for –AsH2(O)< arsinoyl for –SbH(O)< stibonoyl for –SbH2(O)< stibinoyl
Page 937, P-67.1.4.1.1.4, example 3, right version. [corrected 20 March 2019]
Page 938, P-67.1.4.1.1.4, example 5 left on this page. (corrected 27 November 2019)
Page 938, P-67.1.4.1.1.4, example 5 right on this page. (modified 27 November 2019)
Page 941, P-67.1.4.1.3, example 1.
Page 941, P-67.1.4.2, example 2. [corrected 25 August 2016]
Page 943, P-67.1.4.3.2 example 6.
Page 943, P-67.1.4.3.3, line 3. [corrected 25 August 2016]
Page 944, P-67.1.4.4.1, example 10. (corrected 4 December 2019)
Page 945, P-67.1.4.4.2, example 4. [corrected 25 August 2016]
Page 947, P-67.1.5.1, example 2 on this page. [modified 22 April 2020] for N-[tris(phenylamino)phosphoranylidene]benzenesulfonamide For (N,N′,N′′-triphenyl-N′′′-benzenesulfonylphosphorimidic triamide;
for nor N′′′-benzenesulfonylphosphorimimidic tris(phenylamide)
Page 948, P-67.1.6, example 1 on this page. add cyano-N,N-dimethylmethanamine N-oxide (PIN; see P-62.5)
Page 949, P-67.2.1, example 8. [corrected 25 August 2016]
Page 949, P-67.2.1, example 10. [corrected 25 August 2016]
Page 949, P-67.2.1, last example on this page. [corrected 25 August 2016]
Page 950, P-67.2.1, example 2 on this page. [corrected 25 August 2016]
Page 950, P-67.2.1, lines 16/17.
Page 952, P-67.2.2.2, example 1 [corrected 25 August 2016]
Page 952, P-67.2.2.2, example 4. [modified 20 March 2019] for ...name diphosphoric acid...
Page 954, P-67.2.2.2, example 1 on this page. and replace structure with:
Page 955, P-67.2.3, example 5. [corrected 25 August 2016] Replace the structure with:
Page 957, P-67.2.4, example 3 on this page. [corrected 25 August 2016]
Page 958, P-67.2.4, example 2 on this page. [corrected 25 August 2016]
Page 959, P-67.2.5.1.1, example 1. and replace structure with:
Page 959, P-67.2.5.2, example 4. [corrected 11 December 2023]
Page 960, P-67.2.6, example 1.
Page 960, P-67.2.6, example 2. [corrected 25 August 2016]
Page 961, P-67.2.6, example on this page. [modified 20 March 2019]
for (2) 3-(5-methyl-2,4-sulfanylidene-2λ4,4λ4-pentasulfanyl)propanoic acid
Page 961, P-67.3.1, lines 9-13. [corrected 20 March 2019]
Page 962, P-67.3.1, example 5 on this page. [corrected 25 August 2016]
Page 962, P-67.3.1, example 6 on this page. [corrected 25 August 2016]
Page 963, P-67.3.1, example 1 on this page.
Page 963, P-67.3.2, box last line.
Page 963, P-67.3.2, example 1. replace structure with:
Page 963, P-67.3.2, example 2. replace structure with:
Page 963, P-67.3.2, example 3.
Page 964, P-68.0, line 17. [corrected 28 October 2016]
Page 968, P-68.1.1.3.2, example 1.
Page 968, P-68.1.1.3.2, example 8 on this page. [modified 11 November 2020]
for (not 1,3,5-trioxa-2,4,6-cyclotriboroxane)
Page 969, P-68.1.1.3.2.2 (to be P-68.1.1.3.3), example 2. [corrected 16 August 2016]
Page 970, P-68.1.2, example 10.
Page 970, P-68.1.2, example 15. [corrected 20 March 2019]
Page 971, P-68.1.3, example 2.
Page 972, P-68.1.4.1, example 4 on this page. [modified 20 March 2019] for (not N,N-ethane-1,2-diylbis(diphenylborinic amide); for not N,N’-ethane-1,2-diylbis(diphenylboranamine); replace the structure with:
Page 973, P-68.1.5, suheadings. for P-69.1.5.2 Prefix nomenclature
Page 973, P-68.1.5.1, example 3. [corrected 11 March 2020]
Page 974, P-68.1.5.2.1, example 6.
Page 974, P-68.5.2.1, example 7. [modified 11 March 2020] for O,O′-[(ethane-1,2-diylbis(oxymethylene))bis(dimethylborane)]bis(N,N-diethylhydroxylamine)
add N,N′-[ethane-1,2-diylbis(boranediyloxy)]bis(N-ethylethanamine) (PIN)
Page 977, P-68.1.5.2.3, example 1 on this page.
Page 977, P-68.1.5.2.3, example 2 on this page.
Page 977, P-68.1.5.2.3, example 3 on this page. replace structure with:
Page 977, P-68.1.5.2.3, last example on this page.
Page 978, P-68.1.5.2.3, example 1 on this page.
Page 978, P-68.1.5.2.3, example 4 on this page. [corrected 30 Mach 2022]
Page 981, P-68.1.6.2 (formerly P-68.1.6.1.1), example 3 on this page. [corrected 28 October 2016]
Page 981, P-68.1.6.2 (formerly P-68.1.6.1.1), example 4 on this page. [modified 11 September 2919]
Page 981, P-68.1.6.2 (formerly P-68.1.6.1.1), example 5 on this page. [corrected 20 March 2019]
Page 982. P-68.1.6.2 (formerly P-68.1.6.1.1), example 1 on this page. [corrected 11 September 2919]
Page 982, P-68.1.6.2 (formerly P-68.1.6.1.1), example 2 on this page. [modified 20 March 2019] delete 1,1,3,3-tetramethylguanidine(N2—Ga)gallane (1/ 1) for trihydrido[1,1,3,3-tetramethylguanidine-κN2]gallium
Page 982, P-68.1.6.3 (formerly P-68.1.6.1.2), example 1.
Page 982, P-68.1.6.3 (formerly P-68.1.6.1.2), example 2.
Page 983, P-68.1.6.3 (formerly P-68.1.6.1.2), example 3 on this page.. [modified 12 May 2021]
add (N3—B)[2-(1H-1,3-benzimidazol-2-yl)phenyl]boronic acid
for [2-(1H-benzimidazol-2-yl-κN3)benzenido-κC1]dihydroxidoboron
add [2-(1H-1,3-benzimidazol-2-yl-κN3)benzen-1-ido-κC1]dihydroxidoboron
Page 983, P-68.1.6.3 (formerly P-68.1.6.1.2), example 1 on this page. [corrected 28 October 2016]
Page 983, P-68.1.6.3 (formerly P-68.1.6.1.2), example 2 on this page. [corrected 28 October 2016] for difluorido(N-nitroso-κO-N-oxido-kO-methanamine)boron
Page 983, P-68.1.6.3 (formerly P-68.1.6.1.2), example 3 on this page. for [2-(1H-benzimidazol-2-yl-κN 3)benzenido-κC1]dihydoxidoboron
Page 984, P-68.1.6.3 (was P-68.1.6.1.2), example 2 on this page (example 8 of this section). [modified 9 April 2025]
Page 985, P-68.2.1.1, example 3. [corrected 28 October 2016]
Page 986, P-68.2.1.2, example 4.
Page 987, P-68.2.2, example 17 on this page.
Page 988, P-68.2.3, lines 1/2. [corrected 28 October 2016]
Page 989, P-68.2.4, last example.
Page 989, P-68.2.5, example 1.
Page 989, P-68.2.5, example 2.
Page 989, P-68.2.5, example 7. [corrected 18 September 2019] replace the structure with:
Page 989, P-68.2.5, last example on this page. [corrected 28 October 2016]
Page 990, P-68.2.5, example 1 on this page. for dibutyl[(3-methoxy-3-oxopropanoyl)-3-oxy]stannane
Page 990, P-68.2.5, last example. for hydroxybis[(trimethylsilyl)methyl]stanane
Page 990 P-68.2.6.1, example 1.
Page 991 P-68.2.6.2, example 1.
Page 991, P-68.2.6.1, example 2 on this page. [corrected 5 June 2019]
Page 992, P-68.2.6.2, example 1 on this page. [modified 1 July 2020]
Page 992, P-68.2.6.2, example 2 on this page.
Page 992, P-68.2.6.2, example 3 on this page.
Page 992, P-68.2.6.2, last example. add bis[bis(trimethylsilyl)methyl]stannanol (PIN) for (Si preferred to Sn)
Page 992, P-68.3, line 3.
Page 992, P-68.3 (1) (formerly P-68.3.1), line 5. [corrected 31 July 2019]
Page 992, P-68.3, line 9.
Page 992, P-68.3, line 13.
Page 993, P-68.3.1.1.1.1, example 1.
Page 993, P-68.3.1.1.1.1, example 2.
Page 994, P-62.3.1.1.1.1, example 3 on this page. [corrected 24 July 2019]
Page 994, P-68.3.1.1.1.2, example 3.
Page 994, P-68.3.1.1.1.2, example 4. [modified 3 March 2021]
for (not azanyl acetate)
add aminooxy(phenyl)methanone (PIN)
Page 994, P-68.3.1.1.1.2, last example on this page. [corrected 28 October 2016]
Page 995, P-68.3.1.1.1.3, example 1.
Page 995, P-68.3.1.1.1.3, example 3.
Page 996, P-68.3.1.1.1.5, example 2.
Page 997, P-68.3.1.1.2, lines 1/2 on this page. [corrected 11 December 2019]
Page 997, P-68.3.1.1.2, box, line 2. [corrected 11 December 2019]
Page 997, P-68.3.1.1.2, example 1. [corrected 24 July 2019] add N-hydroxypentan-2-imine (PIN)
Page 997, P-68.3.1.1.2, example 2. [corrected 26 September 2018]
Page 997, P-68.3.1.1.2, example 3. [corrected 24 July 2019] add N2,N3-dihydroxybutane-2,3-diimine (PIN)
Page 997, P-68.3.1.1.2, example 6.
Page 998, P-68.3.1.1.2, example on this page.
Page 998, P-68.3.1.1.3, example 1. [corrected 24 July 2019] add N-hydroxy-1-nitropropan-1-imine (PIN)
Page 998, P-68.3.1.1.3, example 2. [modified 4 September 2019] add N-hydroxy-1-nitrosoethan-1-imine (PIN)
Page 998, P-68.3.1.2.1, line 2.
Page 998, P-68.3.1.2.1, box line 4.
Page 1000, P-68.3.1.2.2, example 3. [modified 18 May 2023]
Page 1002, P-68.3.1.2.4, example 2. [corrected 28 October 2016]
Page 1002, P-68.3.1.2.4, example 5 on page. replace structure with:
Page 1003, P-68.3.1.2.5, example 1. [corrected 28 October 2016] For (1-ethylbutylidene)-N,N-diphenylhydrazinecarboxamide
Page 1003, P-68.3.1.2.5, last example on this page. [corrected 28 October 2016]
Page 1004, P-68.3.1.2.6, line 1. [corrected 28 October 2016]
Page 1004, P-68.3.1.2.6, example 1. [corrected 28 October 2016]
Page 1004, P-68.3.1.2.6, 2nd paragraph, lines 1/2. (corrected 27 November 2019)
Page 1004, P-68.3.1.2.6, 2nd paragraph, last example. (corrected 27 November 2019) add 3-(hydrazinecarbohydrazido)propanoic acid (PIN)
Page 1005, P-68.3.1.3.1, example 4.
Page 1007, P-68.3.1.3.2.2, method (1) example 2,
Page 1008, P-68.3.1.3.2.3, example 1. [modified 30 March 2022]
for {not [7-(anthracen-2-yldiazenyl)naphthalen-2-yl]phenyldiazene
Page 1010, P-68.3.1.3.3.2, example 2.
Page 1011, P-68.3.1.3.4, second and third paragraph. [corrected 11 March 2020]
Page 1011, P-68.3.1.3.4, third paragraph, lines 1/2. [corrected 28 October 2016]
Page 1011, P-68.3.1.3.4, last example. [corrected 28 October 2016] add ethyl 3-(diazenecarbohydrazido)propanoate (PIN)
Page 1011, P-68.3.1.3.5, line 3. [corrected 20 March 2019]
Page 1011, P-68.3.1.3.5, structure 2. [modified 5 June 2019] replace structure with:
Page 1012, P-68.3.1.3.5.1, example 1. [corrected 20 March 2019]
Page 1012, P-68.3.1.3.5.1, example 2. [corrected 20 March 2019]
Page 1013, P-68.3.1.3.5.2, column 3 first name. [corrected 28 October 2016]
Page 1014, P-68.3.1.3.5.2, example 1 on this page. [corrected 28 October 2016] for {not 3-(2-{cyano-[(2-hydroxy-5-sulfophenyl)hydrazinylidene]methyl}diazenyl)-4-hydroxybenzene-1-sulfonic acid
Page 1014, P-68.3.1.3.5.2, example on this page. [corrected 5 July 2018]
Page 1014, P-68.3.1.3.6, second paragraph, line 3. [corrected 28 October 2016]
Page 1014, P-68.3.1.3.6, box. [corrected 28 October 2016]
Page 1015, P-68.3.1.4.1, box, line 1. [corrected 28 October 2016]
Page 1015, P-68.3.1.4.1, box line 5
Page 1015, P-68.3.1.4.1, structure 3. [corrected 28 October 2016]
Page 1015, P-68.3.1.4.1, structure 4. [corrected 28 October 2016] for (not triaz[2]eno)
Page 1015, P-68.3.1.4.1, last example on this page. [corrected 28 October 2016]
Page 1016, P-68.3.1.4.2, line 4. [corrected 28 October 2016]
Page 1016, P-68.3.1.4.2, example 3. [corrected 28 October 2016]
Page 1017, P-68.3.2.2, example 6. [corrected 1 July 2020]
Page 1017, P-68.3.2.2, example 9. [modified 7 August 2019] replace the structure with:
Page 1018, P-68.3.2.3.1, example 6.
Page 1018, P-68.3.2.3.1, example 8. [corrected 5 June 2019]
Page 1018, P-68.3.2.3.1, last example.
Page 1019, P-68.3.2.3.2.1, example 3.
Page 1019, P-68.3.2.3.2.1, example 5. for naphthalen-2-ylarsine
Page 1019, P-68.3.2.3.2.1, example 7.. [corrected 12 May 2021]
for ethyl(methyl)phenylphosphine
Page 1019, P-68.3.2.3.2.1, example 8. for 1-benzofuran-2-ylphosphine
Page 1019, P-68.3.2.3.2.1, example 9. for thiophen-2-ylphosphine
Page 1020, P-68.3.2.3.2.1, example 1 on this page. for ethane-1,2-diylbis(dimethylphosphine)
Page 1020, P-68.3.2.3.2.1, example 2 on this page. for butane-1,2,4-triyltris(phosphine)
Page 1020, P-68.3.2.3.2.1, example 3 on this page. for dibenzo[b,d]furan-3,7-diylbis(phosphine)
Page 1020, P-68.3.2.3.2.1, example 4 on this page. for 1,2-phenylenebis(arsine)
Page 1020, P-68.3.2.3.2.2, structure 3. [corrected 28 October 2016]
Page 1020, P-68.3.2.3.2.2, example 2.. [modified 12 May 2021]
for (arsinomethyl)diphenylphosphine
Page 1021, P-68.3.2.3.2.2, example 2 on this page.
Page 1022, P-68.3.2.3.2.2, example 3 on this page. [modified 8 July 2017)
Page 1023, P-68.3.3, example 2 (structure 8 on this page)
Page 1023, P-68.3.3, example 4. [corrected 5 June 2019]
Page 1023, P-68.3.3, last example on this page. [modified 20 March 2019] for dichloro(triphenyl)bismuthorane for C6H5)3BiCl2
Page 1025, P-68.4.1.1, example 3 on this page.
Page 1025, P-68.4.1.1, example 4 on this page.
Page 1025, P-68.4.1.2, example 1.
Page 1026, P-68.4.1.3, example 4. [corrected 27 March 2019]
Page 1026, P-68.4.2.1, example 4. [corrected 28 October 2016]
Page 1027, P-68.4.2.1, example 2 on this page. [corrected 5 July 2018]
Page 1028, P-68.4.2.4, example 2.
Page 1028, P-68.4.2.4, example 4.
Page 1029, P-68.4.2.4, example 1 on this page.
Page 1029, P-68.4.2.4, example 2 on this page. [corrected 20 March 2019] for 1,1′-[dithioperoxybis(oxy)]dipropan-1-one
Page 1030, P-68.4.3.1, example 2. delete (formerly tetramethyl orthosulfate; Rule D-6.92, ref. 1)
Page 1031, P-68.4.3.2, example 2 on this page. [corrected 20 March 2019] for 1-[(ethylseleninyl)selenonyl]ethane
Page 1031, P-68.4.3.3, example 2 . [corrected 20 March 2019]
Page 1034, P-68.5.1, example 7.
Page 1034, P-68.5.1, last example.
Page 1035, P-68.5.3, example 1. [modified 21 April 2021]
Page 1035, P-68.5.3, example 2. [corrected 21 April 2021]
Page 1036, P-69.1, example 3. [corrected 5 June 2019]
Page 1038, P-69.2.3, example 1.
Page 1038, P-69.2.3, example 2. [corrected 28 October 2016]
Page 1038, P-69.2.3, example 5. [corrected 9 April 2025]
Page 1039, P-69.2.4, example 2. [corrected 21 August 2019]
Page 1039, P-69.2.4, example 3. for dicarbonyl[(1–3-η)-cyclohepta-2,4,6-dien-1-yl](η5-cyclopenta-1,4-dien-1-yl)molybdenum for
dicarbonyl[(1–3-η)-cyclohepta-2,4,6-dien-1-ido](η5-cyclopentadienido)molybdenum
Page 1040, P-69.2.5, example. [corrected 28 October 2016]
Page 1040, P-69.2.6, example 1.
Page 1041, P-69.2.6, example on this page. [modified 22 April 2020]
for [(1,2,5,6-η)-cycloocta-1,5-diene)](η6-phenyltriphenylborato)rhodium
Page 1041, P-69.2.7, example 2. [modified 5 June 2019]
for 1-(2-(hydroxyethyl)osmocene
Page 1041, P-69.2.7, example 3. [modified 1 July 2020]
For 1-[1-(dimethylamino)ethyl]vanadocene
Page 1042, P-69.2.7, example 2 on this page. [modified 20 March 2019]
Page 1042, P-69.2.7, example 3. [corrected 16 August 2016]. for 3,5-(ferrocene-1,1′ -diyl)pentanoic acid
Page 1043, P-69.3, example 4. [corrected 28 October 2016] for tetra-μ3-methyltetralithium
Page 1043, P-69.3, example 7. [corrected 28 October 2016]
Page 1043, P-69.3, last example on this page. [corrected 28 October 2016] For poly[iodo(methyl)magnesium]
Page 1045, P-69.4, example 2 on this page. [modified 24.8.2017] for 1-carbonyl-3,5-dimethyl-bis(triethylphosphane)-1-iridabenzene
Page 1045, P-69.4, example 3 on this page
Page 1046, P-69.5.2, example. [corrected 28 October 2016] replace the structure with:
Page 1050, P-70.3.3.2.2, example [corrected 7 April 2021]
Page 1050, P-70.4, section (4) line 4.
Page 1050, P-70.4, section (4), lines 5-6. [modified 19 September 2018]
Page 1051, P-71.2.1.1, example 1.
Page 1051, P-71.2.1.1, example 2.
Page 1051, P-71.2.1.1, example 4.
Page 1052, P-71.2.1.2, example 5. [modified 12 June 2019] for [(2-methylpropan-2-yl)oxy]triphenyl-λ5-phosphanyl for (1,1-dimethylethoxy)triphenyl-λ5-phosphanyl
Page 1053, P-71.2.1.3, example. [corrected 19 September 2018]
Page 1053, P-71.2.2.1, line 10.
Page 1054, P-71.2.2.1, example 4.
Page 1057, P-71.2.6, example 3.
Page 1057, P-71.2.6, example 4. [corrected 3 October 2018] for 1,9-dihydro(C60-Ih)[5,6]fulleren-1-yl
Page 1058, P-71.3.1, example 2. [corrected 12 June 2019]
Page 1058, P-71.3.1, example 6. [modified 7 August 2019]
replace the structure with:
Page 1058, P-71.3.1, example 8. [corrected 7 August 2019]
Page 1060, P-71.3.3, example 1. [modified 7 August 2019]
Page 1060, P-71.3.3, example 3. [corrected 7 August 2019]
Page 1061, P-71.3.4, line 9.
Page 1061, P-71.3.4, example 2. add (1) (chloroacetyl)oxyl (PIN)
Page 1062, P-71.3.4, example 2 on this page. for butanoyloxidanyl (PIN)
Page 1062, P-71.3.4, example 4 on this page.
Page 1062, P-71.3.4, last example.
Page 1062, P-71.4, example 1.
Page 1062, P-71.4, example 2.
Page 1062, P-71.4, example 3.
Page 1062, P-71.4, example 4. for (2) cyclobutane-1,3-diylbis(dioxidanyl)
Page 1063, P-71.5, example 4. [corrected 19 September 2018] Replace the structure with:
Page 1063, P-71.5, line before examples. [corrected 21 April 2021]
Page 1063, P-71.5, example 2. [corrected 21 April 2021]
for ylooxidanyl
Page 1064, P-71.7 (a) example.
Page 1064, P-71.7 (b), example. [corrected 10 October 2018]
Page 1064, P-71.7 (c), example.
Page 1065, P-71.7 (d) (1) example. [modified 21 April 2021]
add {not 3-[(ylooxidanyl)carbonyl]phenoxyl; ylooxidanyl is senior to phenoxyl}
replace the structure with:
Page 1065, P-71.7 (d), example 2 on this page. [corrected 26 September 2018]
Page 1066, P-72.2.1, example 3.
Page 1067, P-72.2.2.1, example 1.
Page 1068, P-72.2.2.1.1, example 1. [corrected 19 September 2018]
Page 1068, P-72.2.2.1.1, example 2. [corrected 19 September 2018]
Page 1068, P-72.2.2.1.1, example 3. delete naphthalene-1,4-diide (PIN)’ for 1,4-dihydronaphthalene-1,4-diide
Page 1068, P-72.2.2.1.1, example 4. [corrected 3 October 2018] for 1,9-dihydro(C60-Ih)[5,6]fulleren-1-ide
Page 1070, P-72.2.2.2.1.1, example 2 on this page. [corrected 28 October 2016]
Page 1071, P-72.2.2.2.1.2, example.
Page 1071, P-72.2.2.2.2, third paragraph. [corrected 28 October 2016]
Page 1072, P-72.2.2.2.2, example 5 on this page. [corrected 27 March 2019] for (not benzenesulfenate; sulfenic acids ...
Page 1072, P-72.2.2.2.2, example 6. [corrected 7 August 2019]
Page 1072, P-72.2.2.2.2, example 7. [corrected 7 August 2019]
Page 1072, P-72.2.2.2.2, example 8. [corrected 7 August 2019]
Page 1073, P-72.2.2.2.3, example 3. [corrected 7 August 2019]
Page 1073, P-72.2.2.2.4, example 1. [corrected 19 September 2018]
Page 1073, P-72.2.2.2.4, example 2.
Page 1073, P-72.2.2.2.4, example 4. [corrected 26 September 2018]
Page 1077, P-72.4, example 1 on this page. for
(not 6λ5-phosphataspiro[5.5]nonan)
Page 1077, P-72.5.1.1, example 1. [modified 7 August 2019] Delete second 1,4-phenylenebis(phosphanide) (PIN)
Page 1078, P-72.5.3, example 1. [corrected 10 October 2018]
Page 1080, P-72.7 (b), example. replace structure with:
Page 1080, P-72.7 (c), example. [corrected 7 November 2018]
Page 1081, P-72.7 (e), example 2.
Page 1082, P-72.8.2, example 2. [corrected 17 June 2020]
for 1λ4, 3λ4-dithiocane-1,3-diuide
for 1λ4, 3λ6-dithiocan-3-id-1-uide
Page 1083, P-73.1.1.1, example 2. [corrected 27 March 2019] for chloro(trimethyl)phosphanium (PIN)
Page 1084, P-73.1.1.2, line 1 on this page.
Page 1085, P-73.1.1.2, example 1 on this page. Change structure to:
Page 1085, P-73.1.1.2, example 4 on this page, move to P-73.1.2.1. [modified 30.6.2017)
Page 1086, P-73.1.2.1, example 2. [corrected 12 June 2019]
Page 1086, P-73.1.2.1, example 5. [corrected 12 June 2019]
Page 1086, P-73.1.2.1, example 7. add N,N,N-trimethylanilinium (PIN)
Page 1087, P-73.1.2.1, example 5 on this page.
Page 1087, P-73.1.2.1, example 6 on this page. [corrected 12 June 2019] for (cyclohexanecarbonyl)dimethyloxonium
Page 1087, P-73.1.2.1, example 7 on this page. [corrected 27 March 2019] for (1-hydroxyethylidene)(dimethyl)ammonium
Page 1088, P-73.1.2.1, example 1 on this page. [corrected 12 June 2019] for benzoyldimethylsulfonium
Page 1088, P-73.1.2.1, example 2 on this page.
Page 1088, P-73.1.2.2, right structural formula. [corrected 12 June 2019]
Page 1088, P-73.1.2.2, example 1. [modified 11 March 2020] Replace the structures with:
Page 1089, P-73.2.1, example 2.
Page 1089, P-73.2.1, example 3.
Page 1089, P-74.2.2, line 4.
Page 1091, P-73.2.2.2, line 4.
Page 1091, P-73.2.2.2, line 10.
Page 1092, P-73.2.2.2, example 1 on this page. for Y = H 4a,8a-dihydronaphthalen-4a-ylium Replace structure with:
Page 1092, P-73.2.2.2, example 2 on this page. [corrected 3 October 2018, modified 10 October 2018] for 1,9-dihydro(C60-Ih)[5,6]fulleren-1-ylium replace the structure with:
Page 1092, P-73.2.2.3, example 3. [corrected 7 August 2019]
Page 1093, P-73.2.3.1, example 6. [corrected 17 June 2020]
Page 1093, P-73.2.3.2, example 3.
Page 1094, P-73.2.3.3, paragraph above examples, lines 1/2. [corrected 1 November 2023]
Page 1094, P-73.2.3.3, example 3. add (chloroacetyl)oxylium (PIN)
Page 1094, P-73.2.3.3, example 5. [corrected 12 June 2019]
Page 1094, P-73.2.3.3, last example.
Page 1095, P-73.2.3.4, example 2 on page. Replace the structure with: CH3–CH2–S–O+
Page 1095, P-73.2.3.4, example 5 on this page. [corrected 19 September 2018]
Page 1095, P-73.2.3.4, example 6.
Page 1095, P-73.3.1, example 1.
Page 1096, P-73.3.1, example 2 on this page. [corrected 12 June 2019]
Page 1096, P-73.3.1, last example.
Page 1097, P-73.3.2, Table 7.5, structure 3. [corrected 27 March 2019] for E = Se 2λ4-benzoselenopyran-1-ylium (PIN) for E = Te 2λ4-benzotelluropyran-1-ylium (PIN)
Page 1097, P-73.3.2, Table 7.5, structure 4. [modified 30.6.2017) for 2H-thienylium (PIN) for 2H-selenofuranylium (PIN) for 2H-tellurofuranylium (PIN)
Page 1097, P-73.3.2, Table 7.5, structure 5. for E = S thioflavylium (PIN) for E = Se selenoflavylium (PIN) for E = Te telluroflavylium (PIN)
Page 1099, P-73.4, example 1 on this page. [modified 30 March 2022]
for 2-ethoxy-N-{2-[(2-ethoxyethyl)sulfanyl]ethyl}-N-methylethanamine for N-(2-ethoxyethyl)-2-[(2-ethoxyethyl)sulfanyl]-N-methylethanamine
Page 1099, P-73.4, example 2 on this page.
Page 1099, P-73.4, last example on this page.
Page 1100, P-73.4, example 3 on this page. [corrected 10 October 2018]
Page 1101, P-73.5.1.1, example 1. [corrected 7 August 2019] for 1,4-phenylenebis(phosphonium)
Page 1101, P-73.5.1.1, example 3. [corrected 12 June 2019]
Page 1101, P-73.5.1.2, example 1. [corrected 7 August 2019] for ethane-1,2-diylbis(oxidanylium)
Page 1101, P-73.5.1.2, example 3. [corrected 7 August 2019] for pentane-2,4-diylidenebis(oxonium)
Page 1102, P-73.5.1.2, example 4 on this page. [corrected 7 August 2019] for pyridine-2,6-diylbis(thioxylium)
Page 1102, P-73.5.1.2, example 5 on this page. [corrected 7 August 2019]
Page 1105, P-73.6, example 5.
Page 1105, P-73.6, example 8. [corrected 17 June 2020]
Page 1106, P-73.7.1 (c), example 1. [modified 12 June 2019]
Page 1106, P-73.7.1 (c), example 2. [modified 11 November 2020]
Page 1107, P-73.8.2, example 2. for 1λ6,3λ4-dithiocan-3-ium-1-ylium
Page 1108, P-74.1.1, example 1. replace structure with:
Page 1109, P-74.1.1, example 1 on this page. [corrected 9 January 2019]
Page 1109, P-74.1.1, example 3 on this page.. [modified 12 May 2021]
add 6,6-dihydroxy-6,11-dihydro-5λ5-[1,3]benzimidazolo[1,2-b][2,1]benzazaborol-5-ylium-6-uide (PIN)
Page 1109, P-74.1.1, example 4 on this page. [modified 21 November 2018] for not 2-methyl-3,4-dihydro-1H-2-selenobenzopyran-2-ium-3-id-4-one for 2-methyl-4-oxo-3,4-dihydro-1H-isoselenochromen-2-ium-3-ide (PIN)
Page 1110, P-74.1.1, example 1 on this page. [corrected 14 November 2018]
Page 1110, P-74.1.2, example 1. [modified 11 November 2020]
Page 1111, P-74.1.3, example 1. [modified 27 March 2019] for trimethyl[2-(methyldiphenylphosphoniumyl)ethenyl]boranuide
Page 1111, P-74.1.3, example 2. [corrected 17 June 2020]
Page 1111, P-74.1.3, example 3. [modified 17 June 2020]
for [not 1-methyl-3-(methylazaniumyl)diborazan-2-ium-1,3-diide;
Replace the structure with:
Page 1112, P-74.2.1.1.1, example. [corrected 17 June 2020]
Page 1112, P-74.2.1.1.2, example. [modified 12 June 2019]
Page 1113, P-74.2.1.2, example. for trimethyl)amine oxide
Page 1114, P-74.2.1.5, example.
Page 1116, P-74.2.2.1.2, structures.
Page 1116, P-74.2.2.1.3, example. [corrected 17 June 2020]
Page 1116, P-74.2.2.1.4, example.
Page 1117, P-74.2.2.1.6, structures.
Page 1117, P-74.2.2.1.8, example 2. [modified 27 March 2019]
for 1-oxo-1λ4-thiophene
Page 1118, P-74.2.2.1.8, example on this page. [modified 27 March 2019] for 1,2-thiazine 1-oxide for 1,2-thiazine S-oxide
Page 1118, P-74.2.2.1.9 example.
Page 1118, P-74.2.2.2, structures. for where X = C or O; and Z = C, N or O
Page 1119, P-74.2.2.2.1.3, heading.
Page 1119, P-74.2.2.2.1.2, example 1.
Page 1119, P-74.2.2.2.1.2, example 2. add acetonitrile sulfide (PIN)
Page 1119, P-74.2.2.2.1.3, example. [corrected 17 June 2020]
Page 1119, P-74.2.2.2.2, example. [modified 3.7.2017)
Page 1120, P-74.2.2.3, structures.
Page 1121, P-74.2.2.3.4. line 1. [corrected 26 September 2018]
Page 1121, P-74.2.3, example 1. [modified 27 March 2019] for 2-{4-[oxido(diphenyl)phosphoniumyl]phenyl}propane-1,3-diyl replace the structure with:
Page 1121, P-74.2.3, example 2. [corrected 26 September 2018]
Page 1123, P-75.2.1, example 5. [corrected 26 September 2018]
Page 1124, P-75.2.3, example 1. [modified 11 November 2020]
Page 1124, P-75.2.3, example 2. [modified 19.7.2017)
Page 1125, P-75.2.4, example 1 on this page. [corrected 10 October 2018]
Page 1125, P-75.2.4, example 2 on page.
Page 1125, P-75.2.4, example 3 (X = • ; Y = +) on this page. [corrected 3 October 2018] for 1,9-dihydro(C60-Ih)[5,6]fulleren-1,9-diyl
Page 1125, P-75.2.4, example 3 (X = • ; Y = –) on this page. [corrected 3 October 2018] for 1,9-dihydro(C60-Ih)[5,6]fulleren-9-id-1-yl
Page 1125, P-75.3.1 example 2. [corrected 26 September 2018]
Page 1125, P-75.2.4, example 3 on this page. [corrected 10 October 2018]
Page 1126, P-75.3.2, example 4.
Page 1126, P-75.3.2, example 3.
Page 1126, P-75.3.2, example 4.
Page 1126, P-75.4, example 1.
Page 1126, P-75.4, example 2.
Page 1127, P-77.1.1, example 1. add anilinium chloride (PIN)
Page 1127, P-77.1.1, example 1,
Page 1127, P-77.1.1, example 2,
Page 1127, P-77.1.1, example 3,
Page 1127, P-77.1.2, line 2.
Page 1128, P-77.1.3 (1), line 1. (corrected 4 December 2019)
Page 1128, P-77.1.3, example. [modified 21 November 2018] for (b) bis(N,N-dimethyl-1,3-thiazolidin-2-amine) sulfate
Page 1128, P-77.2.1, example 1. [corrected 21 November 2018] for sodium methoxide delete a retained name) and methanolate.
Page 1128, P-77.2.1, example 2.
Page 1129, P-77.3.1, example. [corrected 10 October 2018]
Page 1130, P-80, lines 3/4. [corrected 7 August 2019]
Page 1132, P-82.2.1, example 4.
Page 1132, P-82.2.1, example 6. [modified 3 April 2019] for (1,2-13C)acetophenone
Page 1133, P-82.2.1, example 2 on page.
for (α,1-13C2)-1,2-xylene delete (α,1-13C2)-o-toluene
Page 1133, P-82.2.1, last example.
Page 1134, P-82.2.2.1, example 1. [corrected 7 November 2018]
Page 1134, P-82.2.2.1, example 3.
Page 1135, P-82.2.2.2, example 1 on this page.
Page 1136, P-82.2.3, example 4.
Page 1136, P-82.2.3, last example. for
[not 6-methyl-2,3-(2-2H2,3)dihydro-(2,3-2H2)napthalen-1-ol; for not 6-methyl-2-(2H)hydro-3-hydro-(2,3-2H2)napthalen-1-ol;
Page 1136, P-82.2.4, examples 1 and 2.
Page 1137, P-82.2.4, example 1 on this page.
Page 1137, P-82.2.4, example 2 on this page. (corrected 27 November 2019)
Page 1137, P-82.2.4, example 6 on this page. Replace structure with:
Page 1137, P-82.2.5, example 1. (modified 1 December 2021)
Delete acet(2H)amide
Page 1138, P-82.4.1, example 3.
Page 1139, P-82.4.2, example 3.
Page 1141, P-82.5.2, example 1 on this page, structural formula. [corrected 19 June 2019]
Page 1141, P-82.5.2, example 3 on this page.
Page 1141, p-82.6.1.2, example. [modified 8 July 2020]
Page 1142.
Page 1142, P-82.6.1.4, example.
Page 1142, P-82.6.2, example 3. (modified 1 December 2021) Delete D-(O-2H)glycer(2H)aldehyde
Page 1143, P-82.6.3.1, example 3.
Page 1143, P-82.6.3. (corrected 1 December 2021)
Page 1143, insert new P-82.6.3.2 after P-82.6.3.1 (corrected 1 December 2021)
P-82.6.3.2 When the nuclide is located at a position in a retained name that is not numbered a systematic name that identifies separately the relevant atom is used for the IUPAC preferred name. For general nomenclature an italicized prefixes or Greek letters may be used to denote its position.
Page 1143, P-82.6.3.3 (was P-82.6.3.2), example 2.
Page 1144, P-82.6.3.3 (was P-82.6.3.2), example 3.
Page 1144, P-82.6.4, example 5. (modified 27 November 2019)
Page 1144, P-82.6.4, example 6. [corrected 3 April 2019]
Page 1147, P-83.1.2.2, example 1.
Page 1147, P-83.1.2.2, example 2. [modified 7 August 2019] replace the structure with:
Page 1147, P-83.1.2.2, example 3. [corrected 8 July 2020]
Page 1148, P-83.1.2.2, example 1 on this page.
Page 1148, P-83.1.2.2, example 3 on this page.
Page 1148, P-83.1.2.2, example 4 on this page. [modified 19 June 2019]
Page 1148, P-83.1.2.2, example 5 on this page. (modified 27 November 2019)
Page 1148, P-83.1.2.2, example 6 on this page. [corrected 19 September 2018]
Page 1148, P-83.1.2.2, example 5 on this page. [corrected 1 December 2021]
Page 1148, P-83.1.2.2, example 6 on this page [modified 30 March 2022]
Page 1150, P-83.2.1.1, example 4 on this page, the last of the three structures. [corrected 9 January 2019]
Page 1155, P-84, example 3.
Page 1155, P-84, example 5. [corrected 3 April 2019]
Page 1157-1292, running heading.
Page 1158, P-91.2.1.2.1 (a) (i) , line 4. (modified 27 November 2019)
Page 1159, P-91.2.1.2.1 (c) (vi) line 4.
Page 1159, P-91.2.1.2.1, last paragraph on this page, line 1. [corrected 28 October 2016]
Page 1160, P-91.2.1.2.1, paragraph on this page. [corrected 28 October 2016]
Page 1160, P-91.3, example 1. [corrected 28 April 2023]
Page 1161, P-91.3, example 2 on this page.
Page 1161, P-91.3, example 5 on this page.
Page 1164, P-92.1.1 (d), example 2. [corrected 2 January 2019]
Page 1167, P-92.1.2.2, lines 6-8. [corrected 10 April 2019]
Page 1169, P-92.1.3.4 (b)(i), line 2. [corrected 19 September 2018]
Page 1170, P-92.1.4.1, example. [modified 7 August 2019]
Page 1170, P-92.1.4.1, complete digraph for center 4.
Page 1173, P-92.1.4.3, example 1 on this page. [corrected 26 September 2018]
Page 1174, P-92.1.4.4, example on this page.
Page 1176, P-92.2.1.1.1, example. [corrected 6 December 2023]
Page 1177, P-92.2.1.1.1, Example 3.
Page 1179, P-92.2.1.1.3, Example 1. [modified 2 January 2019] Replace structure with: replace structure with:
Page 1181, P-92.2.1.1.3, example 2. [corrected 10 April 2019]
Page 1183, P-92.2.1.2, Example 3. [corrected 19 September 2018]
Page 1183, P-92.2.1.3, Example 1. [corrected 26 September 2018]
Page 1185, P-92.2.1.3, Example 3.
Page 1185, P-92.2.1.3, example 3, explanation, line 4. [corrected 26 June 2019]
Page 1185, P-92.2.1.3, Example 4.
Page 1186, P-92.2.1.3, Explanation, line 9. [corrected 2 January 2019]
Page 1186, P-92.2.1.3, Explanation, line 12. [corrected 2 January 2019]
Page 1187, P-92.2.2, Example 1.
Page 1188, P-92.3, example 1.
Page 1189, P-92.3, example 2 and explanation line 2/3. (modified 27 November 2019) for...is: ‘OH > CH2[125I] > CH2I > H’ and the configuration is ‘R’ for... Replace the structure with:
Page 1190, P-92.4.1, example.
Page 1191, P-92.4.2.1, example 2. [corrected 26 September 2018]
Page 1192, P-92.4.2.2, Example 1. [corrected 2 September 2020]
Page 1193, P-92.4.2.2, Example 2. [modified 2 September 2020] replace structures with:
Page 1193, P-92.4.2.2, last example.
Page 1194, P-92.5.1, Note, line 2. [corrected 10 April 2019]
Page 1195, P-92.5.1 Example (cont‘d), line 2. [corrected 10 April 2019]
Page 1198, P-92.5.2.2, Example 2, Explanation, line 7. [corrected 2 September 2020]
Page 1199, P-92.5.2.2, Example 3, simplified digraph. [corrected 2 September 2020]
Page 1199, P-92.5.2.2, Example 3, Explanation, lines 2/3. [corrected 2 September 2020]
Page 1199, P-92.5.2.2, Example 3, Explanation, line 5. [corrected 2 September 2020]
Page 1200, P-92.5.2.2, example 4.
Page 1201, P-92.5.2.1, example 5. [modified 10 April 2019] delete CH4 from structure
Page 1202, P-92.5.2.2, example 5 (continued).
Page 1203, P-92.5.2.2, Example 5, paragraph 3, line 2 and 3.
Page 1204, P-92.5.2.2, Example 6.. [corrected 12 May 2021]
Page 1204, P-92.5.3, example. [corrected 10 October 2018]
Page 1205, P-92.6, title. [corrected 2 September 2020]
Page 1206, P-92.6, Example 2.
Page 1206, P-92.6, Example 2, digraph. [corrected 3 December 2025]
Page 1206, P-92.6, example 3. for (3R,4ra,5S)-4-(2-bromoethenylidene)heptane-3,5-dithiol
Page 1207, P-92.6, example 4 (1 on this page).
Page 1207, P-92.6, example 5 (2 on this page).
Page 1208, P-92.6, Example 6. [modified 11 November 2020]
Page 1209, P-92.6, Example 6, last paragraph on this page line 6. [corrected 2 September 2020]
Page 1209, P-92.6, Example 6, Table at bottom of page, Branch B. [modified 2 September 2020]
for 14-'R'
Page 1209, P-92.6, example 6 (cont’d), Branch B, last line. [corrected 2 January 2019]
Page 1211, P-93.1.2.1, Example 1.
Page 1212, P-93.1.2.2, example.
Page 1213, P-93.1.4, example.
Page 1214, P-93.2.1, lines 7/8. [corrected 18 September 2019]
Page 1214, P-93.2.2, example 1.. [modified 12 May 2021]
for (R)-[benzyl(methyl)phenyl(prop-2-en-1-yl)azanium] bromide
for (R)-[benzyl(methyl)phenyl(prop-2-en-1-yl)ammonium] bromide
add (R)-N-benzyl-N-methyl-N-(prop-2-en-1-yl)anilinium bromide (PIN)
Page 1214, P-93.2.2, example 2.
Page 1215, P-93.2.3, example 1.. [corrected 12 May 2021]
Page 1215, P-93.2.3, example 1. [modified 21 April 2021]
for (S)-(N-ethyl-N-methylbenzeneaminiumyl)oxidanide
Page 1215, P-93.2.3, example 2. . [modified 12 May 2021]
for (S)-methyl(phenyl)propylphosphane oxide
Page 1216, P-93.2.4, example 3 on this page. [modified 30 September 2020]
Page 1216, P-93.2.4, example 3 on this page. [modified 3 March 2021]
Page 1216, P-93.2.4, last example.
Page 1217, P-93.2.5, example 1 on this page. [modified 21 August 2019] replace structure with:
Page 1217, P-93.2.5, example 2 on this page. [modified 30 September 2020]
for tetrabutylammonium (S)-[O-phenyl (17O,18O)sulfate]
for tetrabutylazanium (S)-[O-phenyl (17O,18O)sulfate]
replace structure with:
Page 1224, P-93.3.3.6, example 2.
Page 1225, P-93.3.3.6, example 3.
Page 1227, P-93.3.4.1, lines 5-7.
Page 1227, P-93.3.4.1, example 1.
Page 1227, P-93.3.4.1, example 2.
Page 1227, P-93.3.4.1, example 3.
Page 1227, P-93.3.4.1, example 4.. [modified 12 May 2021]
Page 1228, P-93.3.4.2.1, example 1.
Page 1228, P-93.3.4.2.1, Example 1, Explanation, the right-hand structure. [corrected 10 October 2018]
Page 1229, P-93.3.4.2.1, example 2. for {not (TBPY-4-11′-A)-3,3,3′,3′-tetramethyl-3H,3′H-1λ4-spirobi[2,1-benzoxathiole]}
Page 1230, P-93.3.4.2.1, example 4.
Page 1231, P-93.3.4.2.2, example, Explanation. [corrected 24 October 2018]
Page 1231, P-93.4. delete P-93.4.4 Specification of configuration of unsaturated compounds
Page 1232, P-93.4.1.1, example 2 on this page.
Page 1234, P-93.4.1.2, last example. replace diagram with:
Page 1236, P-93.4.2.1.1, example 1 on this page. for (2Z)-2,3-dibromoacrylonitrile
Page 1236, P-93.4.2.1.1, example 3 on this page.
Page 1236, P-93.4.2.1.1, last example.
Page 1236, P-93.4.2.1.2, 3rd paragraph, line 3/5. [corrected 26 June 2019]
Page 1236, P-93.4.2.1.2, example 1. [corrected 10 April 2019]
Page 1237, P-93.4.2.1.2, example 1 on this page. [corrected 10 April 2019]
Page 1237, P-93.4.2.1.2, example 3 on this page. [corrected 10 April 2019]
Page 1237, P-93.4.2.1.3, example 1. for (not trans-diphenyldiazene)
Page 1238, P-93.4.2.1.3, example 1 on this page. [modified 2 September 2020]
for (Z)-[(4-chlorophenyl)phenylmethanone oxime]
add (Z)-N-hydroxy(4-chlorophenyl)(phenyl)methanimine (PIN)
Page 1238, P-93.4.2.1.3, example 2 on this page. [corrected 24 July 2019] add (2E,3Z)-N2,N3-dihydroxypentane-2,3-diimine (PIN)
Page 1239, P-93.4.2.3, example 2. [corrected 10 April 2019]
Page 1240, P-93.4.2.4, example 1 on this page. [corrected 10 April 2019]
Page 1240, P-93.4.2.4, example 2 on this page. [corrected 10 April 2019]
Page 1241, P-93.5.1.1.1, example 1. [corrected 10 October 2018]
Page 1241, P-93.5.1.1.1, example 2.
Page 1242, P-93.5.1.1.1, example 3 on this page
Page 1242, P-93.5.1.1.2, line 2. [corrected 3 December 2025]
Page 1242, P-93.5.1.1.2 (a), example 1.
Page 1242, P-93.5.1.1.2 (a), example 2. add trans-cyclohexane-1,4-diol
Page 1243, P-93.5.1.1.2 (a), example 1 on this page.
Page 1243, P-93.5.1.1.2 (a), example 2 on this page.
Page 1243, P-93.5.1.1.2 (a), example 3 on this page. [modified 26 June 2019] add (cis-4-methylcyclohexyl)bis(trans-4-methylcyclohexyl)phosphane
Page 1243, P-93.5.1.1.2 (a) after example 3 on this page. [corrected 3 December 2025]
Page 1243, P-93.5.1.1.2 (b), line 8.
Page 1243, P-93.5.1.1.2 (b), end of paragraph. [corrected 31 May 2023]
Page 1243, P-93.5.1.1.2 (b), example 1. replace structure with:
Page 1244, P-93.5.1.1.2 (b), first example on this page, 3. [modified 31 May 2023]
Page 1244, P-93.5.1.1.2 (b), second example on this page, 4, add after the name. [corrected 31 May 2023]
Page 1245, P-93.5.1.2, last name on this page, line 2.
Page 1246, P-93.5.1.3.1, lines 3-5. [corrected 3 September 2018]
Page 1246, P-93.5.1.3.1, 2nd paragraph, lines 1-3. (corrected 27 November 2019)
Page 1246, P-93.5.1.3.1, Example 1. [Corrected 3 September 2018] replace structure with:
Page 1246, P-93.5.1.3.1, Example 2. [Corrected 3 September 2018] replace structure with:
Page 1247, P-93.5.1.3.2, Example 1. [Corrected 3 September 2018] replace structure with:
Page 1247, P-93.5.1.3.2, Example 2. [Corrected 3 September 2018] replace structure with:
Page 1247, P-93.5.1.3.2, Example 3. [Corrected 3 September 2018] replace structure (showing positions of r, c and t) with:
Page 1248, P-93.5.1.4.1, example 5.
Page 1248, P-93.5.1.4.1, example 6.
Page 1249, P-93.5.1.4.2.1 (1) (b), line 3. [corrected 2 September 2020]
Page 1249, P-93.5.1.4.2.1 (1), example 1. [modified 2 September 2020]
Page 1250, P-93.5.1.4.2.1 (2), Example 2. [corrected 10 April 2019]
Page 1250, P-93.5.1.4.2.1 (2), example 3. for trans-3,3′-diethylidene-1,1′-bis(cyclobutylidene) for 3,3′-di[(E)-ethylidene]-1,1′-bis(cyclobutylidene)
Page 1251, P-93.5.1.4.2.1, example 3, simplified digraph for 5 and 8. [corrected 2 January 2019]
Page 1251, P-93.5.1.4.2.2, example 1. [modified 24 March 2021]
add (1seqCis,4R)-N-hydroxy-4-methylcyclohexan-1-imine (PIN)
Page 1252, P-93.5.1.4.2.2, Example 2. [corrected 9 January 2019] Add (Sa)-1-(bromomethylidene)-3-propylcyclobutane
Page 1252, P-93.5.1.4.2.2, example 3. for (1S,4sa)-1-ethyl-4-(prop-1-en-1-ylidene)cyclohexane replace digraph for C-1 with:
Page 1253, P-93.5.1.4.2.2, example 4 (cont’d). digraph for C-4 with:
Page 1253, P-93.5.1.4.2.2, Example 4, Explanation, lines 8-10. [corrected 10 April 2019]
Page 1254, P-93.5.2.1, example 1. [corrected 2 January 2019]
Page 1255, P-93.5.2.1, last example. replace structure with:
Page 1255, P-93.5.2.2, lines 1/2. [corrected 2 September 2020]
Page 1256, P-93.5.2.2.1, example 1 on this page. [modified 2 September 2020]
for rel-(2R,7S)-2-bromo-7-fluorobicyclo[2.2.1]heptane (PIN)
replace structure with:
Page 1256, P-93.5.2.2.1, example 2. for (5S,7R)-dimethylbicyclo[2.2.1]hept-2-ene (PIN) replace structure with:
Page 1256, P-93.5.2.2.1, example 3.
Page 1257, P-93.5.2.2.1, example on this page. [corrected 13 January 2021]
Page 1257, P-93.5.2.2.2, example. for (1R*,10S*)-1-methylbicyclo[8.3.1]tetradecane replace structure with:
Page 1258, P-93.5.2.3, example 1 on this page. [corrected 2 January 2019]
Page 1258, P-93.5.2.3, example 3 on this page (second cumulene). [corrected 31 October 2018]
Page 1258, P-93.5.2.3, example 4 on this page (third cumulene).
Page 1258, P-93.5.2.3, last example on this page. [modified 7 November 2018] replace structure with:
Page 1259, P-93.5.3.1, example 1.
Page 1260, P-93.5.3.1, example 4, simplified digraph. [corrected 26 June 2019]
Page 1261, P-93.5.3.1, Example 5. For ...[5a,9a](epiminomethano)cyclopenta[f]indolizin-10′-one (PIN) replace structure with:
Page 1261, P-93.5.3.2, Example 1, Explanation, line 4. [corrected 10 April 2019]
Page 1262, P-93.5.3.2, example 3. [corrected 24 October 2018]
Page 1263, P-93.5.3.2, Example 5. [modified 2 September 2020]
Page 1263, P-93.5.3.2, Example 5, explanation, part (b) [formerly (c)]. [corrected 2 September 2020]
Page 1263, P-93.5.3.3 example 2. [corrected 2 January 2019]
Page 1264, P-93.5.3.4, example 1 on this page. [corrected 10 April 2019]
Page 1264, P-93.5.3.4, example 2.
Page 1264, P-93.5.3.5, Example 1. (corrected 4 December 2019)
Page 1265, P-93.5.3.5, Example 1, first digraph. (modified 4 December 2019)
replace digraph 2 with:
Page 1265, P-93.5.3.5, example 1, second digraph. [modified 2 September 2020]
Page 1265, P-93.5.3.5, Example 2. [modified 10 April 2019] for
(5M )-1,12-dioxatrispiro[4.2.2.411.28..25]nonadecane
Page 1266, P-93.5.4.1, example 1.
Page 1267, P-93.5.4.1, example 1 on this page. [corrected 2 January 2019]
Page 1267, P-93.5.4.1, example 2 on this page. [modified 5 August 2017) for (10aRa)-1,11-dinitro-5,7-dihydrodibenzo[a,c][7]annulen-6-one replace structure with:
Page 1268, P-93.5.4.1, example 1 on this page.
Page 1268, P-93.5.4.1, example 2 on this page.
Page 1269, P-93.5.4.2, example 1.. [corrected 12 May 2021]
Page 1269, P-93.5.4.2, example 2, right version. [corrected 2 January 2019]
Page 1269, P-93.5.4.2, example 2.. [corrected 12 May 2021]
Page 1270, P-93.5.5.1, example 1.
Page 1270, P-93.5.5.1, example 2. [corrected 28 May 2024]
add (1M)-17-bromo-2,13-dioxabicyclo[12.2.2]octadeca-1(16),14,17-triene-15-carboxylic acid (PIN)
Page 1270, P-93.5.5.1, example 3.
Page 1271, P-93.5.5.2, example. [corrected 12 September 2018]
Page 1271, P-93.5.5.3, lines 3/4. [corrected 4 September 2024]
Page 1271, P-93.5.5.3, example 2. [corrected 4 September 2024]
Page 1272, P-93.5.6.1, Fig. 9.2, third oblong. [corrected 2 September 2020]
Page 1272, P-93.5.6.1, Fig. 9.2, second ellipse. [corrected 2 September 2020]
Page 1273, P-93.5.6.1, Fig. 9.2, third ellipse. [corrected 2 September 2020]
Page 1273, P-93.5.6.2, line 3. [corrected 7 November 2018]
Page 1275, P-93.5.6.3, example. [corrected 3 October 2018]
Page 1277, P-93.5.6.5, example. [corrected 10 October 2018] Replace the structure with:
Page 1278, P-93.5.6.6, explanation line 4. (corrected 27 November 2019)
Page 1279, P-93.5.7.1, example 1. for (1Ra)-6,6′-dinitro-[1,1′-biphenyl]-2,2′-dicarboxylic acid
Page 1279, P-93.5.7.1, example 2. for (1Sa)-2′,5′-dimethyl-6-nitro-[1,1′-biphenyl]-2-carboxylic acid
Page 1279, P-93.5.7.1, example 3. for (1Ra)-2′,6-diamino-6′-methoxy-[1,1′-biphenyl]-2-carboxylic acid
Page 1280, P-93.5.7.1, example 1 on this page. for (2Sa)-2-[2′-(hydroxymethyl)naphthalen-1-yl]-3,5-dimethylphenol replace structure with:
Page 1280, P-93.5.7.2, example on this page.
Page 1281, P-93.5.7.2, example 1 on this page. for (b) [1(4)cis,1′(4′)cis]-4,4′-dimethyl-1,1′-bi(cyclohexane)
Page 1281, P-93.5.7.2, example 2 on this page. for [11(14)cis,21(24)trans,31(34)cis]-14,34-dimethyl-11,21:24,31-tercyclohexane
Page 1281, P-93.5.7.2, example 3 on this page. add (11s,14r,21s,24s)-24-bromo-14-butyl-34-ethyl-11,12,13,14,15,16,21,22,23,24,25,26-dodecahydro-11,21:24,31-terphenyl (PIN) add (1s,1′s,4r,4′s)-4′-bromo-4-butyl-4′′-ethyl-1,1′,2,2′,3,3′,4,4′,5,5′,6,6′-dodecahydro-1,1′:4′,1′′-terphenyl
Page 1281, P-93.5.7.2, last example on this page. [modified 26 June 2019]
for (1E)-1-[(1′R,1(4)-trans)-1,1′-bi(cyclohexan)-3′-en-4-yl]-N-[(trans)-4-phenylcyclohexyl]methanimine
replace structure with:
Page 1282, P-93.5.7.3, example 1.
Page 1282, P-93.5.7.3, example 2.
Page 1282, P-93.6, example on this page. [corrected 12 September 2018] add
(2R)-1-(cis-4-methylcyclohexyl)-3-(trans-4-methylcyclohexyl)propan-2-ol replace structure with:
Page 1283, P-93.6, first digraph. [corrected 12 September 2018]
Page 1284, P-93.6, example 2. replace structure with:
Page 1284, P-93.6, example 3. replace structure with:
Page 1285, P-93.6, example 4.
Page 1285, P-93.6, example 5. [modified 24 October 2018] replace the structure with:
Page 1285, P-93.6, Example 6. [modified 30 September 2020]
Page 1287, P-94.2.1, top diagram.
Page 1291-2, P-94.3.2.6, Example 1. [corrected 30 September 2020]
Page 1292, P-94.3.2.6, Example 2. [corrected 30 September 2020]
Page 1298, P-101.2.5, example 1. [corrected 12 August 2020]
Page 1298, P-101.2.6, lines 3-5. [corrected 21 October 2020
Page 1300, P-101.2.6.1.2, example. [corrected 17 October 2018]
Page 1301, Table 10.1 (a). add curan, lythranidine, nupharidine, and vincane
Page 1301, Table 10.1 (c). add germacrane
Page 1301, Table 10.1 (c).
Page 1302, Table 10.1, footnote.
Page 1304, P-101.3.1.1, example 1 on this page.
Page 1304, P-101.3.1.1, example 3 on this page. [corrected 26 September 2018]
Page 1304, P-101.3.1.1, structure 4 on this page.
Page 1306, P-101.3.2.2.1, example 2.
Page 1306, P-101.3.2.2.1, example 3.
Page 1306, P-101.3.2.2.1, last example.
Page 1307, P-101.3.2.2.2, last example.
Page 1309, P-101.3.3, structure 1. [corrected 17 October 2018]
Page 1312, P101.3.5.1, example 2. [corrected 14 November 2018]
Page 1313, P-101.3.5.2, example 1. [corrected 23 September 2020]
Page 1313, P-101.3.5.2, example 2. [corrected 23 September 2020]
Page 1315, P-101.3.7.2, line 11. [corrected 23 September 2020]
Page 1316, P-101.3.7.2, example 4. [corrected 17 April 2019]
Page 1318, P-101.4.2, example 1.
Page 1321, P-101.5.1.1, example 3. [corrected 9 January 2019]
Page 1325, P-101.5.2, example 1 on this page.
Page 1325, P-101.5.2, example 3 on this page. [corrected 17 April 2019]
Page 1328, P-101.6.2, example 2. [corrected 17 October 2018]
Page 1329, P-101.6.2, example 3 on this page. [corrected 17 April 2019] for 1,1′-[(1E,3S)-2,3-dimethylbutane-1,4-diyl]dibenzene
Page 1329, P-101.6.3, example. [corrected 7 November 2018]
Page 1330, P-101.6.4, second example.
Page 1331, P-101.6.5, example 2. [corrected 17 October 2018]
Page 1333, P-101.7.1.1.1, example. (modified 27 November 2019)
Page 1334, P-101.7.1.1.2, example. [corrected 23 September 2020]
Page 1334, P-101.7.1.1.3, example 1. [corrected 24 October 2018]
Page 1335, P-101.7.1.1.4, example.
Page 1335, P-101.7.1.2, example 2. [corrected 17 October 2018]
Page 1336, P-101.7.1.2, example 4 on this page. [corrected 23 September 2020]
replace the structure with:
Page 1339, P-101.7.4, example 1. [corrected 24 October 2018]
Page 1339, P-101.7.4, examples 2. [modified 24 October 2018] replace the structure with:
Page 1339, P-101.7.4, example 3. [modified 26 June 2019] For propane-2-one matridine-3β,4β-diyl acetal for acetone matridine-3β,4β-diyl acetal
Page 1339, P-101.7.4, example 3. [corrected 23 September 2020]
Page 1341, P-101.7.5, scheme at top of this page.
Page 1343, P-101.8.4, example 3 (structure II). [corrected 26 September 2018]
Page 1346, P-102.2.1, table 10.2.
Page 1346, P-102.2.1, table 10.2 structure 1, D-glyceraldehyde. replace the structure with:
Page 1347, P-102.2.1, table 10.2 structure 2 on this page, D-arabinose.
Page 1347, P-102.2.1, table 10.2 structure 5 on this page, D-allose.
Page 1347, P-102.2.1, table 10.2 structure 6 on this page, D-altrose. replace the structure with:
Page 1347, P-102.2.1, table 10.2 structure 7 on this page, D-glucose.
Page 1347, P-102.2.1, table 10.2 structure 8 on this page, D-mannose.
Page 1347, P-102.2.1, table 10.2 structure 9 on this page, D-gulose.
Page 1347, P-102.2.1, table 10.2 structure 10 on this page, D-idose.
Page 1347, P-102.2.1, table 10.2 structure 11 on this page, D-galactose.
Page 1347, P-102.2.1, table 10.2 structure 12 on this page, D-talose.
Page 1348, P-102.2.1, Table 10.3 structure 2, D-erythrulose. replace the structure with:
Page 1348, P-102.2.1, Table 10.3 structure 3, D-ribulose.
Page 1348, P-102.2.1, Table 10.3 structure 4, D-xylulose.
Page 1348, P-102.2.1, Table 10.3 structure 6, D-fructose.
Page 1349, P-102.3.1, line 5.
Page 1349, P-102.3.1, structure a.
Page 1350, P-102.3.3, example 4.
Page 1352, P-102.3.4.1.2, lines 3/4. [corrected 23 September 2020]
Page 1353, P-102.3.4.2.2, example 3, left structure. [corrected 18 May 2023]
Page 1358, P-102.5.1.1.4, right hand structure.
Page 1358, P-102.5.2.2, line 1 and 2.
Page 1359, P-102.5.2.3, example 2.
Page 1361, P-102.5.2.3, example 1 left structure on this page. [corrected 3 October 2018]
Page 1362, P-102.5.3.4, example 1.
Page 1371, P-102.5.6.3.2, example 3.
Page 1371, P-102.5.6.3.2, example 4.
Page 1373, P-102.5.6.5.2, line 1. [corrected 18 May 2023]
Page 1374, P-102.5.6.5.2, example 2.
Page 1380, P-102.5.6.6.4.3, example 3.
Page 1381, P-102.5.6.6.5.1, line 3/4. [corrected 18 May 2023]
Page 1382, P-102.5.6.6.5.3, example 3. replace the structure with:
Page 1382, P-102.5.6.7.1, example 1.
Page 1383, P-102.6, line 2.
Page 1384, P-102.6.1, line 2 on this page.
Page 1385, P-102.6.1.2, example 2. [corrected 23 September 2020]
Page 1386, P-102.6.1.2, example on this page.
Page 1387, P-102.6.1.5, example 1. [corrected 3 October 2018]
Page 1387, P-102.6.1.6, section number.
Page 1388, P-102.6.1.6, last example.
Page 1389, P-102.7.1.1, example note line 2.
Page 1392, P-103.1.1.1, table 10.4 entry 2. [corrected 27 July 2018] for 2-amino-4-carbamimidamidopentanoic acid
Page 1392, P-103.1.1.1, Table 10.4, entry 4 aspartic acid [corrected 28 April 2023]
Page 1392, P-103.1.1.1 Table 10.4, entry 6 (glutamine).
Page 1392, P-103.1.1.1, table 10.4 entry 8. (glycine)
Page 1394, P-103.1.1.2, table 10.5, entry 2, symbol.
Page 1394, P-103.1.1.2, Table 10.5, entry 4.
Page 1394, P-103.11.3, Table 10.5 entry 6.
Page 1395, P-103.1.1.2, table 10.5, example 5 on this page.
Page 1395, P-103.1.1.2, table 10.5, last example on this page.
Page 1396, P-103.1.1.3, line 7. [corrected 17 April 2019]
Page 1400, P-103.2.1, example 5. [corrected 17 October 2018]
Page 1400, P-103.2.1, example 6. [corrected 24 October 2018]
Page 1401, P-103.2.2.2, last example on this page. [corrected 17 April 2019]
Page 1405, P-103.2.4.3.2, line 1. [corrected 26 June 2019]
Page 1406, P-103.2.7, line 1. [corrected 19 September 2018]
Page 1407, P-103.2.7, title.
Page 1407, P-103.2.7, example 1.
Page 1407, P-103.2.7, example 2.
Page 1407, P-103.2.7, example 3.
Page 1407, P-103.2.7, example 4 on this page. [corrected 23 September 2020]
Page 1408, P-103.3.1, structure.
Page 1409, P-103.3.4, line 9.
Page 1409, P.103.3.5, line 4. [corrected 17 April 2019]
Page 1410, P-103.3.5, example 2 on this page.
Page 1410, P-103.3.5.1, example 2. [corrected 17 April 2019]
Page 1411, P-103.3.5.1.1, example 1.
Page 1411, P-103.3.5.1.1, example 2.
Page 1411, P-103.3.5.1.2, example.
Page 1412, P-103.3.5.2.1, example 3. [modified 26 June 2019]
replace structure with: move example to P-103.3.5.2.2
Page 1412, P-103.3.5.2.2, example 1. for bradykinin-Leu
Page 1412, P-103.3.5.2.2, example 2. Replace structure with:
Page 1414, P-103.3.5.4, example 1 on this page. for des-PheB1-insulin(cattle)
Replace structure with:
Page 1416, P-104.2.2, example 1. [modified 2 January 2019] replace the structure with:
Page 1417, P-104.2.3, example 1.
Page 1419, P-104.3.1, example 3 on this page. [modified 12 August 2020]
for (1s,2S,3R,4r,5S,6R)-1-methylcyclohexane-1,2,3,4,5,6-hexol
Page 1419, P-104.3.1, example 4 on this page. [modified 21 October 2020
add (not 2-carboxy-2-deoxy-myo-inositol; COOH is senior to OH)
Page 1422, P-105.2.1, example 2.
Page 1423, P-105.2.1, example 3 on this page. [corrected 21 October 2020
Page 1424, P-105.2.1, example on this page. [corrected 19 September 2018] for guanosine 2′,3′,5′-triacetate
Page 1428, P-106.3.1, example 1 on this page.
Page 1430, P-106.3.3, example on this page.
Page 1432, P-107.2, example 2. [corrected 28 October 2016]
Page 1433, P-107.3.1, structure 1.
Page 1433, P-107.3.2, line 9. [corrected 17 April 2019]
Page 1434, P-107.3.3, example. [modified 22 April 2020] for O-({[(2R)-2,3-bis(octadecanoyloxy)propoxy]hydroxyphosphoryl})-L-serine replace the structure with:
Page 1435, P-107.3.5, example.
Page 1435, P-107.3.5 example [corrected 28 April 2023]
Page 1435, P-107.3.6 example. [modified 28 April 2023]
Page 1436, P-107.4.2 example. [modified 28 April 2023]
replace the structure with:
Page 1443, appendix 1, column 2, line 14.
Page 1444, appendix 1, column 1, line 17.
Page 1444, Appendix 1, column 2, elements 15 and 16. [corrected 23 September 2020]
Page 1447, appendix 2, line 1
Page 1447, appendix 2, line 16
Page 1448, appendix 2, line 20. [corrected 14 November 2018]
Page 1449, appendix 2, line 18.
Page 1450, appendix 2, line 6. [corrected 24 April 2019]
Page 1450, appendix 2, line 15. [modified 20 January 2021]
add ONN-azoxy –N(O)=N– P-68.3.1.3.3.1
add NNO-azoxy –N=N(O)– P-68.3.1.3.3.1
Page 1450, appendix 2, lines 20-21. [modified 25 November 2020]
Page 1450, appendix 2, line 27. [corrected 24 April 2019]
Page 1450, appendix 2, lines 22/23. [corrected 24 April 2019]
Page 1451, appendix 2, lines 1/2. [corrected 24 April 2019]
Page 1451, appendix 2, lines 5/6. [corrected 24 April 2019]
Page 1451, appendix 2, lines 16-19. [corrected 24 April 2019]
Page 1452, appendix 2, line 5. [corrected 24 April 2019]
Page 1452, appendix 2, line 6. [corrected 24 April 2019]
Page 1452, appendix 2, line 16, and move to under line 21. [corrected 24 February 2021]
Page 1452, appendix 2, line 18. [corrected 24 April 2019]
Page 1452, appendix 2, lines 22/23. [modified 25 November 2020]
Page 1453, appendix 2, line 4. [corrected 24 April 2019]
Page 1453, appendix 2, column 2, line 16. [corrected 26 September 2018]
Page 1455, appendix 2, line 10. [corrected 24 April 2019]
Page 1455, appendix 2, lines 11/12. [modified 20 January 2021]
Page 1455, appendix 2, column 2, structure 5 from bottom. [corrected 9 January 2019]
Page 1456, appendix 2, line 10/11. [modified 20 January 2021]
Page 1456, appendix 2, line 19. [corrected 24 April 2019]
Page 1457, appendix 2, line 11.
Page 1457, appendix 2, line 17. [corrected 31 October 2018]
Page 1458, appendix 2, line 21, move to after line 24. [corrected 24 April 2019]
Page 1459, appendix 2, line 23. [move after line 24]
Page 1459, appendix 2, column 2, line 13.
Page 1460, appendix 2, line 1. [corrected 25 November 2020]
Page 1460, appendix 2, line 20. [corrected 1 May 2019]
Page 1460, appendix 2, line 21. [corrected 1 May 2019]
Page 1460, appendix 2, lines 24-26. Correction deleted [11 November 2020]
Page 1460, appendix 2, lines 27-29. Correction deleted [11 November 2020]
Page 1460, appendix 2, line 30. [corrected 1 May 2019]
Page 1462, appendix 2, line 1. [corrected 1 May 2019]
Page 1462, appendix 2, line 15.
Page 1462, appendix 2, lines 26/27. [corrected 1 May 2019]
Page 1462, appendix 2, line 28. [corrected 1 May 2019]
Page 1462, appendix 2, lines 29/30. [corrected 1 May 2019]
Page 1462, Appendix 2, column 2, line 20. (corrected 27 November 2019)
Page 1463, appendix 2, line 28. [corrected 1 May 2019]
Page 1463, appendix 2, column 2, structure 2. [corrected 9 January 2019]
Page 1464, appendix 2, line 21. [modified 3 March 2021]
Page 1464, appendix 2, line 25 and move to page 1485 after line 2. [modified 3 March 2021]
Page 1464, appendix 2, column 2, structure 3. [corrected 17 October 2018]
Page 1464, appendix 2, column 2, structure 4. [corrected 17 October 2018]
Page 1464, appendix 2, column 2, structure 20.
Page 1465, appendix 2, line 1. [modified 3 March 2021]
Page 1466, appendix 2, line 19. [corrected 8 May 2019]
Page 1466, Appendix 2, lines 24/25. [modified 22 January 2020]
Page 1467, Appendix 2, lines 11/12. [modified 22 January 2020]
Page 1467, appendix 2, lines 14/15. (modified 27 November 2019)
Page 1468, appendix 2, lines 6/7. (modified 27 November 2019)
Page 1468, appendix 2, lines 8/9. [corrected 8 May 2019]
Page 1468, appendix 2, line 17.
Page 1468, appendix 2, lines 20/21. [modified 20 January 2021]
Page 1468, appendix 2, line 23. [corrected 8 May 2019]
Page 1468, appendix 2, line 26. [corrected 8 May 2019]
Page 1469, appendix 2, lines 6/7. [corrected 15 May 2019]
Page 1469, appendix 2, lines 10/11. [corrected 15 May 2019]
Page 1469, appendix 2, line 14.
Page 1469, appendix 2, line 17. [corrected 15 May 2019]
Page 1470, appendix 2, line 15. [corrected 15 May 2019]
Page 1470, appendix 2, line 17. [corrected 15 May 2019]
Page 1471, appendix 2, line 5.
Page 1471, appendix 2, line 13.
Page 1472, appendix 2, line 1. [corrected 15 May 2019]
Page 1472, appendix 2, line 2. [corrected 15 May 2019]
Page 1473, appendix 2, line 13. [corrected 15 May 2019]
Page 1473, appendix 2, lines 22/23. [modified 30 December 2020]
Page 1473, appendix 2, lines 24-26. [corrected 15 May 2019]
Page 1474, appendix 2, above line 1. [corrected 9 December 2020]
Page 1474, appendix 2, lines 1/2.. [corrected 9 December 2020]
Page 1474, appendix 2, lines 3-5.. [modified 9 December 2020]
Page 1474, appendix 2, below line 3. [corrected 15 May 2019]
Page 1474, appendix 2, line 14. [corrected 15 May 2019]
Page 1474, appendix 2, lines 22-24. [corrected 15 May 2019]
Page 1474, appendix 2, column 3 line 7.
Page 1475, appendix 2, line 13.
Page 1475, appendix 2, line 22. [corrected 28 October 2016]
Page 1475, appendix 2, line 24. [corrected 15 May 2019]
Page 1476, appendix 2, lines 5/6. [modified 10 February 2021]
Page 1476, appendix 2, lines 22/23. [corrected 22 May 2019]
Page 1476, appendix 2, line 26. [corrected 22 May 2019]
Page 1476, appendix 2, column 2, structure 6.
Page 1477, appendix 2, line 18. [corrected 22 May 2019]
Page 1478, appendix 2, line 1. [corrected 22 May 2019]
Page 1478, appendix 2, ccolumn 2, structure 16.
Page 1479, appendix 2, line 6. [corrected 22 May 2019]
Page 1480, appendix 2, column 2, line 5.
Page 1481, appendix 2, line 1. (corrected 4 December 2019)
Page 1481, appendix 2, line 3.
Page 1483, appendix 2, line 17.
Page 1484, appendix 2, line 18. [corrected 29 May 2019]
Page 1484, appendix 2, lines 28-29 and move after line 23. [modified 9 December 2020]
Page 1484, appendix 2, column 2, structure 16. [corrected 29 May 2019]
Page 1485, appendix 2, line 7. (corrected 4 December 2019)
Page 1485, appendix 2, column 2, line 10.
Page 1488, appendix 2, line 1. [modified 9 December 2020]
for 2-naphthyl = naphthalene-2-yl*
Page 1488, appendix 2, line 13. [modified 9 December 2020]
Page 1488, appendix 2, line 15.
Page 1489, appendix 2, line 15.
Page 1489, appendix 2, column 2, structure 10.
Page 1489, appendix 2, column 2, structure 19.
Page 1489, appendix 2, last line. [corrected 18 September 2019]
Page 1490, appendix 2, line 3-4. [modified 29 May 2019]
Page 1490, appendix 2, line 7. [modified 29 May 2019]
Page 1490, appendix 2, line 10.
Page 1490, appendix 2, line 25.
Page 1490, appendix 2, line 28. [corrected 18 September 2019]
Page 1490, appendix 2, column 2, structure 8.
Page 1490, appendix 2, line 28. [corrected 18 September 2019]
Page 1491, appendix 2, column 2, structure 13.
Page 1492, appendix 2, line 9. [modified 29 May 2019]
Page 1492, appendix 2, line 14. [modified 21 April 2021]
Page 1492, appendix 2, column 2, line 6.
Page 1492, appendix 2, column 2, line 22.
Page 1493, appendix 2, line 24. [modified 30 December 2020]
Page 1495, appendix 2, line 3.
Page 1495, appendix 2, column 2, structure 17.
Page 1497, appendix 2, lines 12-14. Correction deleted [11 November 2020]
Page 1499, appendix 2, column 2, structure 3.
Page 1499, appendix 2, column 2, structure 10. [corrected 5 June 2019]
Page 1502, appendix 2, line 21.
Page 1503, appendix 2, line 4. (modified 27 November 2019)
Page 1503, appendix 2, line 5 [modified 22 January 2020]
Page 1503, appendix 2, line 10.
Page 1504, appendix 2, column 2, structure 17.
Page 1505, appendix 2, lines 16-18. [modified 7 August 2019]
Page 1506, appendix 2, lines17-19. [corrected 12 June 2019]
Page 1508, appendix 2, line 2 (modified 4 December 2019)
Page 1508, appendix 2, column 3, line 11.
Page 1508, appendix 2, line 28. [corrected 19 June 2019]
Page 1509, appendix 2, line 1.
Page 1509, appendix 2, lines 13/14. [corrected 19 June 2019]
Page 1509, appendix 2, lines 18/19. [corrected 19 June 2019]
Page 1509, appendix 2, column 2, structure 7.
Page 1510, appendix 2, line 1. [modified 19 June 2019]
Page 1510, appendix 2, line 9.
Page 1510, appendix 2, column 2, line 1.
Page 1511, appendix 2, line 6.
Page 1511, Appendix 2, line 10. [corrected 19 June 2019]
Page 1512, Appendix 2, line 14.
Page 1513, appendix 2, line 3. [corrected 26 June 2019]
Page 1513, appendix 2, lines 8/9. [modified 7 August 2019]
Page 1513, appendix 2, column 2, structure 14. [corrected 26 June 2019]
Page 1514, appendix 2, line 1. [corrected 21 April 2021]
Page 1514, appendix 2, line 3. [corrected 21 April 2021]
Page 1517, Appendix 3, structure 6 on this page. [corrected 17 October 2018]
Page 1518, Appendix 3, structure 2 on this page. [corrected 26 September 2018]
Page 1518, Appendix 3, structure 3 on this page. [corrected 17 October 2018]
Page 1521, Appendix 3, structure 6 on this page. [corrected 17 October 2018]
Page 1522, Appendix 3, ormosanine. [modified 14 October 2020]
Page 1522, Appendix 3, structure 5 on this page. [corrected 17 October 2018]
Page 1523, Appendix 3, structure 1 on this page. [corrected 24 October 2018]
Page 1523, appendix 3, structure 2 on this page.
Page 1524, Appendix 3.
Page 1524, Appendix 3, spirosolane. [modified 14 October 2020] add footnote †† The CAS name for this structure requires the chirality at C-22 to be specified.
Page 1525, Appendix 3, tropane. [modified 14 October 2020]
Page 1525, Appendix 3, structure 4 on this page. [corrected 17 October 2018] replace the structure with:
Page 1526, Appendix 3, structure 4 on this page. [corrected 17 October 2018]
Page 1526, Appendix 3, vobasan. [modified 14 October 2020]
replace the structure with:
Page 1527, Appendix 3, structure 1 on this page. [corrected 17 October 2018] .
Page 1528, appendix 3, structure 1 on this page.
Page 1529, appendix 3, structure 2 on this page.
Page 1529, appendix 3, structure 4 on this page.
Page 1530, appendix 3, structure 2 on this page.
Page 1530, appendix 3, structure 3 on this page.
Page 1531, Appendix 3, structure 1 on this page. [modified 1 January 2019]
Page 1531, appendix 3, structure 2 on this page.
Page 1531, Appendix 3, structure 3 on this page. [corrected 9 January 2019]
Page 1532, appendix 3, structure 3 on this page. [modified 14 October 2020]
Add footnote †† The CAS name for this structure is based on kaurane
Page 1533, appendix 3, example 1 on this page.
Page 1533, appendix 3, example 3 on this page.
Page 1533, appendix 3, example 4 on this page.
Page 1536, appendix 3, footnote ††
Page 1538, Appendix 3.
Page 1539, Appendix 3, structure 2 on this page. [corrected 24 October 2018]
Page 1539, second footnote.
Page 1540, Appendix 3, structure 4 on this page. [corrected 17 October 2018]
Page 1540, Appendix 3, structure 5 on this page. [corrected 17 October 2018]
Page 1552, index.
For [not 16H-1,13,18-(epiethFor [not 16H-1,13,18-(epiethene[1,2]diyl[1]ylidene)-2,11,12-epiprop[1]ene[1,3]diyl[3]ylidene)acephenanthryleno[4,3-bc]tricyclopenta[n,pqr,tuv]picene (II)]
read [not 8,7,2-(epiethane[1,2]diyl[1]ylidene)-1,9,18-(epiprop[1]ene[1,1]diyl[3]ylidene)acephenanthryleno[4,3-bc]tricyclopenta[n,pqr,tuv]picene (II)]
(II)]
For 2b2,8a2:5,8-dimethenecycclohexadeca[1,2,3,4,5,6-cdefg:7,8,9,10,11,12-c′d′e′f′g′]di-as-indacene (I) (PIN)
read 3,2,13,12-(epihexa[1,3,5]trien[1,3,4,6]tetrayl)-6,9-methenocycloundeca[1,11,10-cd:6,7,8-c′d′]diindene (I) (PIN)
Replace the structures with:
Reverse the structures for (I) and (II).
read 2,15:3,14-dimethenoindeno[5'',4'':6',7']cyclododeca[1',2':4,5]indeno[1,2-b]anthracene (PIN)
For 2,2′-bi(bicyclo[2.2.2]octan)-2-yl
read 2,2′-bi(bicyclo[2.2.2]octanyl)
Replace structure with:
For 1,8(2),2,3,4,5,6,7(2,5)-heptathiophenaoctaphane (PIN)
read 1,8(2),2,3,4,5,6,7(2,5)-octathiophenaoctaphane (PIN)
For (a) di(tridecyl)benzene
read 1,2-di(tridecyl)benzene
read [not 1,1′-(1,2-phenylene)di(tridecane);
Replace the structure with:

for cyclododeca-1,3,5,7,9-pentaen-11-yne (PIN)
read (1Z,3E,5Z,7E,9Z)-cyclododeca-1,3,5,7,9-pentaen-11-yne (PIN)
for 1,2-didehydro[12]annulene
read 1,2-didehydro[12]annulene (numbering shown)
Replace the structure with:
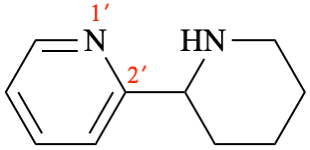
read (1Z,3E,5Z,7E,9Z)-cyclododeca-1,3,5,7,9-pentaen-11-yne (PIN)
for 1,2-didehydro[12]annulene
read 1,2-didehydro[12]annulene (numbering shown)
For 4-[4-(4′-phenyl-[1,1′-bi(cyclohexan)]-4-yl)phenyl]-11,21:24,31-tercyclohexyl
read 14-[4-(4′-phenyl[1,1′-bi(cyclohexan)]-4-yl)phenyl]-11,21:24,31-tercyclohexyl
For 1,4-thiazepinane (PIN)
read 1,4-thiazepane (PIN)
Delete pentazolane
For ...see also P-15.1.3.2, P-31.2, P-58.2), ...
read ...see also P-15.1.5.2, P-31.2, P-58.2), ...
For 1,2-dihydro2,2′-binaphthyl
read 1,2-dihydro-2,2′-binaphthyl
For ...except anisole and tert-butoxy, formic acid, and the formyl group are substitutable with limitations.
read ...except anisole and tert-butoxy, and formic acid and formyl group are substitutable with limitations.
For ...‘SO-thioperoxol’, SeO-selenoperoxol’, ‘SS-dithioperoxol’, TeS-tellurothioperoxol’, ‘SeSe-diselenoperoxol’, ‘SeTe-selenotelluroperoxol’, and ‘TeSe-selenotelluroperoxol’ (see P-63.4.2.1).
read ...‘SO-thioperoxol’, ‘SeO-selenoperoxol’, ‘dithioperoxol’, ‘TeS-tellurothioperoxol’, ‘diselenoperoxol’, ‘SeTe-selenotelluroperoxol’, and ‘TeSe-telluroselenoperoxol’ (see P-63.4.2.1).
For ...suffix ‘sulfenic aced and it chalcogen...
read ...suffix ‘sulfenic acid’ and its chalcogen...
For ...rather thanmethanediyl. CAS...
read ...rather than methanediyl. CAS... [insert space between words]
For (carbonyldiimino, a name...
read (not carbonyldiimino, a name...
For 1-propanylidene
read propan-1-ylidene
For ... isopropylidene for (CH3)C=, ...
read ... isopropylidene for (CH3)2C=, ...
For (2-methylbutyl)
read (2-methylpropyl)
For anthacen-2-yl (preferred prefix)
read anthracen-2-yl (preferred prefix)
For 7-isoquinolyl (also 1-, 3-, 5-, 6-, 7-, and 8- isomers)
read 7-isoquinolyl (also 1-, 3-, 4-, 5-, 6-, and 8-isomers)
read isoquinol-7-yl (also 1-, 3-, 4-, 5-, 6-, and 8-isomers, preferred prefixes)
For naphthalen-2-yl (also 2-isomer;
read naphthalen-2-yl (also 1-isomer;
For ‘Benzyl’ is chosen; it is larger than ‘methyl’ leading to the prefix ‘phenylmethoxy’.
read ‘Benzyl’ is chosen; it is larger than ‘methyl’ leading to the prefix ‘benzyloxy’.
For 4-[4-(benzyloxy)phenyl]methoxy (prefered prefix)
read [4-(benzyloxy)phenyl]methoxy (preferred prefix)
For [1,1′-biphenyl]-4-yloxy (preferred prefix)
read ([1,1′-biphenyl]-4-yl)oxy (preferred prefix)
For ...which results in the prefix ‘[1,1′-biphenyl]-4-yloxy’.
read ...which results in the prefix ‘([1,1′-biphenyl]-4-yl)oxy’.
For [not (dimethylamino)carbonyl]hydrazinylidene]
read [not [(dimethylamino)carbonyl]hydrazinylidene]
For ...name 2-(3-cyanophenoxy])4-isopropylbenzonitrile would...
read ...name 2-(3-cyanophenoxy)-4-isopropylbenzonitrile would...
For 4-chloro-2-(1,3-oxazol-5-ylmethyl)-1,3-oxazole (PIN)
read 4-chloro-2-[(1,3-oxazol-5-yl)methyl]-1,3-oxazole (PIN)
For 1H,2H-3a,7a-methano[2]benzofuran (PIN)
read 1H,2H-3a,7a-methano-2-benzofuran (PIN)
For (not 2,3-dihydro-4H-1-benzoyran;...
read (not 2,3-dihydro-4H-1-benzopyran;...
For napthalene-4a,8a-diyl (preferred prefix)
read naphthalene-4a,8a-diyl (preferred prefix)
For tetrahydro-4H-pyran-4-one (PIN)
read tetrahydro-4H-pyran-4-one
add oxan-4-one (PIN)
For 2-(1,3,4,5-tetrahydro-2H-2-benzazepin-2-yl)ethanol (PIN)
read 2-(1,3,4,5-tetrahydro-2H-2-benzazepin-2-yl)ethan-1-ol (PIN)
For 1,2,3,4,4a,5,7,11b-octahydro-6H-dibenzo[a,c]cycloheptene-6,6-dicarboxylic acid (PIN)
read 1,2,3,4,4a,5,7,11b-octahydro-6H-dibenzo[a,c][7]annulene-6,6-dicarboxylic acid (PIN)
For dihydro-1H,3H,4H-3a, 6a-methanocyclopenta[c]furan-1,3-dione (PIN)
read 5,6-dihydro-1H,3H,4H-3a,6a-methanocyclopenta[c]furan-1,3-dione (PIN) [delete space in name]
read (not 4,5-dihydro-1H,3H,6H-3a,6a-methanocyclopenta[c]furan-1,3-dione; ...
For (not 2H,8H-benzo[1,2-b:5,4-b′]dipyran-4(3H)-one;
read (not 6,7-dihydro-2H,8H-benzo[1,2-b:5,4-b′]dipyran-4(3H)-one;
read ‘2H,6H’ is lower than ‘2H,8H’)
For (not 4,4a-dihydro 2H,4H-benzo[1,2-b:4,3-c′]dipyran-5,6(4aH,6aH)-dione)
read (not 4,4a-dihydro-2H,4H-benzo[1,2-b:4,3-c′]dipyran-5,6(4aH,6aH)-dione)
For ... positions; (6H,8H)-pyrido[3,2,1-ij]quinoline is not a permissible structure), so ...
read ... positions; (6H,8H-pyrido[3,2,1-ij]quinoline is not a permissible structure), so ...
For 1,3-dihydro-1,3-dioxo-2H-isoindole-2,5-diyl (preferred prefix)
read 1,3-dioxo-1,3-dihydro-2H-isoindole-2,5-diyl (preferred prefix)
For N-triazan-1-ylbenzamide (PIN)
read N-(triazan-1-yl)benzamide (PIN)
read [not phenyl(tetraazan-1-yl)methanone; nor 1-benzoyltetraazane]
For tetrazane-1-carboxylic acid (PIN)
read tetraazane-1-carboxylic acid (PIN)
read [not (triazan-1-yl)carbamic acid; the carboxylic acid is senior to the carbonic acid derivative]
For [not 1-(oxomethylidene)carbonyltetrazane]
read [not 1-(oxomethylidene)tetraazane]
For N,N′-hydrazine-1,2-diyldibenzamide (PIN)
read N,N′-(hydrazine-1,2-diyl)dibenzamide (PIN)
For N-isocyanatonitramide
read isocyanatonitramide
For [not trisulfanyl benzenecarbothiaote;
read [not trisulfanyl benzenecarbothioate;
For –S(O2)-R2,6
read –S(O)2-R2,6
For ... as thiocyanate and selenocyanate.
read ... as isothiocyanate and isoselenocyanate.
For ...propan-2-ol (mot propane-2-ol).
read ...propan-2-ol (not propane-2-ol).
For CH3-CH2-CH=CH2CH2-COOH
read CH3-CH2-CH=CH-CH2-COOH
For ethanediol
read ethane-1,2-diol
For 1-propanol
read propan-1-ol
Replace structure with:
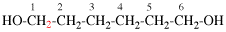
Replace structure with:
-CH2-CH2-CH2-CH2-CH2-CH3
This example should be deleted. The PIN for this example is oxan-3-one. It should be replaced with:
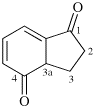
Analysis:
Principal characteristic group: =O dione
Parent hydride: 
1H-indene
Functionalised parent hydride: 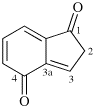
1H-indene-1,4(2H)-dione
Saturation prefix: dihydro
For 3a,9b-dihydro-3H-cyclopenta[a]naphthalene-3,5(2H)-dione
read 3a,9b-dihydro-1H-cyclopenta[a]naphthalene-3,5(2H,4H)-dione
Add allylcyclohexane
For 1,3-di(propan-2-yl)cyclohexane (PIN)
read 1,4-di(propan-2-yl)cyclohexane (PIN)
For {not [1-methyl-3-(4-methylcyclohex-3-en-1-yl) prop-2-en-1-y]benzene;
read {not [1-methyl-3-(4-methylcyclohex-3-en-1-yl)prop-2-en-1-yl]benzene; [delete space from name]
For 1,1′,1′′-(benzene-1,2,4-triyltripropane-3,1-diyl)tris(4-methylbenzene) (PIN)
read 1,1′,1′′-[benzene-1,2,4-triyltri(propane-3,1-diyl)]tris(4-methylbenzene) (PIN)
For 2,3,7,8-tetra(naphthalen-2-yl)anthracene
read 2,3,6,7-tetra(naphthalen-2-yl)anthracene
replace the structure with:
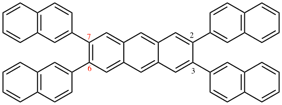
For 2,6-bis(benzo[a]anthrac
read 2,6-bis(benzo[a]anthracen-1-yl)pyridine
Add 1,4-bis(1-chloro-1-methylethyl)benzene
For 1-(5-bromopent-2-en-2-yl)cyclopropane
read (5-bromopent-2-en-2-yl)cyclopropane
read (4-bromo-1-methylbut-1-en-1-yl)cyclopropane
For C60Ih
read C60-Ih
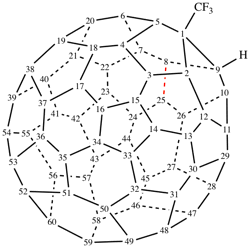
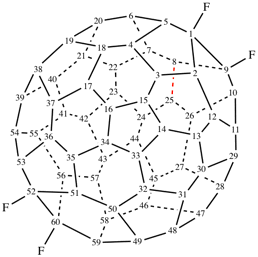
For chloro(dimethyl)borane (PIN)
read chlorodi(methyl)borane (PIN)
For chloro(methyl)phosphane]
read chloro(methyl)phosphane
For N-methylhypochlorous amide (PIN; see P-67.1.2.6)
read methylhypochlorous amide (PIN; see P-68.5.3)
Replace the structure with:
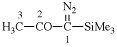
For N-(2-methylpropyl)oxoarsanamine (PIN)s
read N-(2-methylpropyl)-1-oxoarsanamine
For 4-isocyanatobenzenesulfonyl chloride (PIN)
read 4-isocyanatobenzene-1-sulfonyl chloride (PIN)
Replace the structure with:
C6H5-NC
For λ2-methylidenehydroxylamine (PIN)
read λ2-methylidenehydroxylamine
For (quinolin-4-yl)amine
read (quinolin-4-yl)azane
For 1-thiacyclotridecan-3-yl)azane
read (1-thiacyclotridecan-3-yl)azane
For trimethylsilamamine (PIN)
read 1,1,1-trimethylsilanamine (PIN)
For ‘formed by method (2) are set off by parentheses’
read ‘formed by method (3) are set off by parentheses’
For N,N-diethylethan-1-amine
read N,N-diethylethanamine
read triethylazane
For (1) N-silylsilanamine
read (1) N-silylsilanamine (preselected name)
Delete (not 2,2′-dimethyl-N-(2,2-dimethylpropyl)di(prop-2-en-1-amine)
read N-butylcyclopropanamine (PIN)
For N-cyclopropylbutan-1-amine
read (not N-cyclopropylbutan-1-amine)
For (not 5,6,7,8-tetrahydrodi-2-naphthylamine)
read [not 5,6,7,8-tetrahydrodi(2-naphthyl)amine]
For (not disilazan-2-yl acetic acid)
read [not (disilazan-2-yl)acetic acid]. [deleted space from name]
For [(4′-amino[1,1′-biphenyl]-4-yl)amino]
read (4′-amino[1,1′-biphenyl]-4-yl)amino
Replace structure by
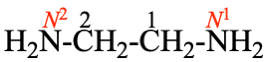
Replace the structure with:
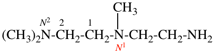
For N1,N1′-(azanediyldiethane-2,1-diyl)di(ethane-1,2-diamine) [PIN;...
read N1,N1′-[azanediyldi(ethane-2,1-diyl)]di(ethane-1,2-diamine) [PIN;...
(substitutive name; alphabetically inferior to the first substitutive name]
For N1,N2-{2-[(2-aminoethyl)amino]ethyl}ethane-1,2-diamine
read N1,N2-bis{2-[(2-aminoethyl)amino]ethyl}ethane-1,2-diamine >
For N1,N1′-(cyclohexane-1,4-diyldiethane-2,1-diyl)di(propane-1,3-diamine) [PIN...
read N1,N1′-[1,4-phenylenedi(ethane-2,1-diyl)]di(propane-1,3-diamine) [PIN...
For N1-(4-aminophenyl)-N1,N1′-(azanediyldi-1,4-phenylene)di(benzene-1,4-diamine)
read N1-(4-aminophenyl)-N1,N1′-(azanediyldi-4,1-phenylene)di(benzene-1,4-diamine)
For N1-(4-aminophenyl)- N1,N1′-(azanediyldi-4,1-phenylene)di(benzene-1,4-diamine)
read N1-(4-aminophenyl)- N1,N1′-[azanediyldi(4,1-phenylene)]di(benzene-1,4-diamine)
For ...reserved to denot e only the...
read ...reserved to denote only the... [remove space in word]
Replace structure with:
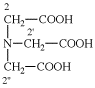
Add 4-(4-cyanoanilino)benzonitrile
For N,N-diethylmethanediamine (PIN)
read N,N′-diethylmethanediamine
read N,N′-methylenediethanamine (PIN)
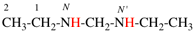 Page 680, P-62.2.5.2 (2) (ii), example 2.
Page 680, P-62.2.5.2 (2) (ii), example 2.
For N1,N1′--methylenedi(benzene-1,4-diamine)
read N1,N1′-methylenedi(benzene-1,4-diamine)
For 2,2′-methylenebis[N12-methyl-1,4(1,4)dibenzenacyclohexaphane-12,13-diamine
read 2,2′-methylenebis[N12-methyl-1,4(1,4)dibenzenacyclohexaphane-12,13-diamine]
For (5,6,7,8-tetrahydronaphthalen-2-y l)azane
read (5,6,7,8-tetrahydronaphthalen-2-yl)azane [delete space in name]
read (5,6,7,8-tetrahydronaphthalen-2-yl)amine [delete space in name]
For 2,4a-dihydronaphthalene-2,4a-diamine
read 2,4a-dihydronaphthalene-2,4a-bis(azane)
read 2,4a-dihydronaphthalene-2,4a-diamine
For N,N′-dimethylnaphthalene-1,4-diimine (PIN; see also P-58.2.2.3)
read N,N′-dimethylnaphthalene-1,4-diimine (see also P-58.2.2.3)
read [not N,N′-(naphthalene-1,4-diylidene)bis(methanamine)]
read [not N,N′-(naphthalene-1,4-diylidene)bis(methylamine)]
read dimethyl(naphthalene-1,4-diylidene)bis(amine)]
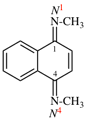
For methylphosphanimine
read 1-methylphosphanimine
For ...(see P-67.1.2.6) . Compounds such as R-NH-OH are named as N-derivatives of hydroxylamine, NH2-OH (see P-68.3.1.1.1). Names based on N-substitution of amines are not recommended in these cases.
read ...(see P-67.1.2.6). Compounds such as R-NH-OH are named as N-derivatives of the senior amine (see P-68.3.1.1.1).
For N-methoxyethan-1-amine (PIN, see P-68.1.1.3)
read N-methoxyethanamine (PIN, see P-68.1.1.1.3)
For N-ethylhydroxylamine (PIN)
read N-ethylhydroxylamine
For N-ethylhypochlorous amide (PIN)
read ethylhypochlorous amide (PIN)
For N-chloroethan-1-amine
read N-chloroethanamine
For N-methylnitrous amide (PIN)
read methylnitrous amide (PIN)
for (not N-nitrosomethanamine)
read N-nitrosomethanamine
For N-methyl-N-nitronitramide (PIN)
read methyl(nitro)nitamide (PIN)
read N,N-dinitromethanamine
For N-methylbromous amide (PIN)
read methylbromous amide (PIN)
read N-bromosylmethanamine
For ...are described in P-63.2.5.1. multiplicative names ...
read ...are described in P-63.2.5. multiplicative names ...
For 3-carbamoyl-5-(2-aminoethyl)benzoic acid N5-oxide
read 3-(2-aminoethyl)-5-carbamoylbenzoic acid N3-oxide
For {not {[2-(3-carbamoyl-5-carboxyphenyl)ethyl]azaniumyl}oxidanide (see also P-71.2.1.2}
read (3) {[2-(3-carbamoyl-5-carboxyphenyl)ethyl]azaniumyl}oxidanide (see also P-71.2.1.2)
For (2) 2-{3-[(dimethyloxo-λ5- azanyl)methyl]phenyl}-N,N-dimethyl-ethan-1-amine N-oxide
read (2)
2-(3-{[dimethyl(oxo)-λ5-azanyl]methyl}phenyl)-N,N-dimethylethan-1-amine N-oxide (PIN) [Deleted space and a hyphen from the name]
read 2-{3-[(dimethylamino)methyl]phenyl}-N,N-dimethylethan-1-amine N1,N3-dioxide
read (3) 2-(3-{[dimethyl(oxido)azaniumyl]methyl}phenyl)-N,N-dimethylethan-1-amine N-oxide (see also P-74.2.1.2)
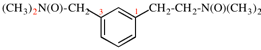
For N,N-diethylethan-1-amine N-sulfide (PIN)
read N,N-diethylethanamine N-sulfide (PIN)
For (2) ethyldimethylazanium iodide
read (2) ethyldi(methyl)azanium iodide
For N-methylbenzenaminium bromide (PIN)
read N-methylbenzenaminium bromide
For N-methylethan-1-iminium chloride (PIN)
read N-methylethaniminium chloride (PIN)
For N-phenylethan-1-iminium bromide (PIN)
read N-phenylethaniminium bromide (PIN)
Replace the structure with:
For ...For instance, H2Si-OH is classified...
read ...For instance, H3Si-OH is classified...
Delete P-63.1.6 Polyfunctional hydroxy compounds
Replace the structure with:
For (C60-Ih)[5,6]fulleren-1(9H)-ol (PIN)
read (C60-Ih)[5,6]fulleren-1(9H)-ol (PIN)
read 1,9-dihydro(C60-Ih)[5,6] fulleren-1-ol
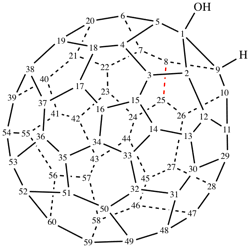
For (1) 2-fluoro-6-[(2-{[(3-fluoro-2-hydroxyphenyl)methylidene]amino}ethyl)imino]methyl}phenol
read (1) 2-fluoro-6-{[(2-{[(3-fluoro-2-hydroxyphenyl)methylidene]amino}ethyl)imino]methyl}phenol
For hydroxy(trimethyl)silane
read hydroxytri(methyl)silane
For ...are described in P-63.2.5.1. multiplicative names ...
read ...are described in P-63.2.5. multiplicative names ...
For butan-2-yloxy
read (butan-2-yl)oxy
For pyridin-2-yloxy
read (pyridin-2-yl)oxy
For piperidin-2-yloxy
read (piperidin-2-yl)oxy
For 2-(butan-2-yloxy)ethyl
read 2-[(butan-2-yl)oxy]ethyl
For propan-2-yloxy
read (propan-2-yl)oxy
For butan-2-yloxy
read (butan-2-yl)oxy
For (not 2-methoxyanisole; see P-34.1.1.2 and P-15.1.8.2 for substitution rules for anisole)
read 2-methoxyanisole (see P-34.1.1.4 and P-15.1.8.2 for substitution rules for anisole)
For 2-(pyridin-3-yloxy)pyrazine (PIN)
read 2-[(pyridin-3-yl)oxy]pyrazine (PIN)
For (3) 2,4′-dichloro-1,1′-oxydibenzene
read (3) 2,4′-dichloro-1,1′-oxydibenzene (numbering shown)
For (1) 1-[(penta-1,4-dien-3-yl)sulfanyl)]cyclobutane (PIN)
read (1) [(penta-1,4-dien-3-yl)sulfanyl]cyclobutane (PIN)
read [(penta-1,4-dien-3-yl)thio]cyclobutane
For (1) 2-{[(methylsulfanyl)methyl]sulfanylthio}-1-[({[(methylsulfanyl)methyl]sulfanyl}methyl)sulfanyl]propane
read (1) 2-{[(methylsulfanyl)methyl]sulfanyl}-1-[({[(methylsulfanyl)methyl]sulfanyl}methyl)sulfanyl]propane
For (1) 1-(propan-2-ylselanyl)-2-(propylselanyl)propane (PIN)
read (1) 1-[(propan-2-yl)selanyl]-2-(propylselanyl)propane (PIN)
read 1-[(propan-2-yl)seleno]-2-(propylseleno)propane
For 1-phenoxy-3-[[3-(phenylselanyl)phenyll]sulfanyl]benzene (substitutive name)
read 1-phenoxy-3-{[3-(phenylselanyl)phenyl]sulfanyl}benzene (substitutive name)
read not 1-[(3-phenoxyphenyl)sulfanyl]-3-(phenylselanyl)benzene (substitutive name)
read (the first substitutive name is correct because phenoxy-phenylselanyl is lower alphabetically than phenoxyphenyl-sulfanyl)
For (1) 1-(propan-2-yldiselanyl)propane (PIN)
read (1) 1-[(propan-2-yl)diselanyl]propane (PIN)
For 1-(methyldiseleno)-2-(methyldithio)disilane
read 1-(methyldiseleno)-2-(methylditelluro)disilane
For [4-(4-carboxyphenyl)peroxy]benzoic acid
read 4-[(4-carboxyphenyl)peroxy]benzoic acid
Delete
(1) 1-(2-{[2-(methylsulfanyl)ethyl]sulfanyl}ethyl)-2-[(methylsulfanyl)methyl]disulfane
Delete (1) bis[3-(phenylsulfanyl)phenyl]disulfane
For {not phenyltellanyl)selanyl]benzene; ...
read {not [(phenyltellanyl)selanyl]benzene; ...
Delete (1) 1-[2-({[2-(methylselanyl)ethyl]sulfanyl}ethyl)]-2-[(methylsulfanyl)methyl]disulfane
read [not (methylsufanyl)methyl 2-{[2-(methylselanyl)ethyl]sulfanyl}ethanesufenothioate]
Delete (1) [3-(phenyltelluranyl)phenyl][3-(phenylsulfanyl)phenyl]disulfane
read [not 3-(phenylsulfanyl)phenyl 3-(phenyltellanyl)benzenesulfenothioate]
For 3-(dimethylamino)-2-methylbutan-2-yl hydroperoxide
read 4-(dimethylamino)-2-methylbutan-2-yl hydroperoxide
For O-thioperoxol
read -SO-thioperoxol
read -TeSe-selenotelluroperoxol
For 2-hydroperoxyethanol (PIN)
read 2-hydroperoxyethan-1-ol (PIN)
For sulfinyldibenzene (PIN)
read 1,1′-sulfinyldibenzene (PIN)
For selenonyldibenzene (PIN)
read 1,1′-selenonyldibenzene (PIN)
For (ethylsulfonyl)ethane]
read (ethylsulfonyl)ethane
For 2 -(ethylperoxy)-1-methoxyethane
read 1-(ethylperoxy)-2-methoxyethane. [Deleted space from name and correct locants]
read {not [(2-methoxyethyl)peroxy]ethane;...
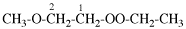
For 1-methoxy 3-(methylthio)propane
read 1-methoxy-3-(methylthio)propane
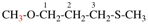 Page 718, P-63.7, example 1 on this page . [corrected 27 February 2019]
Page 718, P-63.7, example 1 on this page . [corrected 27 February 2019]
For ... nor 1-methyl-2-[1-(methylsulfanyl)pent-1-en-1-yl]disulfane; ...
read ... nor methyl[1-(methylsulfanyl)pent-1-en-1-yl]disulfane; ...
Replace the structure with:
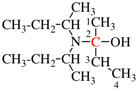
For The traditional names methoxide, ethoxide, proproxide, butoxide, phenoxide, and aminoxide, , for CH3-O–, C2H5-O–, C3H7-O–, C4H9-O–, C6H5-O–, and H2N-O– are retained for use in general nomenclature and may be substituted in the same way as the corresponding alcohols. The traditional names isopropoxide and tert-butoxide for (CH3)CH-O– and (CH3)3C-O– also are retained but cannot be substituted.
read The traditional names methoxide, ethoxide, propoxide, butoxide and phenoxide, for CH3-O–, C2H5-O–, C3H7-O–, C4H9-O– and C6H5-O–, are retained as preferred IUPAC names and aminoxide, H2N-O–, as a preselected name, and they may be substituted in the same way as the corresponding alcohols. The traditional name tert-butoxide for (CH3)3C-O– is also retained as a preferred IUPAC name but cannot be substituted. The traditional name isopropoxide for (CH3)2CH-O– is retained for general nomenclature but cannot be substituted.
For sodium methanolate (PIN)
read sodium methanolate
read sodium methoxide (PIN)
For sodium propan-1-olate (PIN)
read sodium propan-1-olate
read sodium propoxide (PIN)
For llithium phenolate (PIN)
read lithium phenolate
read lithium phenoxide (PIN)
For 2,2′-spirobi[1,3,2]benzodioxagermole
read 2,2′-spirobi[[1,3,2]benzodioxagermole]
For HO-CH3-CH2-CH2-O– Na+
read HO-CH2-CH2-CH2-O– Na+
Replace structure with:
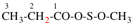
For methyl(dioxo)-λ5-phospane
read methyldi(oxo)-λ5-phospane
For 4-[(1E)-3-(dihydroxyphenyl)-3-oxoprop-1-en-1-yl]benzamide (PIN)
read 4-[(1E)-3-(2,4-dihydroxyphenyl)-3-oxoprop-1-en-1-yl]benzamide (PIN)
For...acetone, 1,4-benzoquinone, naphthoquinone, and anthraquinone are retained...
read ...acetone and 1,4-benzoquinone are retained...
For 1-phenylethanone
read 1-phenylethan-1-one (PIN)
read acetophenone
Add (not benzoquinone)
Add (not naphthoquinone)
Add (not anthraquinone)
Delete as it is a duplicate of example 1 on page 723.
For acenaphthoquinone
read (not acenaphthoquinone)
For isoquinolone (1-isomer shown)
read [not isoquinolone (1-isomer shown)]
For benzal
read [not benzil]
read [not quinolone (2-isomer shown)]
For pyrrolidone (2-isomer shown)
read [not pyrrolidone (2-isomer shown)]
For 1,2-diphenylethane-1,2-dione (PIN)
read diphenylethanedione (PIN)
read [not benzal]
For biacetyl
read [not biacetyl]
For propiophenone
read [not propiophenone]
For 3-methylbutyl methyl ketone
read methyl 3-methylbutyl ketone
For 1,2-di(naphthalen-2-yl)ethane-1,2-dione (PIN)
read di(naphthalen-2-yl)ethanedione (PIN)
For chrysene-1,3,6,8(2H,7H)-tetrone (PIN)
read pyrene-1,3,6,8(2H,7H)-tetrone (PIN)
read 1,2,3,6,7,8-hexahydropyrene-1,3,6,8-tetrone
For ...The names 1,4-benzoquinone, naphthoquinone, and anthraquinone are retained for use...
read...The name 1,4-benzoquinone is retained for use...
For 1,3,6,8(2,5)-tetrafuranacyclododecaphan-11-en-2-one (PIN)
read (11Z)-1,4,7,10(2,5)-tetrafuranacyclododecaphan-11-en-2-one (PIN)
For 2,2-dibromoethen-1-one
read dibromoethenone
For (1,2-dihydroquinolin-2-one)
read (not 1,2-dihydroquinolin-2-one)
For pyrimidine-2,4,6(1H,3H,5H)-trione (PIN)
read pyrimidine-2,4,6(1H,3H,5H)-trione
For 1,3,5-triazine-2,4,6(1H,3H,5H)-trione (PIN)
read 1,3,5-triazine-2,4,6(1H,3H,5H)-trione
For 2′H,4′H-[2,3′-bipyranylidene]-2′,5(6H)-dione
read 2′H,4′H-[2,3′-bipyranylidene]-2′,5(6H)-dione
For 2′H,4′H-[2,3′-bipyranylidene]-4′,6(5H)-dione (PIN)
read 2′H,4′H-[2,3′-bipyranylidene]-4′,6(5H)-dione (PIN)
For 1-(1-oxopropyl)piperidine)
read 1-(1-oxopropyl)piperidine
For acetyl(trimethyl)silane
read acetyltri(methyl)silane
For 1λ4-thiophen-1-one (PIN)
read 1H-1λ4-thiophen-1-one (PIN)
read 1-oxo-1H-1λ4-thiophene
For 5λ6-thianthrene-5,5-dione (PIN)
read 5H-5λ6-thianthrene-5,5-dione (PIN)
read 5,5-dioxo-5H-5λ6-thianthrene
For 2-(2-oxocyclohexyl)propan-2-one
read 1-(2-oxocyclohexyl)propan-2-one
For 3-(2-oxopiperidin-3-yl)propan-2-one
read 1-(2-oxopiperidin-3-yl)propan-2-one
For (not 3-propionylphosphepan-2-one)
read 3-propionylphosphepan-2-one
For (not 1-propionyl pipeeridine)
read 1-propionylpiperidine [delete space in name]
For 1,1′-carbonothioyldi[pyridin-2(1H)-one] (PIN)
read 1,1′-carbonothioyldi(pyridin-2(1H)-one) (PIN)
read 1,1′-thiocarbonyldi(pyridin-2(1H)-one)
For furoic acid (also 3- isomer)
read 2-furoic acid (also 3-isomer)
For naphthoic acid
read 2-naphthoic acid (also 1-isomer)
Add examples 1-3 of page 749, P-65.1.1.2.4 on this page
For propiolic acid
read (not propiolic acid)
read prop-2-ynoic acid (PIN)
For isobutyric acid
read (not isobutyric acid)
read 2-methylpropanoic acid (PIN)
For acetoacetic acid
read (not acetoacetic acid)
For anthranilic acid (1,2-isomer only)
read [not anthranilic acid (1,2-isomer only)]
For benzilic acid
read [not benzilic acid]
read hydroxydi(phenyl)acetic acid (PIN)
For N,N′-ethane-1,2-diylbis[N-(carboxymethyl)glycine]
read N,N′-(ethane-1,2-diyl)bis[N-(carboxymethyl)glycine]
read (HOOC-CH2)2N-CH2-CH2-N(CH2-COOH)2
For glycolic acid
read (not glycolic acid)
For glycoxylic acid
read (not glycoxylic acid)
Delete these examples
Replace the structure with:
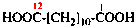
Add the following example:
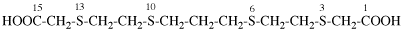
3,6,10,13-tetrathiapentadecane-1,15-dioic acid (PIN)
For benzo[1,2-c:3,4-c′]bis([1,2,5]oxadiazole)-5-carboxylic acid
read [benzo[1,2-c:3,4-c′]bis([1,2,5]oxadiazole)]-4-carboxylic acid
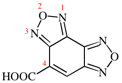
For 4-carboxy-1-methylpyridinium chloride
read 4-carboxy-1-methylpyridin-1-ium chloride
For 1-methyl-3-oxalobicyclo[2.2.2]octan-1-ium (PIN)
read 1-methyl-3-oxalo-1-azabicyclo[2.2.2]octan-1-ium (PIN)
For HO-CH2-CH2-O)2CH-COOH
read (HO-CH2-CH2-O)2CH-COOH
For 4-(hydroxysulfanyl)methyl]benzoic acid (PIN)
read 4-[(hydroxysulfanyl)methyl]benzoic acid (PIN)
Replace the structure with:
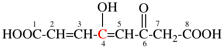
For N,N′-ethane-1,2-diylbis[N-(carboxymethyl)glycine]
read N,N′-(ethane-1,2-diyl)bis[N-(carboxymethyl)glycine]
For N-(carboxymethyl)-N′-(2-hydroxyethyl)-N,N′-ethane-1,2-diyldiglycine
read N-(carboxymethyl)-N′-(2-hydroxyethyl)-N,N′-(ethane-1,2-diyl)diglycine
For (see P-65.1.3.1.1; P-65.1.5.2)
read (see P-65.1.5.2)
Replace the structure with:
H-C(=N-NH2)-OH
Delete this correction.
Delete this correction.
For (not performic acid)
read performic acid
For (not peracetic acid)
read peracetic acid
For (not perbenzoic acid)
read perbenzoic acid
For H{S,O}C-CH2-CH2-CH2-CH2-C{O/S}H
read H{S/O}C-CH2-CH2-CH2-CH2-C{O/S}H
For 4-ethanethioylbenzoic acid (PIN)
read 4-(ethanethioyl)benzoic acid (PIN)
For sulfanyl(oxo)acetic acid (PIN)
read oxo(sulfanyl)acetic acid (PIN)
For 4-[hydroxy(carbonothioyl)]pyridine-2-carboxylic acid (PIN)
read 4-(hydroxycarbonothioyl)pyridine-2-carboxylic acid (PIN)
For N-hydroxycyclohexane-1-carboximidoselenoic acid
read N-hydroxycyclohexanecarboximidoselenoic acid
For 3-amino-3-(ethylsulfanyl)prop-2-enedithioic acid (PIN)
read 3-amino-3-(ethylsulfanyl)prop-2-ene(dithioic acid) (PIN)
For 3-amino-2,3-dioxopropanethoic (O,S)-acid (PIN)
read 3-amino-2,3-dioxopropanethoic acid (PIN)
read H2N-CO-CO-C{O/S}H
For 3-(thiocarboxy)butanoic acid
read 4-(thiocarboxy)butanoic acid
For hydroxyl(sulfanylidene)acetic acid (PIN)
read hydroxy(sulfanylidene)acetic acid (PIN)
For 2-(thiocarboxy)benzenecarbothioic S-acid (PIN)
read 2-(thiocarboxy)benzene-1-carbothioic S-acid (PIN)
For (not 1-selenophthalic Se-acid)
read (not selenoterephthalic Se-acid)
For ethanebis(dithioic) acid (PIN)
read ethanebis(dithioic acid) (PIN)
For –COS2H dithiocarboperoxic acid (preferred suffix; location of sulfur atom unknown)
read –COS2H dithiocarboperoxoic acid (preferred suffix; location of sulfur atom unknown)
Replace the structure with:
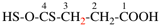
For 3-(dithiocarboperoxoyl)propanoic acid (PIN)
read 3-(dithiocarbonoperoxoyl)propanoic acid (PIN)
For (dithiocarboperoxoyl)formic acid (PIN)
read (dithiocarbonoperoxoyl)formic acid (PIN)
For 4-[(hydroxysulfanyl)carbonyl]cyclohexanecarboxylic acid]
read 4-[(hydroxysulfanyl)carbonyl]cyclohexane-1-carboxylic acid
For 5-(phenylamino)-5-oxopentanoic acid
read 5-oxo-5-(phenylamino)pentanoic acid
Delete oxalaldehydic acid
For ...‘1-oxopropyl’ and ‘iminoethyl’ for CH2-CH2-CO– and ...
read ...‘1-oxopropyl’ and ‘1-iminoethyl’ for CH3-CH2-CO– and ...
For (oxo)phenylmethyl
read oxo(phenyl)methyl
For dioxoethane-1,2-diyl
read dioxoethanediyl
For diiminoethane-1,2-diyl
read diiminoethanediyl
For bis(sulfanylidene)ethane-1,2-diyl
read bis(sulfanylidene)ethanediyl
Delete oxaldehydoyl
For ...nomenclature (see P-65-4.3.2); substitution...
read ...nomenclature (see P-65.1.1.2); substitution...
For 2-sulfanyl-2-sulfanylideneethanethioyl (preferred prefix)
read sulfanyl(sulfanylidene)ethanethioyl (preferred prefix)
For 1,10-dioxodecanediyl
read 1,10-dioxodecane-1,10-diyl
For 1-sulfanylidene propyl
read 1-sulfanylidenepropyl [delete space]
Move carbohydrazonoyl to example 4 (part of name)
delete (not dithiophthaloyl)
delete 1,4-phenylenebis(sulfanylidenemethylene)
delete 1,4-phenylenebis(thioxomethylene)
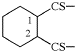
Move carbohydrazonoyl from under example 3 to under the rest of the first name for example 4.
read hydrazinylidene(1-methylcyclopentyl)methyl
For hexane-2,3,5-triyltris(thioxoemethylene)
read hexane-2,3,5-triyltris(thioxomethylene)
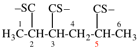
For 1,4-phenylenebis(sulfanylidenemethylene)
read 1,2-phenylenebis(sulfanylidenemethylene)
read 1,2-phenylenebis(thioxomethylene)
For 2,4,6,8-tetraoxanonanedioic acid (PIN)
read 3,5,7-trioxo-2,4,6,8-tetraoxanonanedioic acid (PIN)
Replace structure with:
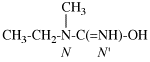
For 2-hydroxypropyl N-(2-aminoethyl)carbamate (PIN)
read 2-hydroxypropyl (2-aminoethyl)carbamate (PIN)
For aminocarbonimidoyl
read C-aminocarbonimidoyl
For (3) (dithiohydroperoxycarbonyl)oxy
read (3) [(dithiohydroperoxy)carbonyl]oxy
For carbononitridothioic
read carbononitridothioic acid
For carbonitridoylacetic acid
read carbononitridoylacetic acid
For 3-carbonitridothioylpropanoic acid
read 3-(carbononitridoylthio)propanoic acid
For [(thiocarboxy)oxy]formothioic S-acid (PIN)
read [(thiocarboxy)oxy]methanethioic S-acid (PIN)
For [(thiocarboxy)oxy]formothioic O-acid (PIN)
read [(thiocarboxy)oxy]methanethioic O-acid (PIN)
For 1-isocyanato-2-imidodicarbonic acid
read 2-imido-1-isocyanatodicarbonic acid
For {not (dithiocarboxy)sulfanyl]thioformyl}
read {not [(dithiocarboxy)sulfanyl]thioformyl}
For R-SO2H seleninic acid
read R-SeO2H seleninic acid
For propane-1-sulfonoselenoic acid (PIN)
read propane-1-sulfinoselenoic acid (PIN)
For ...such as ‘sulfinimidic acid’ for –S(O)(=NH)-OH, ...
read ...such as ‘sulfinimidic acid’ for –S(=NH)-OH, ...
Replace the structure with:
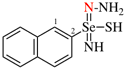
For N-hydroxypropanesulfinamide (PIN)
read N-hydroxypropane-1-sulfinamide (PIN)
Replace structure with:
H{S/O}S-CH2-CH2-SO2-OH
For 2-trithiosulfobenzoic acid (PIN)
read 2-(trithiosulfo)benzoic acid (PIN)
read 2-(sulfanylsulfonodithioyl)benzoic acid
Replace structure with:
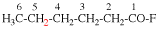
For cyclohexanecarbonimidoyl chloride (PIN)
read cyclohexanecarboximidoyl chloride (PIN)
For cyclohexanecarbonothioyl chloride (PIN)
read cyclohexanecarbothioyl chloride (PIN)
For butanedioyl isocyanate isocyanide (PIN)
read butanedioyl isocyanide isothiocyanate (PIN)
For imidodicarbonic dichloride (PIN)
read 2-imidodicarbonic dichloride (PIN)
For ...appropriate bivalent acyl group...
read ...appropriate divalent acyl group...
For (1) 2-(carbonochloridoyl)benzoic acid (PIN)
read (1) 2-carbonochloridoylbenzoic acid (PIN)
read (2) 2-(chlorocarbonyl)benzoic acid
For (1) 2-(carbonocyanidothioyl)benzoyl chloride (PIN)
read (1) 2-carbonocyanidothioylbenzoyl chloride (PIN)
for (2) 2-(cyanocarbonothioyl)benzoyl chloride
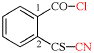
For 4-(2-isocyanato-2-oxoethyl)benzenecarbothioyl cyanide (PIN)
read (4-carbonocyanidothioylphenyl)acetyl isocyanate (PIN)
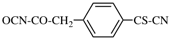
For 2-(carbonocyanidoyl)-5-methylbenzoyl chloride (PIN)
read 2-carbonocyanidoyl-5-methylbenzoyl chloride (PIN)
For (3) 4-bromo-3,4-dioxobutanoic acid
read (3) 4-bromo-3,4-dioxobutanoic acid (PIN)
For (1) 3-[(carbonobromidoyl)oxy]-3-oxopropanoic acid (PIN)
read (1) 3-(carbonobromidoyloxy)-3-oxopropanoic acid (PIN)
For sodium propanedithioate (PIN)
read sodium propane(dithioate) (PIN)
For 3,3′-spirobi[[2,4,3]benzodioxaplumbepin]-1,1′,5,5′-tetrone (PIN)
read 3,3′-spirobi[[2,4,3]benzodioxaplumbepine]-1,1′,5,5′-tetrone (PIN)
For (1) potassium 6-carboxyheptanoate (PIN)
read (1) potassium 6-carboxyhexanoate (PIN)
For (HOOC-CH2-CH2-COO–)3 Sb3
read (HOOC-CH2-CH2-COO–)3 Sb3+
read (2) antimony tris(hydrogen butanedioate)
For CH3-O-CO-CH2-CH2-CO-O CH2-CH3
read CH3-O-CO-CH2-CH2-CO-O-CH2-CH3
Rewrite the second sentence in the box as follows: ‘The bi- or polyvalent functional class name is cited as the organyl (alkanediyl, arylene, etc.) group cited immediately before the name of the acid component... ’
For 1,4-phenylene bis(methyl propanedioate)
read 1,4-phenylene di(methyl propanedioate)
For ...and ‘alkyloxy(alkanyl)...oxo’ or ‘alkyl(alkanyl)oxycarbonyl’ for the group –CO-OR′.
read ...and ‘alkoxy...oxo’, ‘(alkyloxy)...oxo’, ‘(alkanyloxy)...oxo’, ‘alkoxycarbonyl’, ‘(alkyloxy)carbonyl’ or ‘(alkanyloxy)carbonyl’ for the group –CO-OR′.
For [2-(ethoxycarbonyl)ethyl]-N,N,N-trimethylammonium bromide
read [2-(ethoxycarbonyl)ethyl]tri(methyl)ammonium bromide
For [3-ethoxy-3-oxopropyl]-N,N,N-trimethylazanium bromide
read [3-ethoxy-3-oxopropyl]tri(methyl)azanium bromide
For 3-[(phenylcarbony)oxy]propanoic acid
read 3-[(phenylcarbonyl)oxy]propanoic acid
For 3-ethoxy-N,N,N-trimethyl-3-oxopropanaminium bromide
read 3-ethoxy-N,N,N-trimethyl-3-oxopropan-1-aminium bromide
This example is an ester and belongs in P-65.6.3.3.1.
Add S-(2-cyanoethyl) cyclohexanesulfinothioate (PIN)
read {not 3-[(cyclohexanesulfinyl)sulfanyl]propanenitrile;
read nor 3-[(cyclohexylsulfinyl)sulfanyl]propanenitrile; see P-65.4.1for naming acyl groups derived from acids}
Following P-16.5.2.6 replace the structure with:
CH3-[CH2]6-CO-O-C(CH3)3
For ethoxy... is alphabetically preferred to ethyl...)
read butanedioate is preferred to propandioate)
For ...representing the ‘hydroxylic’ are exactly...
read ...representing the ‘hydroxylic’ component are exactly...
For (2) 3,3′-oxydi(methyl benzoate)
read (2) 3,3′-oxybis(methyl benzoate)
For (1) dimethyl butanedioylbis(oxy-2,1-phenylene) dibutanedioate (PIN)
read (1) dimethyl butanedioylbis(oxy-2,1-phenylenedibutanedioate (PIN) [cancelled 28 May 2024]
for (2) butanebis(oxy-2,1-phenylene) di(methyl butanedioate)
read (2) butanebis(oxy-2,1-phenylene)di(methyl butanedioate)
For (1) dimethyl butanedioylbis(oxy-2,1-phenylene)dibutanedioate (PIN)
read (1) dimethyl butanedioylbis(oxy-2,1-phenylene) dibutanedioate (PIN)
For (1) dimethyl butanedioylbis(oxy-2,1-phenylene) dibutanedioate (PIN)
read (1) dimethyl butanedioylbis(oxy-2,1-phenylene)dibutanedioate (PIN)
For (2) butanedioylbis(oxy-2,1-phenylene) di(methyl butanedioate)
read (2) butanedioylbis(oxy-2,1-phenylene) bis(methyl butanedioate)
For (not bis[2-(4-methoxy-4-oxobutanoyl)oxy]phenyl butanedioate; ...
read (not bis{2-[(4-methoxy-4-oxobutanoyl)oxy]phenyl} butanedioate; ...
For methyl 6-chloro-3-[3-(ethoxycarbonyl)phenoxy]benzoate (PIN)
read methyl 2-chloro-5-[3-(ethoxycarbonyl)phenoxy]benzoate (PIN)
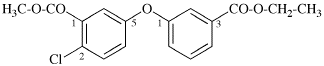
The published structure numbers corresponds to the multiplicative name
For 3,3′-(1,2-phenylene)dipropyl diacetate [PIN; see see P-15.3.0(2) and P-51.3.1)
read 1,2-phenylenedi(propane-3,1-diyl) diacetate [PIN; see P-15.3.0 (2) and P-51.3.1]
Replace structure with:
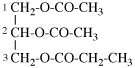
For (2S)-propane-1,2,3-triyl 2-acetate 1-hexadecanoate 3-[(9Z)-octadec-9-enoate] (PIN)
read (1) propane-1,2,3-triyl 2-acetate 1-hexadecanoate 3-[(9Z)-octadec-9-enoate] (PIN)
read (2) 2-(acetyloxy)-3-(hexadecanoyloxy)propyl (9Z)-octadec-9-enoate
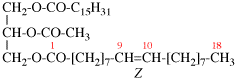
Note deletion of extra closing parenthesis.
For ethane-1,2-diyl bis(methyl butanedioate)
read ethane-1,2-diyl di(methyl butanedioate) [cancelled 28 May 2024]
For ethane-1,2-diyl di(methyl butanedioate)
read ethane-1,2-diyl bis(methyl butanedioate)
Replace the structure with:
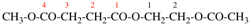
Following P-16.5.2.6 replace the structure with:
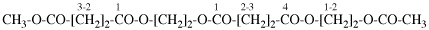
For [not phenyl 3-(phenoxycarbonyl)benzoate; ...
read [not 3-(phenoxycarbonyl)phenyl benzoate; ...
For 3,3′-{2-[3-(formyloxy)propyl]cyclohexane-1,1-diyl}dipropyl diacetate (PIN)
read 2-[3-(formyloxy)propyl]cyclohexane-1,1-diyldi(propane-3,1-diyl) diacetate (PIN)
For (not ethyl 4-[(4-methoxy-4-oxobutanoyl)oxy]phenyl propanedioate; butanedioic acid is preferred to propanoic acid
read (not ethyl 4-[(4-methoxy-4-oxobutanoyl)oxy]phenyl propanedioate; butanedioic acid is preferred to propanedioic acid)
For the organyl groups in the PIN name are lower alphanumerically; see P-14.5
read 3-(methoxycarbonyl)phenyl is alphabetically before 2-methoxy-2-oxoethyl; see P-14.5
For bis[3-(methoxycarbonyl)phenyl butanedioate
read bis[3-(methoxycarbonyl)phenyl] butanedioate
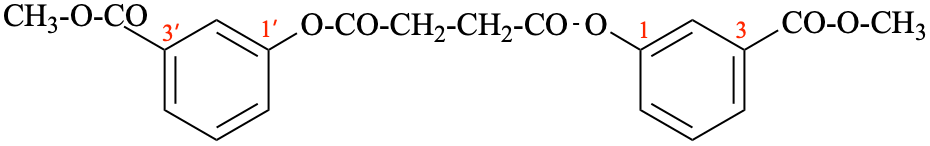
For
1-{2-[2-(acetyloxy)-1-(formyloxy)ethyl]cyclohexyl}-2-(propanoyloxy)ethyl butanoate (PIN)
read 1-{2-[2-(acetyloxy)-1-(formyloxy)ethyl]cyclohexyl}ethane-1,2-diyl 1-butanoate 2-propanoate (PIN)
Replace the structure with:
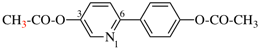
For (not 2-{2-[(acetyloxy)methyl]phenyl}ethyl acetate; ...
read (not 2-{2-[(acetyloxy)ethyl]phenyl}methyl acetate; ...
Delete this example [Duplicate of example 3].
Delete this example [Duplicate of example 4].
For dimethyl ethane-1,2-diylbis(carbonyloxyethyl) dibutanedioate)
read dimethyl ethane-1,2-diylbis(carbonyloxyethane-2,1-diyl) dibutanedioate
For dimethyl 6,8-dioxo-2,5,9,12-tetraoxatridecane-1,13-diyl dipropanedioate (PIN)
read dimethyl 6,8-dioxo-2,5,9,12-tetraoxatridecane-1,13-diyl dipropanedioate
read dimethyl propanedioylbis(oxyethane-2,1-diyloxymethylene) dipropanedioate
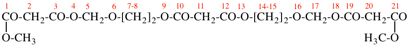
For 2-({[(methoxycarbonyl)acetyl]oxy}ethyl) 3,5,9,11-tetraoxo-2,6,8,12-tetraoxatridecan-1-yl butanedioate (PIN)
read 2-{[(methoxycarbonyl)acetyl]oxy}ethyl 3,5,9,11-tetraoxo-2,6,8,12-tetraoxatridecan-1-yl butanedioate
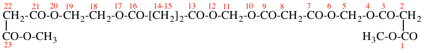
Delete [(not 2-{2-[(acetyloxy)methyl]phenyl}ethyl acetate‘; the ethyl chain is senior to the methyl chain’]
Replace the structure with:
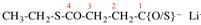
For (1) potassium 2-(2-ethoxy-2-oxoethyl)-2-hydroxybutanedioate (PÏN)
read (1) potassium hydrogen 2-(2-ethoxy-2-oxoethyl)-2-hydroxybutanedioate (PIN)
read (2) potassium 3-ethyl hydrogen 2-hydroxypropane-1,2,3-tricarboxylate
read (2) potassium 3-ethyl hydrogen citrate
For 6-chloro-2-(ethoxycarbonyl)benzoic acid (PIN)
read 2-chloro-6-(ethoxycarbonyl)benzoic acid (PIN)
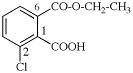
For 3-chloro-2-(ethoxycarbonyl)chlorobenzoic acid (PIN)
read 3-chloro-2-(ethoxycarbonyl)benzoic acid (PIN)
For 2-[(propanimidoyl)selanyl]benzene-1-carboximidic acid (PIN)
read 2-(propanimidoylselanyl)benzene-1-carboximidic acid (PIN)
Replace the structure with:

Replace the structure with:
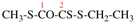
For methyl (ethylsulfanyl)(sulfanylidene)ethanethioate (PIN)
read S-methyl (ethylsulfanyl)(sulfanylidene)ethanethioate (PIN)
For O-methyl 2-{4-[(2-methoxy-2-oxoethanethioyl)oxy]phenoxy}-2-oxoethanethioate (PIN)
read [not O-methyl (4-{[methoxy(oxo)ethanethioyl]oxy}phenoxy)(oxo)ethanethioate; a carboxylic acid is preferred to a thiocarboxylic acid]
read [not methyl (4-{[methoxy(sulfanylidene)acetyl]oxy}phenoxy)(sulfanylidene)acetate; the PIN is lower in alphabetical order]
For 1-S-methyl 3-ethyl 1-thiodicarbonate (PIN)
read 3-ethyl 1-S-methyl 1-thiodicarbonate (PIN)
For 2-{[2-methylsulfanyl-1-oxo-2-sulfanylideneethane-1,2-diyl]oxy}ethanedithoic acid (PIN)
read {[(methylsulfanyl)(sulfanylidene)acetyl]oxy}ethane(dithioic acid) (PIN)
For S-ethyl 3-(cyanosulfanyl)propanethioate (PIN)
read S-ethyl 3-(thiocyanato)propanethioate (PIN)
For (CH3-CO-O)3-B
read (CH3-CO-O)3B
For O-acetyl-N,N-dimethylhydroxylamine (PIN)
read O-acetyl-N,N-dimethylhydroxylamine
For [1-(methylperoxy)sulfanyl]-butan-1-one
read 1-[(methylperoxy)sulfanyl]butan-1-one (PIN)
read (not S-methylperoxyl butanethioate)
For 1-oxadodecan-2-one (PIN)
read 1-oxacyclododecan-2-one (PIN)
read undecano-11-lactone
For 2-oxohexahydro-2H-benzooxete-5,6-dicarboxylic acid
read 2-oxohexahydro-2H-benzoxete-5,6-dicarboxylic acid
For 2λ6-naphtho[1,8-cd][1,2]oxathiole-2,2-dione (PIN)
read 2H-2λ6-naphtho[1,8-cd][1,2]oxathiole-2,2-dione (PIN)
Move pentane-2,5-sultone to example 2
For 1,2λ4-oxathiolan-2-thione (PIN)
read 1,2λ4-oxathiolane-2-thione (PIN)
For (not 3,4-dihydrobenzo[f]dioxocine-1,6-dione)
read (not 3,4-dihydrobenzo[f][1,4]dioxocine-1,6-dione)
For 3,4,6a,7,10,11,14,14a-octahydro[1,4]dioxocino][2,3-c][1,6]dioxecine-2,5,9,12-tetrone (PIN)
read octahydro[1,4]dioxocino[2,3-c][1,6]dioxecine-2,5,9,12-tetrone (PIN)
For 3,4,6a,7a,10,11,14,14a-octahydro[1,5]dioxonino[3,2-b][1,5]dioxonine-2,5,9,12-tetrone (PIN)
read octahydro[1,5]dioxonino[3,2-b][1,5]dioxonine-2,5,9,12-tetrone (PIN)
Delete bis(chloroacetic) anhydride (PIN)
for chloroacetic anhydride
read chloroacetic anhydride (PIN)
For Cl-CH2-CH2-SO)2O
read (Cl-CH2-CH2-SO)2O
read 2-chloroethane-1-sulfinic anhydride
Delete bis(2-chloroethane-1-sulfinic) anhydride (PIN)
for 2-chloroethane-1-sulfinic anhydride
read 2-chloroethane-1-sulfinic anhydride (PIN)
For bis(4-chlorocyclohexane-1-carbothioic) thioanhydride (PIN)
read 4-chlorocyclohexane-1-carbothioic thioanhydride (PIN)
For acetic thioperoxy anhydride (PIN)
read acetic thioperoxyanhydride (PIN) [delete space in name]
For 1,1′-(trioxidanediyl)di(ethan-1-one)
read 1,1′-trioxidanediyldi(ethan-1-one)
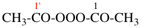
For 1,1′-(dithioxanediyl)di(ethan-1-one)
read 1,1′-dithioxanediyldi(ethan-1-one)
For {not 1-[(acetylsulfanyl)peroxy)]ethan-1-one;
read {not 1-[(acetylsulfanyl)peroxy]ethan-1-one;
For 4-[4-(acetyloxy)-4-oxobutanoic] 2-methyl-4-oxobutanedioic 1-propanoic dianhydride
read 4-[4-(acetyloxy)-4-oxobutanoic] 2-methylbutanedioic 1-propanoic dianhydride
For 6-acetic 3-[4-(acetyloxy)-4-oxo-2-methylbutanoic 2-propanoic naphthalene-2,3,6-tricarboxylic trianhydride (PIN)
read 6-acetic 3-[4-(acetyloxy)-2-methyl-4-oxobutanoic] 2-propanoic naphthalene-2,3,6-tricarboxylic trianhydride (PIN)
For P,P′-diacetic P,P′-dipropanoic ethane-1,2-diylbis(phosphonic) tetraanhydride (PIN)
read P,P′-diacetic P,P′-dipropanoic (ethane-1,2-diyl)bis(phosphonic) tetraanhydride (PIN)
For 5-(acetyloxy)-5-oxopentanoic 6-(butanoyloxy)-6-oxohexanoic 4-(propanoyloxy)-4-oxobutanoic phosphoric trianhydride
read 5-(acetyloxy)-5-oxopentanoic 6-(butanoyloxy)-6-oxohexanoic 4-oxo-4-(propanoyloxy)butanoic phosphoric trianhydride (PIN)
Remove second name
For acetic 4-(ethanethioylsulfanyl)-4-sulfanylidenebutanethioic anhydride (PIN)
read acetic 4-[(ethanethioyl)sulfanyl]-4-sulfanylidenebutanethioic anhydride (PIN)
For acetic butanedioic 4-(acetylsulfanyl)-4-oxobutanoic anhydride (PIN)
read acetic butanedioic 4-oxo-4-(propanoylsulfanyl)butanoic dianhydride (PIN)
For ...‘dianhydride’ or ‘thioanhydride’, thiodianhydride, etc., in the ...
read ...‘dianhydride’ or ‘thioanhydride’, ‘bis(thioanhydride)’, etc., in the ...
For (1) hexahydro-1H-2-benzothiophene-1,3(4H)-dione (PIN)
read (1) hexahydro-2-benzothiophene-1,3-dione (PIN)
read hexahydrobenzo[c]thiophene-1,3-dione
For (1) hexhydro-1H-benzopyran-1,3(4H)-dithione (PIN)
read (1) hexahydro-1H-benzopyran-1,3(4H)-dithione (PIN)
For (1) 5,7-disulfanylidene-5,7-dihydro-1H, 3H-thieno[3,4-f][2]benzofuran-1,3-dione (PIN)
read (1) 5,7-bis(sulfanylidene)-5,7-dihydro-1H, 3H-thieno[3,4-f][2]benzofuran-1,3-dione (PIN)
read (2) 1,3-bis(sulfanylidene)-1,3-dihydro-2-benzothiophene-5,6-dicarboxylic anhydride
read 1,3-dithioxo-1,3-dihydroisobenzothiophene-5,6-dicarboxylic anhydride
For (1) 3b,6a,7,7a- tetrahydro-1H-cyclopenta[1,2-c:3,4-c′]dithiophene-1,3,4,6(3aH)-tetrone (PIN)
read (1) tetrahydro-1H-cyclopenta[1,2-c:3,4-c′]dithiophene-1,3,4,6(3aH)-tetrone (PIN)
Delete this section (conflict with P-65.7.1)
For ...three acyl groups on a single nitrogen atom are generically included and may be designated as primary, secondary, and tertiary amides, respectively.
read ...three acyl groups on a single nitrogen atom are generically included.
Replace structure with:
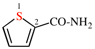
For ... for example, N1, N′1, etc. (see ...
read ... for example, N1, N3, etc. (see ...
For N,N-dimethylformamide (PIN)
read dimethylformamide (PIN)
Replace the structure with:

Replace the structure with:
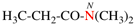
For N,N-diethyl-2-furanamide
read N,N-diethyl-2-furamide
For The suffixes ‘hydroxamic acid’ and ‘carbohydroxamic acid’ are no longer recommended.
read The suffixes ‘hydroxamic acid’ and ‘carbohydroxamic acid’ may be used in general nomenclature.
Replace structure with:
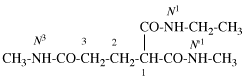
For ...acid’ are no longer recommended.
read ...acid’ may be used in general nomenclature.
Replace structure with:
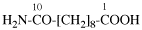
Replace the structure with:
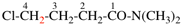
For [ 2,2′-ethane-1,2-diylbis(azanediyl)]di(cyclohexane-1-carboxamide) (PIN)
read 2,2′-[ethane-1,2-diylbis(azanediyl)]di(cyclohexane-1-carboxamide) (PIN) [remove space in name]
Remove one as it is a duplicate of the other
For (2) 3-(methanesulfonylamino)propanoic acid (PIN)
read (2) 3-[(methanesulfonyl)amino]propanoic acid (PIN)
For (2) [(cyclohexylmethyl)amino]acetic acid
read (2) {[(cyclohexylmethyl)sulfonyl]amino}acetic acid
For (1) N-cyclopropyl-N-methanesulfonylglycine
read (1) N-cyclopropyl-N-(methanesulfonyl)glycine
read (2) [cyclopropyl(methanesulfonyl)amino]acetic acid
For 3-{amino(oxo)acetyl]imino}propanoic acid.
read 3-{[amino(oxo)acetyl]imino}propanoic acid
Add N1,N2-bis(cyanomethyl)oxamide (PIN)
read (not 2,2′-[oxalylbis(azanediyl)]diacetonitrile)
read (not 2,2′-[ethanedioylbis(azanediyl)]diacetonitrile)
Replace the structure with:
(CH3-CO)2N–
For N,N-di(cyclohexanecarbonyl)cyclohexane-1-carboxamide (PIN)
read N,N-di(cyclohexanecarbonyl)cyclohexanecarboxamide (PIN)
For (1,4-dihydroquinolin-1(2H)-yl)propan-1-one (PIN)
read 1-(3,4-dihydroquinolin-1(2H)-yl)propan-1-one (PIN)
read 1-propionyl-1,2,3,4-tetrahydroquinoline
For N-ethanethioylethanethioamide (PIN)
read N-(ethanethioyl)ethanethioamide (PIN)
read N-(thioacetyl)thioacetamide
For N-cyclohexyl-N-ethanethioylethanethioamide
read N-cyclohexyl-N-(ethanethioyl)ethanethioamide (PIN)
read N-cyclohexyl-N-(thioacetyl)thioacetamide
For N-propanethioylacetamide (PIN)
read N-(propanethioyl)acetamide (PIN)
For 1-(pyrrolidin-1-yl)ethan-1-thione (PIN)
read 1-(pyrrolidin-1-yl)ethane-1-thione (PIN)
read not 1-(ethanethioyl)pyrrolidine
For 4-ethanethioamidobenzamide (PIN)
read 4-(ethanethioamido)benzamide (PIN)
read 4-[(ethanethioyl)amino]benzamide
For (methanesulfinothioylamino)acetic acid (PIN)
read [(methanesulfinothioyl)amino]acetic acid (PIN)
For [aminos(sulfanylidene)ethanethioyl]benzoic acid (PIN)
read 2-[amino(sulfanylidene)ethanethioyl]benzoic acid (PIN)
For 1λ6-2H-naphtho[1,8-cd][1,2]thiazole-1,1-dione (PIN)
read 1λ6-naphtho[1,8-cd][1,2]thiazole-1,1(2H)-dione (PIN)
For ... H2N-CO-NH2 has the retained named ‘urea’, which...
read ... H2N-CO-NH2 has the retained name ‘urea’, which...
Replace the structure with:
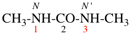
For N-carbamoylbenzene-1-sulfonamide (PIN)
read N-carbamoylbenzenesulfonamide (PIN)
read N-(aminocarbonyl)benzenesulfonamide
For 1-[3-(formylamino)propyl]urea
read N-[3-(formylamino)propyl]urea
For ...derived from isorea are named...
read ...derived from isourea are named...
For carbonothioic diamide (PIN)
read carbonothioic diamide
read thiourea (PIN)
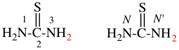
Replace the structure with:
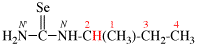
For ethyl N-methylcarbamimidothioate (PIN)
read ethyl N′-methylcarbamimidothioate (PIN)
For (not S-ethyl N-methylisothiourea)
read (not S-ethyl-N-methylisothiourea)
For 3-[amino(sulfanylidene)methyl]aminopropanoic acid
read 3-{[amino(sulfanylidene)methyl]amino}propanoic acid
For N-methyl-2,4-diimido-3-thiotricarbonic diamide (PIN)
read N1-methyl-2,4-diimido-3-thiotricarbonic diamide (PIN)
For 3,5,7-trioxo-2,4,6,8-tetraazanonanediamide (PIN)
read 3,5,7-trioxo-2,4,6,8-tetraazanonane-1,9-diamide (PIN)
For N-(propan-2-yl)dicarbonic diamide (PIN)
read N1-(propan-2-yl)dicarbonic diamide (PIN)
For N-[({[(acetamidomethyl)nitroamino]methyl}nitroamino)methyl]prop-2-enamide (PIN)
read N-{[{[(acetamidomethyl)(nitro)amino]methyl}(nitro)amino]methyl}prop-2-enamide
read N-{[({[(acetylamino)methyl](nitro)amino}methyl)(nitro)amino]methyl}prop-2-enamide
For hexahydro-2H-isoindole-1,3-dione (PIN)
read hexahydro-2H-isoindole-1,3-dione
For 2-phenyl-2H-isoindole-1,3-dione (PIN)
read 2-phenyl-2H-isoindole-1,3-dione
For 7-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)naphthalen-1-carboxylic acid (PIN)
read 7-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)naphthalene-1-carboxylic acid (PIN)
For ...CH3-CH3-CH2-CH2-CO-NH-NH2, not pentanohydrazide
read ...CH3-CH2-CH2-CH2-CO-NH-NH2, not pentanohydrazide.
Replace structure with:
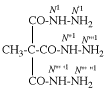
Replace the structure with:
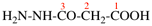
For ...and ‘benzenecarbohydrazido’; the...
read ...and ‘benzohydrazido’; the...
For (1) 4-(acetohydrazido)benzoic acid (PIN)
read (1) 4-acetohydrazidobenzoic acid (PIN)
For ...and '1,4,6' is lower than '1,4,7')
read ...and 'N1,N′4,6' is lower than 'N′1,N4,7')
For not benzenecarbothioylhydrazine);...
read not (benzenecarbothioyl)hydrazine;...
For (hydrazinecarbonyl)oxy}formohydrazide
read [(hydrazinecarbonyl)oxy]formohydrazide
For [(hydrazinecarbonyl)amino)formohydrazide
read [(hydrazinecarbonyl)amino]formohydrazide
For 4-{[(hydrazinecarbonyl)oxy)]formohydrazido}butanoic acid (PIN)
read 4-{[(hydrazinecarbonyl)oxy]formohydrazido}butanoic acid (PIN)
For hydrazinecarbohydrazidoacetic acid (PIN)
read (hydrazinecarbohydrazido)acetic acid (PIN)
read [2-(hydrazinylcarbonyl)hydrazin-1-yl]acetic acid
Delete example as it is the same as example 2
Replace structure with:
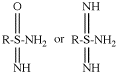
Replace the structure with:
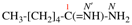
For N′′′1-ethyl-N1,N1-dimethylcyclohexane-1,1-dicarboximidamide (PIN)
read N′′1-ethyl-N1,N1-dimethylcyclohexane-1,1-dicarboximidamide (PIN)
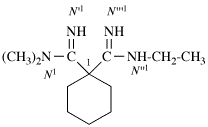
Replace the structure with:
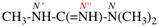
For carbaminidamido
read carbamimidamido
For carbamimidoylurea (PIN)
read N-carbamimidoylurea (PIN)
Replace the structure with:
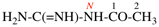
Replace the structure with:
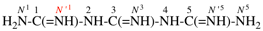
For N,N-diphenyl-N′′-ethylimidodicarbonimidic diamide (PIN)
read N′1-ethyl-N1,N1-diphenylimidodicarbonimidic diamide (PIN)
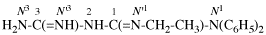
For 3,5,7-triimino-2,4,6,8-tetraazanonanedi(imidamide) (PIN)
read 3,5,7-triimino-2,4,6,8-tetraazanonane-1,9-diimidamide (PIN)
For N′-methyl-N,N-diphenylbenzene-1-carboximidamide (PIN)
read N′-methyl-N,N-diphenylbenzenecarboximidamide (PIN)
For N′-ethyl-N -methylbenzenecarboximidamide (PIN)
read N′-ethyl-N-methylbenzenecarboximidamide (PIN)
read (not N′-ethyl-N-methylbenzenecarboxamidine). [Deleted space from names]
For N-phenylbenzene-1-carboximidamide (PIN)
read N-phenylbenzenecarboximidamide (PIN)
read (not N-phenylbenzenecarboxamidine;
Replace the structure with:

For [not N1-ethyl-N2-methyldisulfanedicarboximidamide; not N-ethyl-N′′-methyl(dithioperoxy)dicarbonimidic diamide (numbering shown); ...
read [not N1-ethyl-N2-methyldisulfanedicarboximidamide (numbering shown); not N-ethyl-N′′-methyl(dithioperoxy)dicarbonimidic diamide; ...
read ...; not N1-ethyl-N3-methyl-α,α′-dithiobisformamidine...
For N1-ethyl-N′′1,N′′1,N3,N3-tetramethylcyclohexane-1,1,3-tricarboximidamide (PIN)
read N′′1-ethyl-N1,N1,N3,N3-tetramethylcyclohexane-1,1,3-tricarboximidamide (PIN)
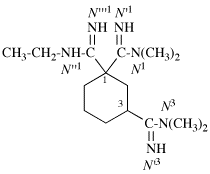
For 2-(hydrazinyl)-2-iminoethane-1-hydrazonamide (PIN)
For 2-hydrazinyl-2-iminoethanehydrazonamide (PIN)
For 4-[C-(2-nitrosohydrazine-1-carbonimidoyl)]tetraaz-3-ene-1-carboximidamide (PIN)
read 4-(2-nitrosohydrazine-1-carboximidoyl)tetraaz-3-ene-1-carboximidamide (PIN)
For N′1,N′1-diethyl-N′′′ ′1, N′′′ ′1-dimethyllcyclohexane-1,1-dicarboximidohydrazide (PIN)
read N′1,N′1-diethyl-N′′′ ′1,N′′′ ′1-dimethylcyclohexane-1,1-dicarboximidohydrazide (PIN) [delete a space from name]
For (hydrazinecarboximidoyloxy)methanehydrazonamide (PIN)
read [(hydrazinecarboximidoyl)oxy]methanehydrazonamide (PIN)
read [(carbamohydrazonoyl)oxy]methanimidohydrazide
read [(hydrazinecarboximidoyl)oxy]formohydrazonamide
For 3-imino-3-hydrazinylpropanoic acid (PIN)
read 3-hydrazinyl-3-iminopropanoic acid (PIN)
read (hydrazinecarboximidoyl)acetic acid
For 3-hydrazinecarboximidoylbenzoic acid (PIN)
read 3-(hydrazinecarboximidoyl)benzoic acid (PIN)
For ...and ‘(hydrazinylidenemethyl)amino’ and ‘methanehydrazonoylamino’. The preferred...
read ...and ‘(hydrazinylidenemethyl)amino’ and ‘(methanehydrazonoyl)amino’. The preferred...
For 3-methanehydrazonamidopropanenitrile (PIN)
read N-(2-cyanoethyl)methanehydrazonamide (PIN)
read (not 3-[(methanehydrazonoyl)amino]propanenitrile;
read nor 3-[(hydrazinylidenemethyl)amino]propanenitrile)
For ...the group H2N-CH=N-NH– is (aminomethylidene)hydrazin-1-yl’.
read ...the group H2N-CH=N-NH– is ‘2-(aminomethylidene)hydrazin-1-yl’.
For ...and ‘2-methanimidoylhydrazin-1-yl’ and...
read ...and ‘2-(methanimidoyl)hydrazin-1-yl’ and...
For ...(preferred prefix) and ‘methanehydrazonoylamino’.
read ...(preferred prefix) and ‘(methanehydrazonoyl)amino’.
For 3-[(aminomethylidene)hydrazinyl]propanoic acid (PIN)
read 3-[2-(aminomethylidene)hydrazin-1-yl]propanoic acid (PIN)
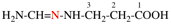
For 3-ethanehydrazonamidopropanoic acid (PIN)
read 3-(ethanehydrazonamido)propanoic acid (PIN)
read 3-[(ethanehydrazonoyl)amino]propanoic acid
For 4-benzenesulfinohydrazonamidobenzoic acid (PIN)
read 4-(benzenesulfinohydrazonamido)benzoic acid (PIN)
read 4-[(benzenesulfinohydrazonoyl)amino]benzoic acid
For 3-methanimidohydrazidopropanoic acid (PIN)
read 3-(methanimidohydrazido)propanoic acid (PIN)
read 3-[2-(methanimidoyl)hydrazin-1-yl]propanoic acid
For methyl ethanimidohydrazidoacetate (PIN)
read methyl (ethanimidohydrazido)acetate (PIN)
read methyl [2-(ethanimidoyl)hydrazin-1-yl]acetate
CH3-C(=NH)-NH-NH-CH2-CO-O-CH3
For 4-[2-benzenecarboximidoyl)hydrazin-1-yl]benzoic acid
read 4-[2-(benzenecarboximidoyl)hydrazin-1-yl]benzoic acid
Replace the structure with:
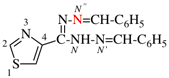
For 1, N′ 2, N2, N6-tetramethylnaphthalene-2,6-dicarbohydrazonohydrazide (PIN)
read N′2,N′2,N6,1-tetramethylnaphthalene-2,6-dicarbohydrazonohydrazide (PIN) [delete spaces from name]
For
N′1, N′1-diethyl-N′′′ ′1,N′′′ ′1,N3-trimethylcyclohexane-1-1-3-tricarbohydrazonohydrazide (PIN)
read N′1,N′1-diethyl-N′′′ ′1,N′′′ ′1,N3-trimethylcyclohexane-1,1,3-tricarbohydrazonohydrazide (PIN) [remove a space from name]
For carbonohydrazonic dihydrazide (P-65.2.1.5)
read carbonohydrazonic dihydrazide (P-65.2.1.3)
For 3-(hydrazin-1-yl)-3-hydrazinylidenepropanoic acid (PIN)
read 3-hydrazinyl-3-hydrazinylidenepropanoic acid (PIN)
read (C-hydrazinylcarbonohydrazonoyl)acetic acid
For 3-[ C-(hydrazinylcarbonohydrazonoyl)]benzoic acid
read 3-(C-hydrazinylcarbonohydrazonoyl)benzoic acid [delete space in name]
read 3-[hydrazinyl(hydrazinylidene)methyl]benzoic acid
For 4-[(carbamimidoyloxy)methanimidohydrazido]benzoic acid (PIN)
read 4-[C-(carbamimidoyloxy)methanimidohydrazido]benzoic acid (PIN)
read 4-{2-[C-(carbamimidoyloxy)methanimidoyl]hydrazin-1-yl}benzoic acid
Delete (2-methanehydrazonoylhydrazin-1-yl)acetic acid (PIN)
read [(hydrazinylmethylidene)hydrazinyl]acetic acid (PIN)
For ...compound is a preferred IUPAC name. When unsubstituted, the preferred IUPAC name is hydrogen cyanide.
read ...compound is a preferred IUPAC name.
For ...formonitrile in the preferred IUPAC name...
read ...formonitrile is the preferred IUPAC name...
For benzonitrile (PIN)
read benzenecarbonitrile
Replace structure with:
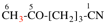
For (not 3,3′-iminodipropionitrile;
read (not 3,3′-azanediyldipropionitrile;
move (not oxomalononitrile; no substitution allowed on malononitrile) from example 2 to example 1 (carbonyl dicyanide)
For dicarbonic dicyanide (PIN)
Add (see P-65.5.3.2)
For methyl 4-[(oxo-λ5-azanylidyne)methyl]benzoate (PIN)
read
4-(methoxycarbonyl)benzonitrile oxide (PIN)
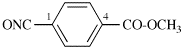
Add sodium 4-[(oxo-λ5-azanylidyne)methyl]benzoate (PIN)
(no longer sodium 4-isofulminatobenzoate)
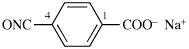
Replace structure with:
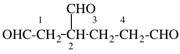
For benzenecarboxaldehyde
read benzenecarbaldehyde
Delete this entry
Replace structures with:
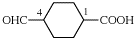
For 7-(2-formylcyclopentyl)heptanaldehyde
read 7-(2-formylcyclopentyl)heptanal
Delete oxydiformaldehyde
Delete [carbonylbis(oxy)diformaldehyde]
For 4-methanethioylbenzoic acid (PIN)
read 4-(methanethioyl)benzoic acid (PIN)
For [2-(1,3-dioxolan-2-yl)ethyl]trimethylsilane (PIN)
read [2-(1,3-dioxolan-2-yl)ethyl]tri(methyl)silane (PIN)
For pentanal diethyl dithioacetal
read pentanal dimethyl dithioacetal
For N(O)(OH)3 azoric acid (preselected name)
read N(O)(OH)3 nitroric acid (preselected name)
For hypoflurous acid
read hypofluorous acid
For naphthalene-2,6-diylbis(phosphonous acid) (PIN)
read (naphthalene-2,6-diyl)bis(phosphonous acid) (PIN)
For N(O)(OH)3 azoric acid (hypothetical)
read N(O)(OH)3 nitroric acid (hypothetical)
For N(OH)
read N(OH)3
For ...telluroperoxo
read ...ditelluroperoxo
For P-67.1.2.3.2 Infixes denoting classes (in decreasing order of seniority except for halides that have the same rank but are cited in alphabetical order and pseudohalides that have the same rank but are cited in alphabetical order)
read P-67.1.2.3.2 Infixes denoting replacement of -OH group in decreasing order of seniority with all halides (class 1) and pseudohalides (class 2) having the same rank within the class.
For P-67.1.2.3.4 Prefixes denoting classes (in decreasing order of seniority except for halides that have the same rank but are cited in alphabetical order and pseudohalides that have the same rank but are cited in alphabetical order)
read P-67.1.2.3.4 Prefixes denoting replacement of -OH group in decreasing order of seniority with all halides (class 1) and pseudohalides (class 2)
having the same rank within the class.
For N,N-dimethylphosphoramidic acid (PIN)
read dimethylphosphoramidic acid (PIN)
For CH3)2N-P(O)(NCS)(SH)
read (CH3)2N-P(O)(NCS)(SH)
For N,N-dimethylphosphoramido(isothiocyanatido)thioic S-acid (PIN)
read N,N-dimethylphosphoramid(isothiocyanatido)thioic S-acid (PIN)
For N,N-dimethyl-N′ -phenylphosphoramidimido(thiocyanatidic) acid (PIN)
read N,N-dimethyl-N′-phenylphosphoramidimido(thiocyanatidic) acid (PIN) [delete space from name]
For phenylphosphonothious S-acid (PIN)
read phenylphosphonothious acid (PIN)
For P(=NH)(NH-NH2)(OH)
read P(=NH)(NH-NH2)(OH)2
For diphenylarsorothious acid (PIN)
read diphenylarsinothious acid (PIN)
For sulfuro(isothiocyanatidic) acid
read sulfur(isothiocyanatidic) acid
read HO-SO2-NCS
For methylphophonocyanatidic acid (PIN)
read methylphosphonocyanatidic acid (PIN)
read CH3-P(O)(OCN)OH
For ...phosphinous, phosponic and phosphinic...
read ...phosphinous, phosphonic and phosphinic...
For ...and not chlorophenylphosphinic acid; (C5H10N)-P(O)Cl(OH) is piperidin-1-ylphosphonochloridic acid and not chloropiperidin-1-ylphosphinic acid; and...
read ...and not chloro(phenyl)phosphinic acid; (C5H10N)-P(O)Cl(OH) is (piperidin-1-yl)phosphonochloridic acid and not chloro(piperidin-1-yl)phosphinic acid; and...
For S(=NH-CH3)Cl2
read S(=N-CH3)Cl2
For bromo(chloro)phenylborane (PIN)
read bromo(chloro)(phenyl)borane (PIN)
Replace structure with:
CH3-SiCl3 [delete space in structure]
Add tetrachlorosilane (preselected name)
For 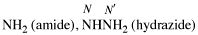
read 
For N,N-diethyl-N′,N′-di methyl-Sb-phenylstibonothioic diamide (PIN)
read N,N-diethyl-N′,N′-dimethyl-Sb-phenylstibonothioic diamide (PIN) [Deleted space from name]
For N,N,N,N′-tetramethyl-N-phenylsulfurimidic diamide (PIN)
read N,N,N′,N′-tetramethyl-N′′-phenylsulfurimidic diamide (PIN)
For 1,1′,1′′-boranetriyltris(hydrazine)
read 1,1′,1′′-boranetriyltrihydrazine
For 1,1′,1′′,1′′′-silanetetrayltetrakis(hydrazine)
read 1,1′,1′′,1′′′-silanetetrayltetrahydrazine
Replace structure with:
Si(NH-NH2)4
For N,N-dipropylnitrous amide (PIN)
read dipropylnitrous amide (PIN)
For N-butyl-N-ethylnitrous amide (PIN)
read butyl(ethyl)nitrous amide (PIN)
For N-(chloromethyl)-N-methylnitramide (PIN)
read (chloromethyl)(methyl)nitramide (PIN)
For N-methyl-N-nitronitramide (PIN)
read methyl(nitro)nitramide (PIN)
For P(O)(CH3)3
read P(O)(O-CH3)3
For O-methyl phenylarsinothioate
read O-methyl phenylarsinothioate (PIN)
For Sb(O)(F2)(S-CH3)
read Sb(O)(F)2(S-CH3)
Replace the structure with:
Si(S-CH3)3(O-CH2-CH3)
For S,S′-diethyl O,O′-dimethyl P,P′-ethane-1,2-diylbis(phosphonothioate)(PIN)
read S,S′-diethyl O,O′-dimethyl P,P′-(ethane-1,2-diyl)bis(phosphonothioate) (PIN)
For –NH(O)< azonoyl
read NH(O)< azonoyl
read NH2(O)– azinoyl
read PH(O)< phosphonoyl
For –PH2(O)< phosphinoyl
read PH2(O)– phosphinoyl
read AsH(O)< arsonoyl
read AsH2(O)– arsinoyl
read SbH(O)< stibonoyl
read SbH2(O)– stibinoyl
For hydrazonostiboroyl
read hydrazonostiboryl
For dimethylphosphoramidic acid (PIN)
read N,N-dimethylphosphoramidic acid (PIN)
For dimethylphosphoramidoyl (preferred prefix)
read N,N-dimethylphosphoramidoyl (preferred prefix)
for (diimethylamido)phosphoryl
read (dimethylamido)phosphoryl
For 2-(phosphonooxy)acetic acid (PIN)
read (phosphonooxy)acetic acid (PIN)
For (not borinoyl)
read (not boryl)
For (sulfanylideneimino)sulfonyl (preselected prefix)
read (sulfanylideneamino)sulfonyl (preselected prefix)
For ...appropriate substituent group ‘hydrazine-1-yl’, ‘hydrazine-1-ylidene’, etc.
read ...appropriate substituent group ‘hydrazin-1-yl’, ‘hydrazin-1-ylidene’, etc.
For sulfurisothiocyanatidoyl
read sulfur(isothiocyanatidoyl)
For [not (sulfonamidoyloxy)propanoic acid; ...
read [not 3-(sulfonamidoyloxy)propanoic acid; ...
For N-[tris(phenylamino)-λ5-phosphanylidene]benzenesulfonamide (PIN)
read N-(trianilino-λ5-phosphanylidene)benzenesulfonamide (PIN)
read N-(trianilinophosphoranylidene)benzenesulfonamide
read [N,N′,N′′-triphenyl-N′′′-benzenesulfonylphosphorimidic triamide;
read nor N′′′-benzenesulfonylphosphorimimidic tris(phenylamide)]
For (dimethylazinoyl)acetonitrile (PIN)
read (dimethylazinoyl)acetonitrile
For HO)2P(O)-O-P(O)(OH)2
read (HO)2P(O)-O-P(O)(OH)2
For HO)2P-O-P(OH)2
read (HO)2P-O-P(OH)2
For HO)2As(O)-O-As(O)(OH)2
read (HO)2As(O)-O-As(O)(OH)2
For HO)2As-O-As(OH)2
read (HO)2As-O-As(OH)2
Delete HO-SO-O-SO-OH disulfurous acid (preselected name; (see P-67.3.2)
For (not 1,3-dithiotriphosphoric 1-S , 3 - S - acid)
read (not 1,3-dithiotriphosphoric 1-S,3-S-acid) [delete spaces in name]
For 1,3-dithiodiphosphonic O1,S2-acid
read 1,3-dithiodiphosphonic O1,S3-acid
read ...name diphosphonic acid...
For N-methyl-1-imido-2-thiodithionous S2-acid (PIN;
read N1-methyl-1-imido-2-thiodithionous S2-acid (PIN;
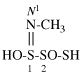
For 1-amido-2-imidodiphosphonic chloride
read 3-amido-2-imidodiphosphonic chloride
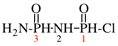
For N1methyl-1,2,3-triimidodiphosphoric tetraamide (PIN)
read N1-methyl-1,2,3-triimidodiphosphoric tetraamide (PIN)
For heptaimidoimidotetraphosphoric hexaamide
read heptaimidotetraphosphoric hexaamide
For disodium N-methyl-1,2-diimidodithionite (PIN)
read disodium N1-methyl-1,2-diimidodithionite (PIN)
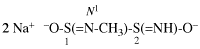
For (CH3)2CH-O-SO-O-SO-O-CH(CH3)2 di(propan-2-yl) disulfite (PIN)
read (CH3)2CH-O-SO-O-SO-O-CH(CH3)2 1,3-bis[(propan-2-yl)oxy]-1λ4,3λ
[not di(propan-2-yl) disulfite, see P-67.3.2]
For (1) 3-{[hydroxy(phosphonooxy)phosphoryl)]oxy}propanoic acid
read (1) 3-{[hydroxy(phosphonooxy)phosphoryl]oxy}propanoic acid
For (1) [({[(methoxysulfinyl)oxy]sulfinyl}oxy)sulfinyl]oxy}acetic acid
read (1) {[({[(methoxysulfinyl)oxy]sulfinyl}oxy)sulfinyl]oxy}acetic acid
For (3) 3,5-disulfanylidene-2,3λ4,4,5λ4,6-pentathianonan-9-oic acid (PIN)
read (3) 3,5-bis(sulfanylidene)-2,3λ4,4,5λ4,6-pentathianonan-9-oic acid (PIN)
read (2) 3-[5-methyl-2,4-bis(sulfanylidene)-2λ4,4λ4-pentasulfanyl]propanoic acid
For ...acids, acid halides, azides, amides, hydrazides, cyanides, isocyanides, isocyanates (and chalcogen analogues in the order O > S > Se > Te), imides, and nitrides and the maximum number of groups representing senior classes.
read ...acids, acid halides, azides, cyanides, isocyanides, isocyanates (and chalcogen analogues in the order O > S > Se > Te), amides, hydrazides, and the maximum number of groups representing the senior class (see P-67.1.2.3.2).
For bis(trimethylphosphanyl)phosphinous acid (PIN)
read bis(dimethylphosphanyl)phosphinous acid (PIN)
For (not orthosulfurodiiodidothiocyanatous acid)
read (not orthosulfurodiiodidothiocyanatidous acid)
For CH3-CO-O-P(OH)2
read CH3-CO-O-P(O)(OH)2
For 1,3-dihydroxydisulfoxane-1,3-dione
read 1,3-dihydroxy-1λ4,3λ4-dithioxane-1,3-dione
For 1,3-dihydroxydisulfoxane-1,3-dione (preselected name)
read 1,3-dihydroxy-1λ4,3λ4-dithioxane-1,3-dione (preselected name)
HO-SO-O-SO-OH
For 1,3-dimethoxydisulfoxane-1,3-dione (PIN)
read 1,3-dimethoxy-1λ4,3λ4-dithioxane-1,3-dione (PIN)
CH3-O-SO-O-SO-O-CH3
For 1,3-diimino-1,3-dimethoxydisulfoxane-1,3-dione (PIN)
read 1,3-dimethoxy-1λ4,3λ4-dithioxane-1,3-diimine (PIN)
For ... is named by functional class nomenclature as an ester, i.e., methyl phenylphosphinate, but the...
read ... is named by functional class nomenclature as an ester, i.e., methyl phenylphosphinite, but the...
For 1,3,6,2-trioxaaluminocane
read 1,3,6,2-trioxaluminocane
For boroxin
read boroxin (see D-7.54, ref. 1)
read (not 1,3,5-trioxa-2,4,6-triboracyclohexane)
For 2,4,8,10-tetraoxa-3,9-diboraspiro[5.5]undecane
read 2,4,8,10-tetraoxa-3,9-diboraspiro[5.5]undecane (PIN)
For H2Ga–
read H2Ga– gallanyl
For [1,3,2]diazaborino[1,2-a][1,3,2]diazaborin-2-yl
read [1,3,2]diazaborinino[1,2-a][1,3,2]diazaborinin-2-yl
For 1-methyldecahydro-1-benzoaluminine
read 1-methyldecahydro-1-benzaluminine
For N,N′-bis(diphenylboranyl)ethane-1,2-diamine) (PIN)
read N1,N2-bis(diphenylboranyl)ethane-1,2-diamine (PIN)
read (not N,N′-(ethane-1,2-diyl)bis(diphenylborinic amide);
read not N,N′-(ethane-1,2-diyl)bis(diphenylboranamine);
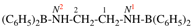
For P-69.1.5.1 Suffix nomenclature
read P-68.1.5.1 Suffix nomenclature
read P-68.1.5.2 Prefix nomenclature
For hydroxydimethylthallane
read hydroxydi(methyl)thallane
For [(ethane-1,2-diylbis(oxymethylene))bis(dimethylborane)
read [ethane-1,2-diylbis(oxymethylene)]bis(dimethylborane)
Delete 3,10-diethyl-4,9-dioxa-3,10-diaza-5,8-diboradodecane (PIN)
read O,O′-[ethane-1,2-diylbis(boranediyl)]bis(N,N-diethylhydroxylamine)
For [not N,N′ -boranediylbis[1,1,1-trimethyl(N-trimethylsilyl)silanamine];
read [not N,N′-boranediylbis[1,1,1-trimethyl-N-(trimethylsilyl)silanamine]; [delete space in name]
For [chloro(2,4,6-tri-tert-butylphenyl)gallanyl]-1,1,1-trimethyl-N-(trimethylsilyl)silanamine (Si is senior to Ga)
read N-[chloro(2,4,6-tri-tert-butylphenyl)gallanyl]-1,1,1-trimethyl-N-(trimethylsilyl)silanamine (Si is senior to Ga)
For N,N,N′,N′-tetrakis(trimethylsilyl)-1-[(2,4,6-tri-tert-butylphenyl)indiganediamine
read 1-(2,4,6-tri-tert-butylphenyl)-N,N,N′,N′-tetrakis(trimethylsilyl)indiganediamine
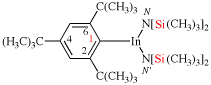
For (phenylgallanediyl)bis(phenylphosphane)
read P,P′-(phenylgallanediyl)bis(phenylphosphane)
For 4,4′,4′′-boranetriyltris(benzeneamine)
read 4,4′,4′′-boranetriyltris(benzen-1-amine)
For (difluoroboranyl)trimethylsilane (PIN)
read (difluoroboranyl)tri(methyl)silane (PIN)
For ethaneamine(N→B2)(N→B4)pentaborane(9) (2/1)
read ethanamine(N→B2)(N→B4)pentaborane(9) (2/1)
For μ-(ethane-1,2-diamine-1κN-2κN′)bis(trihydridoboron)
read μ-(ethane-1,2-diamine-1κN1,2κN2)bis(trihydridoboron)
For trimethylamine—(methylsulfanyl)methane—dodecaborane(10) (1/1/1)
read (trimethyl)amine—(methylsulfanyl)methane—dodecaborane(10) (1/1/1)
For (2,2′-bipyridine-κN1,κN1′)(2,2′-oxybis(benzen-1-ido-κC1)]boron(1+) perchlorate
read (2,2′-bipyridine-κ2N1,N1′)(2,2′-oxybis(benzen-1-ido-κC1)]boron(1+) perchlorate
For N,N,N′,N′-tetramethylguanidine(N′′—Ga)gallane
read N,N,N′,N′-tetramethylguanidine(N′′—Ga)gallane (1/1)
read trihydrido[N,N,N′,N′-tetramethylguanidine-κN′′]gallium
For (2-amino-κN-ethan-1-olato-κO)dimethanidoboron
read (2-amino-κN-ethan-1-olato-κO)dimethanidoboron [κ not italic]
For dichlorido(2-nitro-κO-phenolato-κO)boron
read dichlorido(2-nitro-κO-phenolato-κO)boron [κ not italic]
For (N3—B)[1H-benzimidazol-2-yl)phenyl]boronic acid
read (N3—B)[2-(1H-benzimidazol-2-yl)phenyl]boronic acid
read [2-(1H-benzimidazol-2-yl-κN3)benzen-1-ido-κC1]dihydroxidoboron
For [3,3′,3′′-nitrilo-κN-tris(propane-1-yl-κC1)]boron
read [3,3′,3′′-nitrilo-κN-tris(propan-1-yl-κC1)]boron
For [2(O—B)]-N-[(difluoroboranyl)oxy]-N-nitrosomethanamine
read (O—B)-N-[(difluoroboranyl)oxy]-N-nitrosomethanamine
read difluorido(N-nitroso-κO-N-oxido-κO-methanamine)boron
For (N 3—B)[1H-benzimidazol-2-yl)phenyl]boronic acid
read (N3—B)[2-(1H-benzimidazol-2-yl)phenyl]boronic acid
read [2-(1H-benzimidazol-2-yl-κN3)benzen-l-ido-κC1]dihydoxidoboron
For (μ-{2,2′-[ethane-1,2-diylbis(azanylylidene-1κN:2κN′-methanylylidene)bis(4,6-di-tert-butylphenolato-1κO:2κO′)bis[di(ethanido-1κ2C1,2κ2C1)gallium]
read (μ-{2,2′-[ethane-1,2-diylbis(azanylylidene-1κN1:2κN2-methanylylidene)bis(4,6-di-tert-butylphenolato-1κO:2κO′)]})bis[di(ethanido-1κ2C1,2κ2C1)gallium]
Replace the structure with:
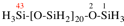
For 2,4,6,8,10-pentathia-1,3,5,7tetrasilaadamantane
read 2,4,6,8,9,10-hexathia-1,3,5,7-tetrasilaadamantane
Replace diagram with:
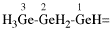
For ...and saturation of mancude structures...
read ...and saturation of double bonds of mancude structures...
For 1,1′,1′′,1′′′-silanetetrayltetrakis(hydrazine)
read 1,1′,1′′,1′′′-silanetetrayltetrahydrazine
For methylsilanetriamine (PIN)
read 1-methylsilanetriamine (PIN)
For N,N′ -disilyl-1-methylsilanediamine (PIN)
read 1-methyl-N,N′-disilylsilanediamine (PIN) [Deleted space from name]
For 4-[(bis(silanyl)methyl]hexan-2-one
read 4-[bis(silanyl)methyl]hexan-2-one
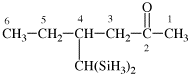
Replace the structure with:
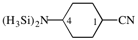
For dimethyl stannanediyl dibutanedioate (PIN;
read dimethyl dibutylstannanediyl dibutanedioate (PIN;
read dibutylbis[(4-methoxy-4-oxobutanoyl)oxy]stannane
For bis[(trimethylsilyl)methyl]stannanol (PIN)
read bis[bis(trimethylsilyl)methyl]stannanol (PIN)
read [(hydroxystannanediyl)dimethanetriyl]tetrakis(trimethylsilane)
Following P-16.5.2.6 replace the structure with:
CH3-[CH2]11-SiH2-[CH2]11-CH3
Following P-16.5.2.6 replace the structure with:
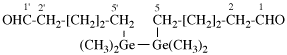
For bis(4,5-dihydrothiophen-2-yl)dimethylgermane (PIN)
read bis(4,5-dihydrothiophen-2-yl)di(methyl)germane (PIN)
For methoxydimethyl[(2-(trimethylgermyl)phenyl]silane (PIN)
read methoxydi(methyl)[2-(trimethylgermyl)phenyl]silane (PIN)
For 1,4-phenylenebis(dimethylsilane) (PIN)
read (1,4-phenylene)bis(dimethylsilane) (PIN)
For thiophene-2,5-diylbis(trimethylstannane)
read (thiophene-2,5-diyl)bis(trimethylstannane)
For [(hydroxystannanediyl)dimethanetriyl]tetrakis(trimethylsilane) (PIN)
read [(hydroxystannanediyl)dimethanetriyl]tetrakis(trimethylsilane)
read [Decided by P-41 table 4.1 class 17, not class 26 or class 28]
For P-68.3.1 Nitrogen compound...
read (1) Nitrogen compound...
For ...similarities, azonic HN(OH)2 and azinic acids H2N(OH), are...
read ...similarities, azonic HN(O)(OH)2 and azinic acids H2N(O)(OH), are...
For P-68.3.2 Phosphorus, arsenic,...
read (2) Phosphorus, arsenic,...
For P-68.3.3 Bismuth compounds...
read (3) Bismuth compounds...
Add N-hydroxymethanamine (PIN)
Add N-hydroxy-N-methylmethanamine (PIN)
For (not acetohydroxamic acid)
read acetohydroxamic acid
For O,O′-ethane-1,2-diylbis(hydroxylamine) (PIN)
read O,O′-(ethane-1,2-diyl)bis(hydroxylamine) (PIN)
For O-benzoylhydroxylamine (PIN)
read O-benzoylhydroxylamine
read (not azanyl benzoate)
For (not 1-methyldioxazane)
read (not 1-methyldiazoxane)
For N,O-dimethoxyhydroxylamine
read N,O-dimethylhydroxylamine
For O,O′-[(ethane-1,2-diylbis(boranediyl)]bis(N,N-diethylhydroxylamine)
read O,O′-[ethane-1,2-diylbis(boranediyl)]bis(N,N-diethylhydroxylamine)
For 2-(aminooxy)ethanamine (PIN)
read 2-(aminooxy)ethan-1-amine (PIN)
For ...substitutively as ‘ylidene’ derivatives of hydroxylamine. Compounds...
read ...substitutively as N-hydroxy derivatives of imines. Compounds...
For ...substitutively as ‘ylidene’ derivatives of hydroxylamine rather...
read ...substitutively as N-hydroxy derivatives of imines rather...
For (pentan-2-ylidene)hydroxylamine (PIN)
read (pentan-2-ylidene)hydroxylamine
Replace the structure with:
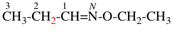
For butane-2,3-diylidenebis(hydroxylamine) (PIN)
read (butane-2,3-diylidene)bis(hydroxylamine)
For 4-[(ethoxyimino)methyl)]benzene-1-sulfonic acid (PIN)
read 4-[(ethoxyimino)methyl]benzene-1-sulfonic acid (PIN)
For pentane-2,4-diylidenebis(O-phenylhydroxylamine) (PIN)
read N2,N4-diphenoxypentane-2,4-diimine (PIN)
For N-(1-nitropropylidene)hydroxylamine (PIN)
read N-(1-nitropropylidene)hydroxylamine
For N-(1-nitrosoethylidene)hydroxylamine (PIN)
read N-(1-nitrosoethylidene)hydroxylamine
For hetrane name
read heterane name
For ... –NH-NH– ‘hydrazinediylidene’. The ...
read ... –NH-NH– ‘hydrazine-1,2-diyl’. The ...
For ethane-1,2-diylidenebis(phenylhydrazine) (PIN)
read 1,1′-ethanediylidenebis(2-phenylhydrazine) (PIN)
For N,1-dimethylhydrazin-1-carboxamide (PIN)
read N,1-dimethylhydrazine-1-carboxamide (PIN)
For 3-(2-carbamoylhydrazin-1-yl)propanenitrile (PIN)
read 3-(2-carbamoylhydrazin-1-yl)propanoic acid (PIN)
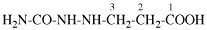
For (hexan-3-ylidene)-N,N-diphenylhydrazinecarboxamide (PIN)
read 2-(hexan-3-ylidene)-N,N-diphenylhydrazine-1-carboxamide (PIN)
read 2-(1-ethylbutylidene)-N,N-diphenylhydrazine-1-carboxamide
For N,N-diphenyl(1-phenylbutylidene)hydrazine-1-carbothioamide (PIN)
read N,N-diphenyl-2-(1-phenylbutylidene)hydrazine-1-carbothioamide (PIN)
For P-68.3.1.2.6.1 The preferred...
read The preferred...
Replace the structure with:
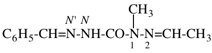
For ...are called ‘2-(hydrazinecarbonyl)hydrazin-1-yl’ and ‘(hydrazinecarbonyl)hydrazinylidene’...
read ...are called ‘hydrazinecarbohydrazido’ and ‘(hydrazinecarbonyl)hydrazinylidene’...
For 3-[2-(hydrazinecarbonyl)hydrazin-1-yl]propanoic acid (PIN)
read 3-[2-(hydrazinecarbonyl)hydrazin-1-yl]propanoic acid
For 3-(diazenyl)propanoic acid (PIN)
read 3-diazenylpropanoic acid (PIN)
For naphthalen-2-yl(phenyl)diazene (PIN)
read (naphthalen-2-yl)(phenyl)diazene (PIN)
For (anthracen-2-yl)[(7-phenyldiazenyl)naphthalen-2-yl]diazene (PIN)
read (1) {7-[(anthracen-2-yl)diazenyl]naphthalen-2-yl}(phenyl)diazene (PIN)
(numbering shown); alphanumerical order ‘anthracen-2-yl’ precedes ‘anthracen-2-yldiazenyl’}
read {not (anthracen-2-yl)[7-(phenyldiazenyl)naphthalen-2-yl]diazene;
each primary substituent has the same locant, i.e., none, so ‘anthracenyldiazenyl’ is alphabtically preferred to ‘anthracenylphenyl’}
For 2-(2-phenyl-2-oxo-2λ5-diazenyl)naphthalen-1-yl preferred prefix)
read 2-(2-phenyl-2-oxo-2λ5-diazenyl)naphthalen-1-yl (preferred prefix)
Move "The name 'carbazono' is not recommended." to the third paragraph
For ...The group H2N-NH-CO-N=NH– is named...
read ...The group H2N-NH-CO-N=N– is named...
For ethyl 3-(2-diazenecarbonylhydrazin-1-yl)propanoate (PIN)
read ethyl 3-[2-(diazenecarbonyl)hydrazin-1-yl]propanoate
For ...derivative of the parent hydride ‘diazene’; its...
read ...derivative of the parent hydride ‘hydrazine’; its...
For(hydrazinylidenemethyl)diazene
read (diazenylmethylidene)hydrazine
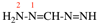
For [hydrazinylidene(phenyl)methyl]phenyldiazene
read [phenyl(phenyldiazenyl)methylidene]hydrazine
For [phenyl(phenylhydrazinylidene)methyl]diazene
read [diazenyl(phenyl)methylidene]phenylhydrazine
For hydrazinylidenemethyl)diazenyl
read (hydrazinylidenemethyl)diazenyl
For 3-(2-{cyano-[(2-hydroxy-5-sulfophenyl)diazenyl]methylidene}hydrazinyl)-4-hydroxybenzene-1-sulfonic acid
read 3-(2-{cyano[(2-hydroxy-5-sulfophenyl)diazenyl]methylidene}hydrazinyl)-4-hydroxybenzene-1-sulfonic acid
read {not 3-({cyano[(2-hydroxy-5-sulfophenyl)hydrazinylidene]methyl}diazenyl)-4-hydroxybenzene-1-sulfonic acid
Replace the structure with:
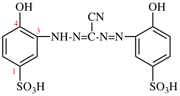
For ...and telluro are used with the name carbadiazone in general nomenclature.
read ...and telluro are used with the name carbodiazone in general nomenclature.
Delete the box.
For ...systematic prefix names, i.e., triaazan-1-yl, etc.,
read ...systematic prefix names, i.e., triazan-1-yl, etc.,
For ...names ‘epitrazano’, etc.
read ...names ‘epitriazano’, etc.
For triazan-1-yl- (preselected prefix)
read triazan-1-yl (preselected prefix)
For triaz-2-en-1-yl- (preselected prefix
read triaz-2-en-1-yl (preselected prefix)
read (not triaz-2-eno)
For (not 4-triazenobenzoic acid)
read [not 4-(triaz-2-eno)benzoic acid]
For ... the group –N=NH-NH– is no longer recommended.
read ... the group –N=N-NH– is no longer recommended.
Replace the structure with:
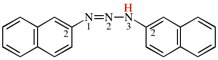
For cyclotriphosphazene
read (not cyclotriphosphazene, which has only one double bond)
Add 3,5-dioxa-4-phospha-2-silaheptane
delete all of the Note:

Delete [not (methylimino)phosphane] (repeat from example 5).
For trimethyl-λ5-arsanimine (PIN)
read As,As,As-trimethyl-λ5-arsanimine (PIN)
For 2,4,6-triethoxy-1,3,5,2λ5,4λ5, 6λ5-triazatriphosphinane-2,4,6-trione (PIN)
read 2,4,6-triethoxy-1,3,5,2λ5,4λ5,6λ5-triazatriphosphinane-2,4,6-trione (PIN) [remove space in name]
Delete (formerly pentamethyl holophosphate
For naphthalen-2-ylarsane (PIN)
read (naphthalen-2-yl)arsane (PIN)
read (naphthalen-2-yl)arsine
For ethyl(methyl)phenylphosphane (PIN)
read ethyl(methyl)(phenyl)phosphane (PIN)
read ethyl(methyl)(phenyl)phosphine
For 1-benzofuran-2-ylphosphane (PIN)
read (1-benzofuran-2-yl)phosphane (PIN)
read (1-benzofuran-2-yl)phosphine
For thiophen-2-ylphosphane (PIN)
read (thiophen-2-yl)phosphane (PIN)
read (thiophen-2-yl)phosphine
For ethane-1,2-diylbis(dimethylphosphane) (PIN)
read (ethane-1,2-diyl)bis(dimethylphosphane) (PIN)
read (ethane-1,2-diyl)bis(dimethylphosphine)
For butane-1,2,4-triyltris(phosphane) (PIN)
read (butane-1,2,4-triyl)tris(phosphane) (PIN)
read (butane-1,2,4-triyl)tris(phosphine)
For dibenzo[b,d]furan-3,7-diylbis(phosphane) (PIN)
read (dibenzo[b,d]furan-3,7-diyl)bis(phosphane) (PIN)
read (dibenzo[b,d]furan-3,7-diyl)bis(phosphine)
For 1,2-phenylenebis(arsane) (PIN)
read (1,2-phenylene)bis(arsane) (PIN)
read (1,2-phenylene)bis(arsine)
For –H2P-PH2– read –HP-PH–
For (arsanylmethyl)diphenylphosphane (PIN)
read (arsanylmethyl)di(phenyl)phosphane (PIN)
read (arsinomethyl)di(phenyl)phosphine
For 4-[ethyl(methyl)phosphino]-1H-imidazole (PIN)
read 4-[ethyl(methyl)phosphino]-1H-imidazole
For methyl (dimethoxyphosphorimidoyl)acetate (PIN)
read methyl (P,P-dimethoxyphosphinimidoyl)acetate (PIN)
For trimethylbusmuthine
read trimethylbismuthine
For triphenyl-λ5-bismuthanimine (PIN)
read Bi,Bi,Bi-triphenyl-λ5-bismuthanimine (PIN)
For dichloro(triphenyl)-λ5-bismuthane (PIN)
read dichlorotri(phenyl)-λ5-bismuthane (PIN)
read dichlorotri(phenyl)bismuthorane
read (C6H5)3BiCl2
For 1-methyl-3-phenyltriselane (PIN)
read methyl(phenyl)triselane (PIN)
For 1,3-diphenyltriselane (PIN)
read diphenyltriselane (PIN)
For methylenebis(trisulfane) (PIN)
read 1,1′-methylenebis(trisulfane) (PIN)
Delete 1-(acetylpentathio)propan-1-one
For 1-methyl-3-phenyldithioxane (PIN)
read methyl(phenyl)dithioxane (PIN)
For (not oxydisulfanol)
read [not oxybis(sulfanol)]
For [(methylperoxy)sulfanyl)]methane (PIN)
read [(methylperoxy)sulfanyl]methane (PIN)
For [sulfanediylbis(oxy)]dibenzene
read 1,1′-[sulfanediylbis(oxy)]dibenzene
For [(acetylsulfanyl)peroxy]propan-1-one (PIN)
read 1-[(acetylsulfanyl)peroxy]propan-1-one (PIN)
For 1,1′-[disulfanediylbis(oxy)]dipropan-1-one (PIN)
read 1,1′-[disulfanediylbis(oxy)]di(propan-1-one) (PIN)
read 1,1′-[dithioperoxybis(oxy)]di(propan-1-one)
For (trimethoxy-λ4-sulfanyl)methane
read [(trimethoxy-λ4-sulfanyl)oxy]methane
For 1-[(ethaneseleninyl)selenonyl]ethane
read [(ethaneseleninyl)selenonyl]ethane
read [(ethylseleninyl)selenonyl]ethane
For S,S-diphenyl-N-benzenesulfonylsulfimide
read S,S-diphenyl-N-(benzenesulfonyl)sulfimide
For 1H-1λ3-bromirane (PIN)
read 1λ3-bromirane (PIN)
For 1λ3-benziodole (PIN)
read 1H-1λ3-benziodole (PIN)
For N-methylbromic amide(PIN)
read methylbromic amide (PIN) [add space before (PIN)]
For N-ethylhypochlorous amide (PIN, see P-62.4)
read ethylhypochlorous amide (PIN, see P-62.4)
For bromodiethenylstibane (PIN)
read bromodi(ethenyl)stibane (PIN)
For (methyl)titanium
read trichlorido(methyl)titanium
For (methyl)bis(triethylphosphane)platinum
read acetyl(methyl)bis(triethylphosphane)platinum
For (4-carboxyphenyl)methylmercury
read (4-carboxyphenyl)(methyl)mercury
For [(2,3,5,6-η4)-bicyclo[2.2.1]hepta-2,5-diene]tricarbonyliron
read [(2,3,5,6-η)-bicyclo[2.2.1]hepta-2,5-diene]tricarbonyliron
For dicarbonyl[(1–3-η)-cyclohepta-2,4,6-dien-1-ido](η5-cyclopenta-2,4-dien-1-ido)molybdenum
read dicarbonyl[(1–3-η)-cyclohepta-2,4,6-trien-1-ido](η5-cyclopenta-2,4-dien-1-ido)molybdenum
read dicarbonyl[(1–3-η)-cyclohepta-2,4,6-trien-1-yl](η5-cyclopenta-2,4-dien-1-yl)molybdenum
read
dicarbonyl[(1–3-η)-cyclohepta-2,4,6-trien-1-ido](η5-cyclopentadienido)molybdenum
For {μ-[2(1-3,3a,8a-η):1(4–6-η)]azulene}-(pentacarbonyl-1κ3C,2κ2C)diiron(Fe—Fe)
read {μ-[2(1–3,3a,8a-η):1(4–6-η)]azulene}-(pentacarbonyl-1κ3C,2κ2C)diiron(Fe—Fe)
For tricarbonyl{N,N-dimethyl-1-[2-(diphenylphosphanyl)-η6-phenyl]ethan-1-amine}chromium
read tricarbonyl{1-[2-(diphenylphosphanyl)-η6-phenyl]-N,N-dimethylethan-1-amine}chromium
For [(1,2,5,6-η)-cycloocta-1,5-diene)](η6-phenyltriphenylboranuide)rhodium
read [(1,2,5,6-η)-cycloocta-1,5-diene][triphenyl(η6-phenyl)boranuido]rhodium
read [(1,2,5,6-η)-cycloocta-1,5-diene][triphenyl(η6-phenyl)borato]rhodium
For 2-(osmocen-1-yl)ethanol (PIN)
read 2-osmocenylethan-1-ol (PIN)
read 1-(2-hydroxyethyl)osmocene
For N,N-dimethyl(vanadocen-1-yl)ethan-1-amine (PIN)
read N,N-dimethyl-1-vanadocenylethan-1-amine (PIN)
read [1-(dimethylamino)ethyl]vanadocene
For 1(1,1′),3(1,1′)-diferrocenacylotetraphane (PIN)
read 1,3(1,1′)-diferrocenacyclotetraphane (PIN)
For 1,1′-(4-carboxybutane-1,3-diyl)ferrocene (PIN)
read 1,1′-(4-carboxybutane-1,3-diyl)ferrocene
read 3,5-(ferrocene-1,1′-diyl)pentanoic acid (PIN) [space removed from name]
For tetra-μ3-methanidotetralithium
read tetra-μ3-methanido-tetralithium
read tetra-μ3-methyl-tetralithium
For MgI(Me)]
read [MgI(Me)]
For [(MgI(Me)]n
read [MgI(Me)]n
read poly[iodido(methyl)magnesium]
Delete 1-carbonyl-3,5-dimethyl-bis(triethylphosphane)iridine (Hantzsch-Widman type name)
read 1-carbonyl-3,5-dimethyl-1,1-bis(triethylphosphane)-1-iridabenzene
For 9,9-[methylenebis(dimethylphosphane)-10H-9-platinaanthracene
read 9,9-[methylenebis(dimethylphosphane)]-10H-9-platinaanthracene
For [(4-diphenylstibanyl)phenyl](phenyl)mercury
read [4-(diphenylstibanyl)phenyl](phenyl)mercury
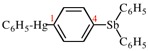
Delete this correction (leave as printed)
For ...not ‘acetyl cation’. For CH3-C(O)-O+;...
read ...not ‘acetyl cation’, for CH3-C(O)+;...
For ... and ‘methyliumyl’ not ‘methyl radical cation’, for CH4•–.
read ... and ‘methaniumyl’ not ‘methyl radical cation’, for CH4•+.
Replace structure with:
•CH3
Replace structure with:
•CH2-CH2-CH3
Replace structure with:
For tert-butoxy(triphenyl)-λ5-phosphanyl (PIN)
read tert-butoxytri(phenyl)-λ5-phosphanyl (PIN)
read [(2-methylpropan-2-yl)oxy]tri(phenyl)-λ5-phosphanyl
read (1,1-dimethylethoxy)tri(phenyl)-λ5-phosphanyl
For anthracene-9-eliumuide
read anthracen-9-eliumuide
For ... aminylene, silylene, and carbyne, ...
read ... aminylene, and carbyne, ...
For diphenylnethanediyl
read diphenylmethanediyl
For naphthalen-4a(8aH)-yl
read naphthalen-4a(8aH)-yl
For (C60-Ih)[5,6]fulleren-1(9H)-yl (PIN)
read (C60-Ih)[5,6]fulleren-1(9H)-yl (PIN)
read 1,9-dihydro(C60-Ih)[5,6]fulleren-1-yl
For dimethyloxo-λ5-phosphanyl
read dimethyl(oxo)-λ5-phosphanyl
For 1,4-phenylenebis(oxo-λ4-sulfanyl)
read (1,4-phenylene)bis(oxo-λ4-sulfanyl)

For 1,4-phenylenebis(oxomethyl)
read (1,4-phenylene)bis(oxomethyl)
For (1) ethane-1,2-bis(aminyl) (PIN)
read (1) (ethane-1,2-diyl)bis(aminyl) (PIN)
For (2) benzene-1,2-dicarbonylbis(azanyl)
read (2) (benzene-1,2-dicarbonyl)bis(azanyl)
For ...phenoxy, and aminooxyl, which...
read ...phenoxy, and aminoxyl, which...
For (1) chloroacetoxyl (PIN)
read chloroacetoxyl
For butanoyloxyl
read butanoyloxyl (PIN)
read butanoyloxidanyl
For acetylselanyl (PIN)
read methylselanyl (PIN)
For [chloro(ethanethioyl)]sulfanyl (PIN)
read (chloroethanethioyl)sulfanyl (PIN)
For cyclopropane-1,2-diyldimethyl (PIN)
read (cyclopropane-1,2-diyl)dimethyl (PIN)
For naphthalene-2,6-diylbis(disulfanyl) (PIN)
read (naphthalene-2,6-diyl)bis(disulfanyl) (PIN)
Replace structure with:
•O-C(CH3)2-CH2-C(CH3)2-O•
For (1) cyclobutane-1,3-diylbis(peroxyl) (PIN)
read (1) (cyclobutane-1,3-diyl)bis(peroxyl) (PIN)
read (2) (cyclobutane-1,3-diyl)bis(dioxidanyl)
For oxylcarboxyl (preferred prefix)
read oxylcarbonyl (preferred prefix)
—CO-O•
Delete this line
For oxyl (preselected name)
read ylooxidanyl (preselected name)
read ylooxy
Add [not 4-(1,2-diyloethyl)cyclohexyl; ethane has two radical centers, cyclohexane only has one]
Replace the structure with:
Replace the structure with:
•CH2-C(CH3)2-O•
For (3-oxylbenzoyl)oxyl (PIN)
read [3-(ylooxidanyl)benzoyl]oxyl (PIN)
Replace the structure with:
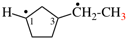
For oxophenyl-λ4-sulfanide (PIN)
read oxo(phenyl)-λ4-sulfanide (PIN)
Replace structure with:
(NC)3C –
For 1,2-dihydropyridine-1-ide
read 1,2-dihydropyridin-1-ide
For 1-methyl-1,2-dihydro-1-benzoazocine-2,2-diide
read 1-methyl-1,2-dihydro-1-benzazocine-2,2-diide
Delete X = Y = –
read 1,4-dihydronaphthalene-1,4-diide (PIN)
Replace structure with:
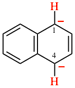
For (C60-Ih)[5,6]fulleren-1(9H)-ide (PIN)
read (C60-Ih)[5,6]fulleren-1(9H)-ide (PIN)
read 1,9-dihydro(C60-Ih)[5,6]fulleren-1-ide
For ethane-OS-(thioperoxoate) (PIN)
read ethane(OS-thioperoxoate) (PIN)
For phenyl hydrogen phosphonate (PIN)
read phenyl hydrogen phosphate (PIN)
For The traditional names methoxide, ethoxide, propoxide (but not isopropoxide), butoxide, tert-butoxide, phenoxide and aminoxide, for CH3-O–, C2H5-O–, C3H7-O–, C4H9-O–, (CH3)3C-O–, C6H5-O– and H2N-O–, are retained as preferred IUPAC names.
read The traditional names methoxide, ethoxide, propoxide, butoxide, tert-butoxide, phenoxide and aminoxide, for CH3-O–, C2H5-O–, C3H7-O–, C4H9-O–, (CH3)3C-O–, C6H5-O– and H2N-O– are retained as preferred IUPAC names. tert-Butoxide cannot be substituted. Isopropoxide, (CH3)2CH-O–, is retained for general nomenclature but cannot be substituted.
For ethylsulfanyl)oxidanide
read (ethylsulfanyl)oxidanide
read (not ethanesulfenate; sulfenic acids ...
For ethane-1,2-diylbis(dioxidanide)
read (ethane-1,2-diyl)bis(dioxidanide)
For 1,4-phenylenebis(disulfanide)
read (1,4-phenylene)bis(disulfanide)
For 1,4-phenylenebis(oxy)bis(sulfanide)
read (1,4-phenylene)bis(oxy)bis(sulfanide)
For ethane-1,2-diylbis(amide)
read (ethane-1,2-diyl)bis(amide)
For (2-methylpropan-2-y)trioxidanide
read (2-methylpropan-2-yl)trioxidanide
For 1-oxoethanide
read 1-oxoethan-1-ide
Replace the structure with:
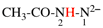
For 6λ5-phosphanuidaspiro[5.5]nonan
read 6λ5-phosphanuidaspiro[5.5]undecane
read (not 6λ5-phosphataspiro[5.5]undecane)
For 1,4-phenylenebis(phosphanide) (PIN)
read (1,4-phenylene)bis(phosphanide) (PIN)
Replace the structure with:
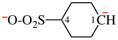
Page 1080, P-72.7 (b) example. [modified 21 April 2021]
Replace example with
2-(2-carboxylatoethyl)-1,1,5,5-tetracyanopentane-1,5-diide (PIN)
[not 1,1-dicyano-4-(dicyanomethanidyl)heptan-1-id-7-oate; diide is senior to idoate]
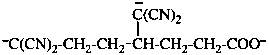
For (2-phosphanidylethyl))arsanuide
read (2-phosphanidylethyl)arsanuide
For oxidonaphthalene-2-carboxylate (PIN)
read 3-oxidonaphthalene-2-carboxylate (PIN)
For 1λ6, 3λ6-dithiocane-1,3-diide (PIN)
read 1λ6,3λ6-dithiocane-1,3-diide (PIN)
read1λ4,3λ4-dithiocane-1,3-diuide
read (not 1λ4,3λ6-dithiocan-3-id-1-uide; identical groups must not be named differently within a name, if possible)
[remove space in names]
For chloro(trimethyl)phosphonium
read chlorotri(methyl)phosphonium (PIN)
read chlorotri(methyl)phosphanium (PIN)
For ...multiplying locants ‘di’,...
read ...multiplying prefixes ‘di’,...
For 1,1,3,3-tetraphenyl-4,5-dihydro-3H-1λ5,2,3-triphosphol-3-ium (PIN)
read 1,1,3,3-tetraphenyl-4,5-dihydro-1H-1,2,3λ5-triphosphol-1-ium (PIN)
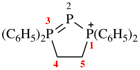
For 1,1,1,5,5,5-hexamethyltrisilazane-2,4-diium (PIN)
read N,N′-bis(trimethylsilyl)silanebis(aminium) (PIN)
For benzoyltrimethylammonium
read benzoyltri(methyl)ammonium
For (1,4-dithian-2-yl)trimethylammonium
read (1,4-dithian-2-yl)tri(methyl)ammonium
For N,N,N-trimethylbenzenaminium (PIN)
read N,N,N-trimethylbenzenaminium
For [1-(oxidaniumyl)ethylidene]oxidanium (PIN)
read (1-oxidaniumylethylidene)oxidanium (PIN)
For (cyclohexanecarbonyl)dimethyloxidanium (PIN)
read (cyclohexanecarbonyl)di(methyl)oxidanium (PIN)
read (cyclohexanecarbonyl)di(methyl)oxonium
For (1-hydroxyethylidene)(dimethyl)azanium (PIN)
read (1-hydroxyethylidene)di(methyl)azanium (PIN)
read (1-hydroxyethylidene)di(methyl)ammonium
For benzoyldimethylsulfanium
read benzoyldi(methyl)sulfanium
read benzoyldi(methyl)sulfonium
For benzoyldioxidanium (PIN)
read 2-benzoyldioxidan-1-ium (PIN)
Replace the structure with:
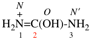
For N-(methylamino)phenoxymethylidene]methanaminium
read N-[(methylamino)phenoxymethylidene]methanaminium
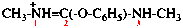
Add phenylium
For 1-oxoethylium (PIN)
read acetylium (PIN)
For P-73.2.2.2 Cationic centres on characteristic groups
read P-73.2.2.2 ‘Added indicated hydrogen’
P-73.2.2.3 Diazonium ions
For ... Such a radical can also...
read ... Such a cation can also...
For ...for the radical center...
read ...for the cationic center...
For X = + naphthalen-4a(8aH)-ylium (PIN)
read naphthalen-4a(8aH)-ylium (PIN)
read 4a,8a-dihydronaphthalen-4a-ylium
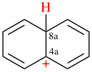
For (C60-Ih)[5,6]fulleren-1(9H)-ylium (PIN)
read (C60-Ih)[5,6]fulleren-1(9H)-ylium (PIN)
read 1,9-dihydro(C60-Ih)[5,6]fulleren-1-ylium
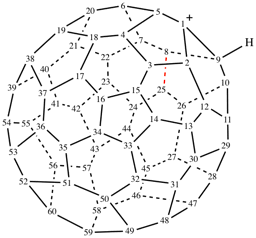
For 1,4-phenylenebis(diazenylium) (PIN)
read (1,4-phenylene)bis(diazenylium) (PIN)
For methylphosphonoylium (PIN)
read methylphosphonobis(ylium) (PIN)
Replace structure with:
For The names methoxylium, ethoxylium, propoxylium, butoxylium, phenoxylium, and aminoxylium are retained as preferred IUPAC names....
read The names methoxylium, ethoxylium, propoxylium, butoxylium, and phenoxylium are retained as preferred IUPAC names, and aminoxylium as a preselected name....
For chloracetoxylium (PIN)
read chloroacetoxylium
For dimethylaminoxylium (PIN)
read N-methylmethanaminoxylium (PIN)
Replace the structure with:
For 2-benzoyldiazane-1-ylium
read 2-benzoyldiazan-1-ylium
For (not ethyltelluorperoxylium)
read (not ethyltelluroperoxylium)
Add (not 3H-thienylium)
For 1λ3-benzoiodol-1-ylium
read 1λ3-benziodol-1-ylium
Add (not 3H-thienylium)
For E = S 2λ4-benzothiopyran-1-ylium (PIN)
read E = S 2λ4-benzothiopyran-2-ylium (PIN)
read E = Se 2λ4-benzoselenopyran-2-ylium (PIN)
read E = Te 2λ4-benzotelluropyran-2-ylium (PIN)
For 2H-furylium (PIN)
read 2H-1λ4-furan-1-ylium (PIN)
read 2H-1λ4-thiophen-1-ylium (PIN)
read 2H-1λ4-selenophen-1-ylium (PIN)
read 2H-1λ4-tellurophen-1-ylium (PIN)
For E = O flavylium (PIN)
read E = O flavylium
read E = S thioflavylium
read E = Se selenoflavylium
read E = Te telluroflavylium
For 2-ethoxy-N-{2-[(2-ethoxyethyl)sulfaniumyl]ethyl}-N,N-dimethylethanaminium (PIN)
read 2-ethoxy-N-{2-[(2-ethoxyethyl)(methyl)sulfaniumyl]ethyl}-N,N-dimethylethanaminium (PIN)
read 2-ethoxy-N-{2-[(2-ethoxyethyl)(methyl)sulfanyl]ethyl}-N-methylethanamine
read N-(2-ethoxyethyl)-2-[(2-ethoxyethyl)(methyl)sulfanyl]-N-methylethanamine
Replace the structure with:
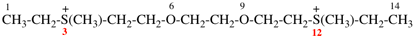
For 5H-5λ5-arsaspiro[4.4]nonan-5-ylium (PIN)
read 5λ5-arsaspiro[4.4]nonan-5-ylium (PIN)
Replace the structure with:
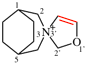
For 1,4-phenylenebis(phosphanium) (PIN)
read (1,4-phenylene)bis(phosphanium) (PIN)
read (1,4-phenylene)bis(phosphonium)
Delete 2,2′-benzene-1,3-diyldi(propan-2-ylium)
For ethane-1,2-diylbis(oxylium) (PIN)
read (ethane-1,2-diyl)bis(oxylium) (PIN)
read (ethane-1,2-diyl)bis(oxidanylium)
For pentane-2,4-diylidenebis(oxidanium) (PIN)
read (pentane-2,4-diylidene)bis(oxidanium) (PIN)
read (pentane-2,4-diylidene)bis(oxonium)
For pyridine-2,6-diylbis(sulfanylium) (PIN)
read (pyridine-2,6-diyl)bis(sulfanylium) (PIN)
read (pyridine-2,6-diyl)bis(thioxylium)
For 1,4-phenylenebis(dioxo-λ6-sulfanylium) (PIN)
read (1,4-phenylene)bis(dioxo-λ6-sulfanylium) (PIN)
For sulfoniumyl
read selenoniumyl
For dimethylazaniumylidene (preferred prefix)
read N-methylmethanaminiumylidene (preferred prefix)
For trimethyl[6-(dimethylsulfaniumyl)hexyl]phosphanium (PIN)
read [6-(dimethylsulfaniumyl)hexyl]tri(methyl)phosphanium (PIN)
For trimethyl[(6-dimethylsulfaniumyl)hexyl]ammonium (PIN)
read 6-(dimethylsulfaniumyl)-N,N,N-trimethylhexan-1-aminium (PIN)
For λ6,3λ6-dithiocane-1,3-bis(ylium)
read 1λ6,3λ6-dithiocane-1,3-bis(ylium)
read (not 1λ6,3λ4-dithiocan-3-ium-1-ylium; identical groups, if possible, must not be named differently within a name)
For 1,2,2-trimethylhydrazin-2-ium-1-ide (PIN)
read 1,2,2,2-tetramethylhydrazin-2-ium-1-ide (PIN)
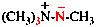
For 5H-11λ5-5H-indolo[2,3-b]quinolizin-11-ylium-5-ide (PIN)
read 5H-11λ5-indolo[2,3-b]quinolizin-11-ylium-5-ide (PIN)
For 6,6-dihydroxy-6,11-dihydro-5λ5-benzimidazolo[1,2-b][2,1]benzazaborol-5-ylium-6-uide (PIN)
read 6,6-dihydroxy-6,11-dihydro-5λ5-benzimidazolo[1,2-b][2,1]benzazaborol-5-ylium-6-uide
For 2-methyl-4-oxo-3,4-dihydro-1H-2-selenobenzopyran -2-ium-3-ide (PIN)
read 2-methyl-4-oxo-3,4-dihydro-1H-2-benzoselenopyran-2-ium-3-ide (PIN)
read not 2-methyl-3,4-dihydro-1H-2-benzoselenopyran-2-ium-3-id-4-one
read 2-methyl-4-oxo-3,4-dihydro-1H-isoselenochromen-2-ium-3-ide
For 5λ5,7λ5-spiro[1,3,2-diazaborolo[3,4-a:5,1-a′]dipyridine-6,10′-phenoxaborinine]-5,7-bis(ylium)-6-uide
read 5λ5,7λ5-spiro[[1,3,2]diazaborolo[3,4-a:5,1-a′]dipyridine-6,10′-phenoxaborinine]-5,7-bis(ylium)-6-uide
Replace the structure with:
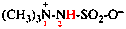
For trimethyl[2-(methyldiphenylphosphaniumyl)ethenyl]boranuide (PIN)
read trimethyl{2-[methyldi(phenyl)phosphaniumyl]ethen-1-yl}boranuide (PIN)
read trimethyl{2-[methyldi(phenyl)phosphoniumyl]ethen-1-yl}boranuide
For (trimethylazaniumyl)acetate (PIN)
read (N,N-dimethylmethanaminiumyl)acetate (PIN)
For N-methyl-N′-(methylboranuidyl)boranuidediylbis(aminium) (PIN)
read methyl{[(methanaminiumyl)boranuidyl]azaniumyl}boranuide (PIN)
read [not 1-methyl-3-(methanaminiumyl)diborazan-2-ium-1,3-diide;
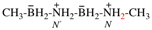
For 2-(trimethylazaniumyl)propan-2-ide (PIN)
read 2-(N,N-dimethylmethanaminiumyl)propan-2-ide (PIN)
For trimethyl(isopropylidene)phosphorane
read isopropylidenetri(methyl)phosphorane
For N,N-dimethylmethanamine oxide (PIN)
read N,N-dimethylmethanamine N-oxide (PIN)
read (trimethyl)amine oxide
Replace structure with:
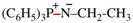
For R-N––+(R)=CR2 ↔
R-N=N+(R)-C–R2
read R-N––N+(R)=CR2 ↔ R-N=N+(R)-C–R2
For 2-[methyl(propan-2-ylidene)azaniumyl]propan-2-ide (PIN)
read 2-(N-methylpropan-2-iminiumyl)propan-2-ide (PIN)
For diphenyldiazeniumyl)oxidanide
read (diphenyldiazeniumyl)oxidanide
For
R2C––O+=O ↔ R2C––O-O+
read R2C––O+=O ↔ R2C=O+–O–
For 1λ4-thiophen-1-one (PIN)
read 1H-1λ4-thiophen-1-one (PIN)
read 1-oxo-1H-1λ4-thiophene
For 1λ4,2-thiazin-1-one (PIN)
read 1H-1λ4,2-thiazol-1-one (PIN)
read 1,2-thiazole 1-oxide
read 1,2-thiazole S-oxide
For N-methylpropan-2-imine oxide
read N-methylpropan-2-imine N-oxide
For X≡N+–Z– ↔ –X=N+=Z ↔ –X=N-Z+ ↔ X-N=Z,
read X≡N+–Z– ↔ X–=N+=Z ↔ X–=N–Z+,
read where X = C, N or O; and Z = C, N or O
For P-74.2.2.2 Nitrile ylides.
read P-74.2.2.2.1.3 Nitrile ylides.
Add (ethylidyneazaniumyl)oxidanide
For (ethylidyneazaniumyl)oxidanide
read (ethylidyneazaniumyl)sulfanide
For 2-(ethylidyneazaniumyl)propan-2-ide (PIN)
read 2-(acetonitriliumyl)propan-2-ide (PIN)
For phenyltriazadien-2-ium
read 3-phenyltriazadien-2-ium-1-ide
For X–C=Z ↔ +X=C–Z–
read
X2•–C=Z ↔ X+=C–Z–
For RR′=CR′′-C2•-R′′′
read RR′C=CR′′-C2•-R′′′
For 2-{4-[oxido(diphenyl)phosphaniumyl]phenyl}propane-1,3-diyl (PIN)
read 2-{4-[oxidodi(phenyl)phosphaniumyl]phenyl}propane-1,3-diyl (PIN)
read 2-{4-[oxidodi(phenyl)phosphoniumyl]phenyl}propane-1,3-diyl
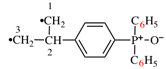
Replace the structure with:
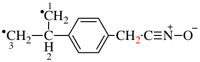
Replace the structure with:
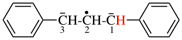
For (triethylazaniumyl)boranuidyl (PIN)
read (N,N-diethylethanaminiumyl)boranuidyl (PIN)
Replace structure with:
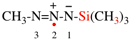
Replace the structure with:
For 1-ethyl-2-oxopyridin-1-ium-1(2H)-yl (P
read 1-ethyl-2-oxopyridin-1-ium-1(2H)-yl (PIN)
For (C60-Ih)[5,6]fulleren-9-ylium-1(9H)-yl (PIN)
read (C60-Ih)[5,6]fulleren-9-ylium-1(9H)-yl (PIN)
read 1,9-dihydro(C60-Ih)[5,6]fulleren-9-ylium-1-yl
For (C60-Ih)[5,6]fulleren-9-id-1(9H)-yl (PIN)
read (C60-Ih)[5,6]fulleren-9-id-1(9H)-yl (PIN)
read 1,9-dihydro(C60-Ih)[5,6]fulleren-9-id-1-yl
Replace the structure with:
Replace the structure with:
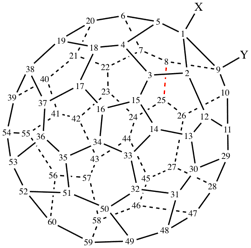
For 2-(benzenesulfonyl)diazane-1-id-1-yl
read 2-(benzenesulfonyl)diazan-1-id-1-yl
Replace structure with:
CH3-CO-N•–
Replace structure with:
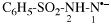
For 2-oxidaniumylidyneethyl(PIN)
read (oxidaniumylidyne)ethyl (PIN)
For ethoxy(oxido)benzyl
read α-ethoxy-α-oxidobenzyl
For benzenaminium chloride (PIN)
read benzenaminium chloride
Replace structure with:
Replace structure with:

Replace structure with:

For ... Adduct names (see P-77.1.2) may be...
read ... Adduct names (see P-77.1.3) may be...
For as adducts, leading to preferred IUPAC names;
read as adducts. The names of these adducts are preferred IUPAC names only when acid components of the adducts are organic compounds.
For (a) N,N-dimethyl-1,3-thiazolidin-2-amine—sulfuric acid (2/1) (PIN)
read (1) N,N-dimethyl-1,3-thiazolidin-2-amine—sulfuric acid (2/1) (PIN)
read (2) bis(N,N-dimethyl-1,3-thiazolidin-2-amine) sulfate
For sodium methanolate (PIN)
read sodium methanolate
read sodium methoxide (PIN)
For sodium 4-methylbenzenethiolate (PIN)
read sodium 4-methylbenzene-1-thiolate (PIN)
For H3-CO-O–
read CH3-CO-O–
For ...see ref. 29). Comparative examples of the application of these rules are given in Table 8.1, below). This...
read ...see ref. 29). Examples of isotopic nuclides for hydrogen are given in Table 8.1, below. This...
For 2H2Cl2
read C2H2Cl2
For 1-phenyl(1,2-13C2)ethanone (PIN)
read 1-phenyl(1,2-13C2)ethan-1-one (PIN)
read (1,2-13C2)acetophenone
For 2-(13C)methyl-(1-13C)benzene (PIN)
read 1-(13C)methyl(2-13C)benzene (PIN)
read (α,2-13C2)toluene
Replace structure with:
For 2-(35Cl)chloro-3-[(2H3)methyl](1-2H1)pentane (PIN)
read 2-(35Cl)chloro-3-(2H3)methyl(1-2H1)pentane (PIN)
For N-[(7-131I)iodo-6-iodo-9H-fluoren-2-yl]acetamide (PIN)
read N-[7-(131I)iodo-6-iodo-9H-fluoren-2-yl]acetamide (PIN)
Replace structure with:
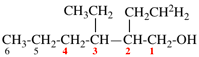
For cyclohexanedi[(14C)-carboxylic acid] (PIN)
read cyclohexane-1,1-di[(14C)carboxylic acid] (PIN)
For 2,3-di[(2H)hydro]-(2,3-2H2,15N)-1H-indole
read 2,3-di[(2H)hydro](2,3-2H2,15N)-1H-indole
For 6-methyl-2,3-di[(2H2)dihydro](2,3-2H2)napthalen-1-ol (PIN)
read 6-methyl-2,3-di[(2H)hydro]-1,4-dihydro(2,3-2H2)naphthalen-1-ol (PIN)
read {not 6-methyl-1,4-dihydro-2,3-di[(2H)hydro](2,3-2H2)naphthalen-1-ol;
read not 6-methyl[(2,3-2H2)-1,2,3,4-tetrahydro](2,3-2H2)naphthalen-1-ol;
Move acetic (2H)acid from under example 1 to under example 2
For (O-2H,18O)acetic acid (PIN)
read (O-2H,18O)acetic acid (PIN)
For (18O-2H)acetic acid (PIN)
read (18O-2H,18O)acetic acid (PIN)
For 4-[(2-14C)ethyl]benzoic acid (PIN)
read 4-(2-14C)ethylbenzoic acid (PIN)
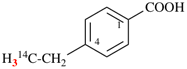
For (N-2H)acetamide (PIN)
read (N-2H1)acetamide (PIN)
Replace structure with:
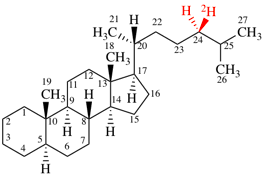
For 2-(18F) fluoro-2-deoxy-β-D-glucopyranose
read 2-deoxy-2-(18F)fluoro-β-D-glucopyranose [Deleted space from name]
Replace the structure with:
For (2R)-[(1-131I)iodo]-3-iodopropan-2-ol (PIN)
read (2R)-1-(131I)iodo-3-iodopropan-2-ol (PIN)
For (2,3-2H2,15N)pyridine (PIN)
read (2,4-2H2,15N)pyridine (PIN)
For P-82.6.1.4 (at top of page)
read P-82.6.1.3
For 2-(79Br)bromo-1-(13C)benzene
read 1-(79Br)bromo(2-13C)benzene (PIN)
Replace structure with:
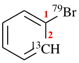
For (2R)-2(O-2H)hydroxy-3-hydroxy(1-2H)propanal (PIN)
read (2R)-2-(2H)hydroxy-3-hydroxy(1-2H)propanal (PIN)
For L-(α-2H)-phenylalanine (PIN)
read L-(α-2H)phenylalanine (PIN)
For P-82.6.3.2
read P-82.6.3.3
benzene(13C)carbonitrile (PIN)
(cyano-13C)benzonitrile
benzene(13C)carboxylic acid (PIN)
(carboxyl-13C)benzoic acid
[benzene[14C]carbonyl]phosphonic acid (PIN)
[phenyl[14C]carbonyl]phosphonic acid
[carbonyl-14C]benzoylphosphonic acid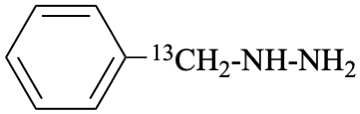
[phenyl(13C)methyl]hydrazine (PIN)
[(α-13C)benzyl]hydrazine
For 1-propylidene(1-15N)diazene (PIN)
read 1-propylidene(1-15N)hydrazine (PIN)
For 3-[3-ethyl(2-34S)trisulfan-1-yl]propanoic acid (PIN)
read 3-[ethyl(2-34S)trisulfanyl]propanoic acid (PIN)
For (18O-2H)acetic acid (PIN)
read (18O-2H,18O)acetic acid (PIN)
delete (the ‘18O’ is both an isotopic descriptor and a locant).
For (18O,O-2H)acetic acid
read (O-2H,18O)acetic acid (PIN)
For benzene-1,3,5-tri[13C]carboxylic acid (PIN)
read benzene-1,3,5-tri([13C]carboxylic acid) (PIN)
For 3-carboxy-5-[13C]carboxybenzene-1-[18O]carboxylic acid (PIN)
read 3-[13C]carboxy-5-carboxybenzene-1-[18O]carboxylic acid (PIN)
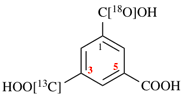
For (not benzene-1,3,5-[1,5-13C2,1,3-18O2]tricarboxylic acid)
read (not benzene-1,3,5-[1,3-13C2,1,5-18O2]tricarboxylic acid)
For 2-carboxy[1-14C]benzene-1-[1-14C]carboxylic acid (PIN)
read 2-carboxy[1-14C]benzene-1-[14C]carboxylic acid (PIN;
For acetic [14C]acid (PIN;
read [1-14C]acetic acid (PIN)
For 2-[15N]amino-3-hydroxy[2,3-2H2 ,3-14C]propanoic acid
read (2S)-2-[15N]amino-3-hydroxy[2,3-2H2,3-14C]propanoic acid [delete space from name]
For 1-(naphthalene-2-yl)-2-phenyl[2-15N]diazene (PIN)
read 1-(naphthalen-2-yl)-2-phenyl[2-15N]diazene (PIN)
For O-ethyl 18O-methyl naphthlene-2-yl[18O]phosphonate (PIN)
read O-ethyl 18O-methyl naphthlen-2-yl[18O]phosphonate (PIN)
For ...(see P-82.6.3.2)
read ...(see P-82.6.3.3)
For O-ethyl 18O-methyl naphthalene-2-yl[18O]phosphonate (PIN)
read O-ethyl 18O-methyl (naphthalene-2-yl)[18O]phosphonate (PIN)
Replace the structure with:
For C[2H2]-CH2-O[2H]
read C[2H3]-CH2-O[2H]
For [2-2H2;2]ethan-1-[18O0;]]ol
read [2-2H2;2]ethan-1-[18O0;1]ol (PIN)
For P-9 Specification of Configuration and Confirmation
read P-9 Specification of Configuration and Conformation
For ...example, R1R2C= and =C{[CH2}n}C=NOH. The...
read ...example, R1R2C=NOH and HON=C{[CH2]n}C=NOH. The...
For ...a 50:0 mixture.
read ...50:50 mixture. >
For In preferred IUPAC names, nonstereogenic units are not specified and in preferred...
read In preferred...
Delete the last sentence.
For (R)-bromo(chloro)fluoromethane
read (R)-bromo(chloro)(fluoro)methane
For 1-[(1R)-(1-chloropropyl)]benzene (PIN)
read [(1R)-1-chloropropyl]benzene (PIN)
For (2Z,6S)-6-chlorohept-2-ene
read (2Z,6S)-6-chlorohept-2-ene (PIN)
For A pseudoasymmetric stereogenic unit denoted by ‘m’ for ‘a > F > ꟻ > d’ ...
read A pseudoasymmetric stereogenic unit denoted by ‘m’ for ‘a > b and F > ꟻ’ ...
For ...considers the torsion angle between two specified (fiducial) groups are attached to the atoms linked by that bond. The sign of the smaller torsion angle between the fiducial groups defines the sense of chirality sense of the helix (see torsion angle, P-94.2).
read ...considers the torsion angle between two reference groups attached to the atoms at each end of a bond or an axis. The sign of the smaller torsion angle between the reference groups defines the chirality sense of the corresponding stereogenic unit (see torsion angle, P-94.2)
For ‘seqTran/seqTrans’
read ‘seqTrans/seqTrans’
For 6-chlorohexane-2,4-diol [PIN; represented by a Fischer projection formula [see Prelog and Helmchen, subsection 3.1, ref. 36 and see also P-102.3.1)]
read 6-chlorohexane-2,4-diol (PIN)
Replace structure with:
Replace both structures with:
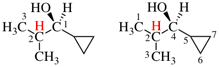
For 3-[(S)-(cyclopenta-1,3-dien-2-yl)(hydroxy)methyl]cyclopenta-2,4-dien-1-ide (PIN)
read 3-[(S)-(cyclopenta-1,4-dien-1-yl)(hydroxy)methyl]cyclopenta-2,4-dien-1-ide (PIN)
For (R)-bromo(chloro)fluoromethane (PIN)
read (R)-bromo(chloro)(fluoro)methane (PIN)
For (R)-germyl(methoxy)(methylsulfanyl)methyl]silane (PIN)
read [(R)-germyl(methoxy)(methylsulfanyl)methyl]silane (PIN)
For (2R,3S,4S,5R,6S)-3-(2-bromoethyl)-1-chloro-2,6,7-trifluoro-5-(2-iodoethyl)heptan-4-ol (PIN)
read (2R,3S,4R,5R,6S)-3-(2-bromoethyl)-1-chloro-2,6,7-trifluoro-5-(2-iodoethyl)heptan-4-ol (PIN)

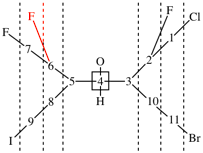
For (2R,3S,4S,5R,6S)-3-(bromoethyl)-1,2,6,7-tetrafluoro-6-(2-iodoethyl)heptan-4-ol (PIN)
read (2R,3S,4S,5R,6S)-3-(bromoethyl)-1,2,6,7-tetrafluoro-5-(2-iodoethyl)heptan-4-ol (PIN)
For (2R)-2-hydroxypent-3-enenitrile (PIN)
read (2R)-2-hydroxybut-3-enenitrile (PIN)
Replace both structures with:
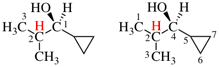
For (2S)-cyclopropyl(hydroxy)acetaldehyde (PIN)
read (S)-cyclopropyl(hydroxy)acetaldehyde (PIN)
For ... branch it is ‘C,H,H’, leading to...
read ... branch it is ‘C,C,H’, leading to...
For 1-[(1R)-1-chloropropyl]cyclopropane (PIN)
read [(1R)-1-chloropropyl]cyclopropane (PIN)
For ... leading to the overall priority of ‘C-2 > C-2 > C-7 >, H’
read ... leading to the overall priority of ‘C-2 > C-6 > C-7 > H’
For ... resulting in the priority order ‘C-6 > C-3 > C-8 > H’
read ... resulting in the priority order ‘C-1 > C-3 > C-8 > H’
For (1S)-1-(bicyclo[2.2.2}octan-1-yl)-4-cyclopropyl-2,2-bis(2-cyclopropylethyl)butan-1-ol (PIN)
read (1S)-1-(bicyclo[2.2.2]octan-1-yl)-4-cyclopropyl-2,2-bis(2-cyclopropylethyl)butan-1-ol (PIN)
For (1R)-1-(1-2H1)-ethan-1-ol
read (1R)-(1-2H1)-ethan-1-ol (PIN)
For
For (2R)-1,3-[1-125I]diiodopropan-2-ol (PIN)
read (2S)-1-[125I]iodo-3-iodopropan-2-ol (PIN)
read ...is: ‘OH > CH2I > CH2[125I] > H’ and the configuration is ‘S’ for...
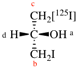
Replace the structure with:
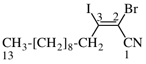
Replace the structure with:
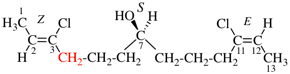
For 1,3-di[(1E)-ethylidene]cyclobutane
read 1,3-di[(E)-ethylidene]cyclobutane
For (2Z,5Z,7S,8Z,11Z)-9-[(2Z)-but-2-en-1-yl]-5-[(2E)-but-2-en-1-yl]trideca-2,5,8,11-tetraen-7-ol (PIN)
read (2Z,5Z,7S,11Z)-5-[(2E)-but-2-en-1-yl]-9-[(2Z)-but-2-en-1-yl]trideca-2,5,8,11-tetraen-7-ol (PIN)
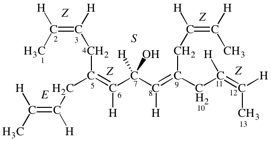
For (2Z,5Z,7S,8Z,11Z)-9-[(2Z)-but-2-en-1-yl]-5-[(2E)-but-2-en-1-yl]trideca-2,5,8,11-tetraen-7-ol (PIN)
read (2Z,5Z,7S,11Z)-5-[(2E)-but-2-en-1-yl]-9-[(2Z)-but-2-en-1-yl]trideca-2,5,8,11-tetraen-7-ol (PIN)
For ... the stereodescriptor set ‘2R,3S,6R’ in the principal chain ...
read ... the stereodescriptor set ‘2R,4S,6R’ in the principal chain ...
For ...at ‘C-2’, ‘C-5’, ‘C-6’, ‘C-8’ and ‘C-10’,...
read ...at ‘C-2’, ‘C-3’, ‘C-6’, ‘C-8’ and ‘C-10’,...
For ... ‘u’. like pairs being ‘RR’ or ‘SS’, unlike pairs...
read ...‘u’, like pairs being ‘RR’ or ‘SS’, unlike pairs...
Replace the structure with:

For ...auxiliary descriptors specified in place of usual stereodescriptors R and S as described...
read ...auxiliary descriptors, R0 and S0, specified in place of usual stereodescriptors R and S as described...
For ...auxiliary descriptors (R0 and S0). The methodology...
read ...auxiliary descriptors (R0 and S0) illustrated here for C-1. The auxiliary descriptors for every node are determined for this digraph. For example, the auxiliary descriptor for C-6 node is R0 as Cl > C-1 > C-5 > H. Once all necessary auxiliary descriptors are assigned, the methodology...
For (2R,3S,6R,9R,10S)-6-chloro-5-[(1R,2S)-1,2-dihydroxypropoxy]-7-[(1S,2S)-1,2-dihydroxypropoxy]- 4,8-dioxa-5,7-diazaundecane-2,3,9,10-tetrol (PIN; [in the principal chain, both pairs of stereodescriptors are unlike; thus, stereodescriptor R is assigned to the lowest possible position, ‘2’)
read (2R,3S,6R,9R,10S)-6-chloro-5-[(2R,3S)-2,3-dihydroxybutyl]-7-[(2S,3S)-2,3-dihydroxybutyl]-4,8-dioxa-5,7-diazaundecane-2,3,9,10-tetrol
(PIN; in the principal chain, named by skeletal replacement nomenclature, both pairs of stereodescriptors are unlike so the stereodescriptor R is assigned the lowest locant, ‘2’). [delete space in name and change explanation]
For (2R,3R,5R,7R,8R)-4.4-bis[(2S,3R)-3-chlorobutan-2-yl]-6,6-bis[(2S,4S)-3-chlorobutan-2-yl)]-2,8-dichloro-3,7-dimethylnonan-5-ol (PIN; ...
read (2R,3R,5R,7R,8R)-2,8-dichloro-4,4-bis[(2S,3R)-3-chlorobutan-2-yl]-6,6-bis[(2S,3S)-3-chlorobutan-2-yl]-3,7-dimethylnonan-5-ol (PIN; ...
Replace digraph for C-5 with
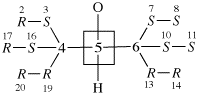
For ...pair S,8 and S,11 (like pair) over the pair S,2 and S,17 (unlike pair)...
read ...pair S,8 and S,11 (like pair) over the pair S,2 and S,17 (unlike pair)...
For (2R)-2-{bis[(1R)-1-hydroxyethyl]amino}{[(1R)-1-hydroxyethyl]-2-[(1S)-1-hydroxyethyl]amino}acetic acid (PIN)
read (R)-{bis[(1R)-1-hydroxyethyl]amino}{[(1R)-1-hydroxyethyl][(1S)-1-hydroxyethyl]amino}acetic acid (PIN)
Replace the structure with:
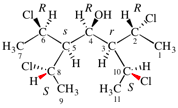
For ...and ‘seqCis’ precedes ‘seqTrans’.
read ...and ‘seqCis’ precedes ‘seqTrans’, and similarly for auxiliary descriptors.
Replace structure with:
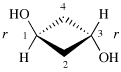
Replace by
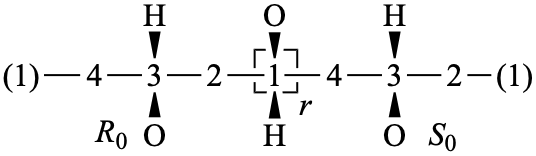
For (3R,4m,5S)-4-(2-bromoethenylidene)heptane-3,5-dithiol (PIN)
read (3R,4m,5S)-4-(2-bromoethen-1-ylidene)heptane-3,5-dithiol (PIN)
read (3R,4ra,5S)-4-(2-bromoethen-1-ylidene)heptane-3,5-dithiol
For (11Sp)-12-[(1R)-1-bromoethyl]-16-[(1S)-1-bromoethyl]-1,4(1,4)-dibenzenacyclohexaphane (PIN)
read (11sp)-12-[(1R)-1-bromoethyl]-16-[(1S)-1-bromoethyl]-1,4(1,4)-dibenzenacyclohexaphane
Replace structures with:
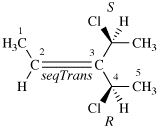
For (1s)-1-{[(1R,2R)-1,2-dichloropropyl][(1S,2R)-1,2-dichloropropyl]amino}-1-{[(1R,2S)-1,2-dichloropropyl][(1S,2S)-1,2-dichloropropyl]amino}methan-1-ol
read (s)-{[(1R,2R)-1,2-dichloropropyl][(1S,2R)-1,2-dichloropropyl]amino}{[(1R,2S)-1,2-dichloropropyl][(1S,2S)-1,2-dichloropropyl]amino}methanol
replace the structure with:
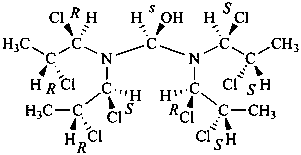
For ...ranking node 8 is 'S in 'branch B';...
read ..ranking node 14 is 'S in 'branch B';...
For 8-'S' (first difference encountered)
read 14-'S' (first difference encountered)
read 8-'S'
For 14-‘R’
read 14-‘S’
For cis-2-chlorocyclopentane-1-carboxylic acid (see P-93.5.1.2 for use of the non-CIP stereodescriptor ‘cis’)
read trans-2-chlorocyclopentane-1-carboxylic acid (see P-93.5.1.2 for use of the non-CIP stereodescriptor ‘trans’)
For (1R*,3R*,5S*)-1-[(2S)-butan-2-yloxy]-3-chloro-5-nitrocyclohexane (PIN)
read (1R*,3R*,5S*)-1-{[(2S)-butan-2-yl]oxy}-3-chloro-5-nitrocyclohexane (PIN)
For 2-ambo-(2R)-2,5,7,8-tetramethyl-2[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydro-2H-1-benzopyran-6-ol (PIN)
read 2-ambo-(2R)-2,5,7,8-tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydro-2H-1-benzopyran-6-ol (PIN)
For ...atom is B, Al, Ga, In, Tl, S, Se, Te, N, P, As, Sb, Bi is written...
read ...atom is B, S, Se, Te, N, P, As, Sb, Bi is written...
For (R)-[N-benzyl-N-methyl-N-(prop-2-en-1-yl)benzenaminium] bromide (PIN)
read (R)-N-benzyl-N-methyl-N-(prop-2-en-1-yl)benzenaminium bromide
read (R)-benzyl(methyl)(phenyl)(prop-2-en-1-yl)azanium bromide
read (R)-benzyl(methyl)(phenyl)(prop-2-en-1-yl)ammonium bromide
For (2S)-(4-hydroxyphenyl)-2-phenyl-1,2,3,4-tetrahydrophosphinolin-2-ium bromide (PIN)
read (2S)-2-(4-hydroxyphenyl)-2-phenyl-1,2,3,4-tetrahydroisophosphinolin-2-ium bromide (PIN)
For (S)-N-ethyl-N-methylbenzenaminiumyloxidanide
read [(S)-N-ethyl-N-methylbenzenaminiumyl]oxidanide
For (S)-N-ethyl-N-methylbenzenamine N-oxide (PIN;
read (S)-N-ethyl-N-methylaniline N-oxide (PIN;
read (S)-[ethyl(methyl)phenylazaniumyl]oxidanide
For (S)-[methyl(phenyl)propyl-λ5-phosphanone] (PIN;
read (S)-methyl(phenyl)(propyl)-λ5-phosphanone (PIN;
read (S)-methyl(phenyl)(propyl)phosphane oxide
For dihydrogen (S)-[O-methyl (17O,18O)phosphate] (PIN)
read dihydrogen (S)-[O-methyl (17O1,18O1)phosphate] (PIN)
Replace the structure with:
For dihydrogen (S)-[O-methyl (17O,18O)phosphate] (PIN)
read dihydrogen (S)-[O-methyl (17O,18O)phosphate] (PIN)
For 1-methyl-4-{(R)-phenyl[(18O1)methanesulfonyl}benzene (PIN)
read 1-methyl-4-[(R)-phenyl(18O1)methanesulfonyl]benzene (PIN)
For N,N,N-tributylbutanaminium (S)-[O-phenyl (17O,18O)sulfate] (PIN)
read N,N,N-tributylbutan-1-aminium (S)-[O-phenyl (17O1,18O1)sulfate] (PIN)
read tetrabutylammonium (S)-[O-phenyl (17O1,18O1)sulfate]
read tetrabutylazanium (S)-[O-phenyl (17O1,18O1)sulfate];
For (SPY-5-21′)-2-phenyl-2λ5,2′-spirobi[1,3,2-benzodioxaphosphole] (PIN)
read (SPY-5-21′)-2-phenyl-2H-2λ5,2′-spirobi[[1,3,2]benzodioxaphosphole] (PIN)
For (SPY-5-21′)-2-phenyl-2,2′-spirobi[naphtho[2,3-d][1,3,2]dioxasilol]-2-uide (PIN)
read (SPY-5-21′)-2-phenyl-2H-2,2′-spirobi[naphtho[2,3-d][1,3,2]dioxasilol]-2-uide (PIN)
Delete the sentence:
As no locants are present, the name following the stereodescriptor is placed in parentheses, or bracket, according to the required nesting order.
For (S)-[(methanesulfinyl)ethane]
read (S)-(methanesulfinyl)ethane (PIN)
For (S)-[(methanesulfinyl)benzene] (PIN)
read (S)-(methanesulfinyl)benzene (PIN)
For ethyl (R)-(4-nitrobenzene-1-sulfinate) (PIN)
read ethyl (R)-4-nitrobenzene-1-sulfinate (PIN)
For (R)-[methyl(phenyl)propylphosphane] (PIN)
read (R)-methyl(phenyl)(propyl)phosphane (PIN)
For (TPBY-5-12-C)-2-phenoxy-2λ5-spiro[1,3,2-dithiaphosphinane-2,2′-phenanthro[9,10-d][1,3,2]dioxaphosphole]
read (TPBY-5-12-C)-2-phenoxy-2H-2λ5-spiro[[1,3,2]dithiaphosphinane-2,2′-phenanthro[9,10-d][1,3,2]dioxaphosphole]
Replace structure with:
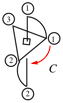
For (SS-4-11′-A)-3,3,3′,3′-tetramethyl-3H,3′H-1λ4-spirobi[2,1-benzoxathiole] (PIN)
read (SS-4-11′-A)-3,3,3′,3′-tetramethyl-3H,3′H-1λ4,1′-spirobi[[2,1]benzoxathiole] (PIN)
read {not (TBPY-4-11′-A)-3,3,3′,3′-tetramethyl-3H,3′H-1λ4,1′-spirobi[[2,1]benzoxathiole]}
For (OC-6-22′-A)-1,1-difluoro-3,3,3′,3′-tetrakis(trifluoromethyl)-3H,3′H-1λ6-spirobi[2,1-benzoxathiole] (PIN)
read
(OC-6-22′-A)-1,1-difluoro-3,3,3′,3′-tetrakis(trifluoromethyl)-1,3′-dihydro-3H-1λ6,1′-spirobi[[2,1]benzoxathiole] (PIN)
Replace left structure with:
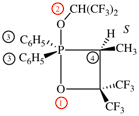
Delete P-93.4.3 Specification of configuration of cumulenes
For (1R)-(1-2H)ethan-1-ol (PIN)
read (1R)-(1-2H1)ethan-1-ol (PIN)
For (2R,3R)-2,3-dichloro-(2-2H)butan-2-ol (PIN)
read (2R,3R)-2,3-dichloro-(2-2H)butane (PIN)
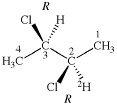
For (2Z)-2,3-dibromoprop-2-enenitrile (PIN)
read (2Z)-2,3-dibromo-3-iodoprop-2-enenitrile (PIN)
read (2Z)-2,3-dibromo-3-iodoacrylonitrile
Replace structure with:
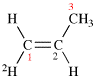
For (1E)-1,1′-[1-(4-chlorophenyl)ethene-1,2-diyl]dibenzene (PIN)...
read 1,1′-[(1E)-1-(4-chlorophenyl)ethene-1,2-diyl]dibenzene (PIN)...
For ... The stereodescriptors, ‘cis’ and ‘trans’, placed in parentheses, are cited at the front of the name, preceded by the appropriate locant followed by a hyphen.
read ... The stereodescriptors, ‘cis’ and ‘trans’, are cited at the front of the name, preceded by the appropriate locant, if necessary, followed by a hyphen.
For (2-cis,4-trans)-octa-2,4-diene
read 2-cis,4-trans-octa-2,4-diene
For (2-trans,4-cis)-hexa-2,4-dienoic acid
read 2-trans,4-cis-hexa-2,4-dienoic acid
For (3-cis,5-trans)-octa-3,5-diene
read 3-cis,5-trans-octa-3,5-diene
For (Z)-(diphenyldiazene) (PIN)
read (Z)-diphenyldiazene (PIN)
read (not cis-diphenyldiazene)
For (Z)-{N-[(4-chlorophenyl)(phenyl)methylidene]hydroxylamine} (PIN)
read (Z)-N-[(4-chlorophenyl)(phenyl)methylidene]hydroxylamine
read (Z)-(4-chlorophenyl)(phenyl)methanone oxime
For (2E,3Z)-pentane-2,3-diylidenebis(hydroxylamine) (PIN)
read (2E,3Z)-(pentane-2,3-diylidene)bis(hydroxylamine)
For (2-trans,6-trans)-octa-2,3,4,6-tetraene
read 2-trans,6-trans-octa-2,3,4,6-tetraene
For (5S,6-cis,8-trans,10-trans,12R,14-cis)-5,12-dihtydroxyicosa-6,8,10,14-tetraenoic acid
read 6-cis,8-trans,10-trans,14-cis-(5S,12R)-5,12-dihtydroxyicosa-6,8,10,14-tetraenoic acid
For (2R)-3-hydroxypropane-1,2-diyl di[(3-trans,5-trans)-hepta-3,5-dienoate]
read (2R)-3-hydroxypropane-1,2-diyl di(3-trans,5-trans-hepta-3,5-dienoate)
Replace the structure with:
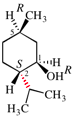
Replace structure with:
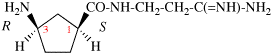
Replace structure with:
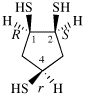
Add For the use of cis or trans see P-93.5.1.2.
Add cis-cyclohexane-1,4-diol
For 1r,4r)-cyclohexane-1,4-diol (PIN)
read (1r,4r)-cyclohexane-1,4-diol (PIN)
Add trans-4-chloro-4-methylcyclohexan-1-ol
Add bis(trans-4-methylcyclohexyl)phosphane
For bis[(1r,4r)-4-methylcyclohexyl]-(1s,4s)-4-methylcyclohexylphosphane (PIN)
read bis[(1r,4r)-4-methylcyclohexyl][(1s,4s)-4-methylcyclohexyl]phosphane (PIN)
Add
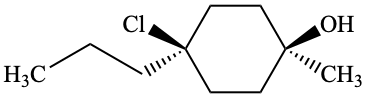
(1r,4s)-4-chloro-1-methyl-4-propylcyclohexan-1-ol (PIN)
1,4-cis-4-chloro-1-methyl-4-propylcyclohexan-1-ol
Explanation. The CIP preferred group at both substitution positions are cis.
For (1R,2R3S,4R,5S,6S)-1,2,3,4,5,6-hexachlorocyclohexane
read (1R,2R,3S,4R,5S,6S)-1,2,3,4,5,6-hexachlorocyclohexane
Add The numbering for these examples is decided by application of criterion P-14.4 (j).
For 1 1R,2R,3S,4S,5R,6S)
read 1 (1R,2R,3S,4R,5S,6S)
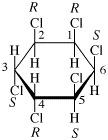
For 3 (1R,2s,3S,4R,5s,6S)
read 3 (1R,2r,3S,4R,5s,6S)
Note R > r > S > s [see P-14.4 (j)]
replace the structure with:
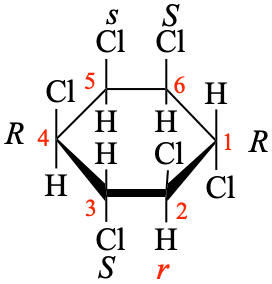
Add Note R > r > S > s [see P-14.4 (j)]
For ... 1,1,2,2-tetracarbonitrile (I) (PIN; ...
read ... 1,1,2,2-tetracarbonitrile (PIN; ...
For ...by adding ‘r’ (the reference ligand) followed by a hyphen and the locant of the lowest numbered of these ligands, and ‘c’ for ‘cis’ and ‘t’ for ‘trans’ (as required) followed by a hyphen and the locant of another ligand,...
read ...by adding ‘r’ (the reference ligand) after the locant of the lowest numbered of these ligands, and ‘c’ for ‘cis’ and ‘t’ for ‘trans’ (as required) after the locant of another ligand,...
For ...reference substituent) followed by a hyphen and the locant of the lowest numbered of these substituents and ‘c’ (for cis) and ‘t’ (for trans) (as appropriate) followed by a hyphen and the locant...
read ...reference substituent) after the locant of the lowest numbered of these substituents and ‘c’ (for cis), ‘t’ (for trans) (as appropriate) and the locant...
For 1-r,2-c,4-c-tribromocyclohexane
read 1r,2c,4c-tribromocyclohexane
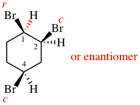
For 2-r,3-t,4-c-trichlorocyclopentane-1,1-dicarboxylic acid
read 2r,3t,4c-trichlorocyclopentane-1,1-dicarboxylic acid
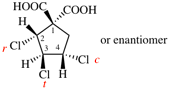
For 1,2-t-dichlorocyclopentane-1-r-carboxylic acid
read 1,2t-dichlorocyclopentane-1r-carboxylic acid
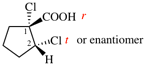
For 1-r-bromo-1-chloro-3-t-ethyl-3-methylcyclohexane
read 1r-bromo-1-chloro-3t-ethyl-3-methylcyclohexane
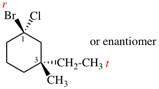
For 1-r,2-c,3-c,4-t,5-t,6-t-hexachlorocyclohexane
read 1r,2c,3c,4t,5t,6t-hexachlorocyclohexane
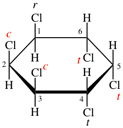
For (1-cis,3-trans)-cyclodeca-1,3-diene
read 1-cis,3-trans-cyclodeca-1,3-diene
For (1R-trans,4S,7-cis)-4-(propan-2-yl)cyclodeca-2,7-dien-1-ol
read 2-trans,7-cis-(1R,4S)-4-(propan-2-yl)cyclodeca-2,7-dien-1-ol
For ...substituent group; without a locant; the name of...
read ...substituent group; the name of...
For 1-[(1E)-butan-2-ylidene] 1H-indene
read 1-[(2E)-butan-2-ylidene]-1H-indene
For 1,4-di[(1Z)-ethylidene]cyclohexane
read 1,4-di[(Z)-ethylidene]cyclohexane
For [1(1′)E,3E′,3E)]3,3′-diethylidene-1,1′-bis(cyclobutylidene) (PIN)
read [1(1′)E,3E′,3E]-3,3′-diethylidene-1,1′-bi(cyclobutylidene) (PIN)
read trans-3,3′-diethylidene-1,1′-bi(cyclobutylidene)
read 3,3′-di[(E)-ethylidene]-1,1′-bi(cyclobutylidene)
Replace structure with:
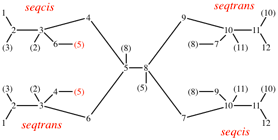
For N-[(1seqCis,4R)-4-methylcyclohexylidene]hydroxylamine (PIN)
read [(1seqCis,4R)-4-methylcyclohexylidene]hydroxylamine
For (3P)-1-(bromomethylidene)-3-propylcyclobutane
read (P)-1-(bromomethylidene)-3-propylcyclobutane
For (1S,4p)-1-ethyl-4-(prop-1-en-1-ylidene)cyclohexane
read (1s,4p)-1-ethyl-4-(prop-1-en-1-ylidene)cyclohexane
read (1s,4sa)-1-ethyl-4-(prop-1-en-1-ylidene)cyclohexane
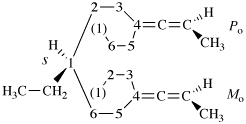
Replace digraph for C-1=C-1′ with:
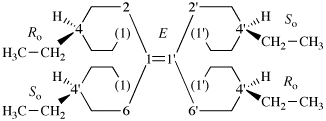
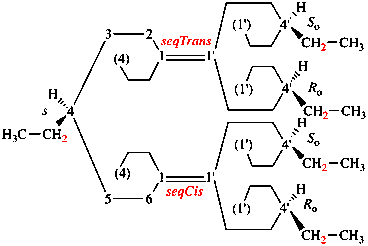
For ... the double bonds ‘C-1–C-1′’; priority being assigned to Sequence Rule 5, the ligand ‘C-4’ has priority over ligand ‘C-3’, ...
read ... the double bonds ‘C-1=C-1′’; priority being assigned to Sequence Rule 5, the ligand ‘C-5’ has priority over ligand ‘C-3’, ...
Replace the structure with:
For (1S,10R)-1-methylbicyclo[8.3.1]tetradecane (PIN)
read (1R,10R)-1-methylbicyclo[8.3.1]tetradecane (PIN)
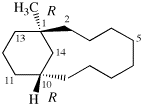
For ...stereodescriptors 'R' and 'S' and prefixes...
read ...stereodescriptors 'R' and 'S' and prefixes...
For (2-endo)-bromo-(7-anti)-fluorobicyclo[2.2.1]heptane
read 2-endo-bromo-7-anti-fluorobicyclo[2.2.1]heptane
read rel-(1R,2S,4R,7R)-2-bromo-7-fluorobicyclo[2.2.1]heptane (PIN)
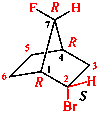
For (5-exo)-bromo-(5-endo,7-anti)-dimethylbicyclo[2.2.1]hept-2-ene
read (±)-5-exo-bromo-5-endo,7-anti-dimethylbicyclo[2.2.1]hept-2-ene
read rac-(1R,4S,5S,7R)-5-bromo-5,7-dimethylbicyclo[2.2.1]hept-2-ene (PIN)
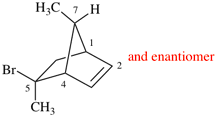
For syn-8-methylbicyclo[3.2.1]octane
read 8-syn-methylbicyclo[3.2.1]octane
For 8-methyl-8-aza-bicyclo[3.2.1]heptan-3-endo-yl (2S)-3-hydroxy-2-phenylpropanoate
read 8-methyl-8-aza-bicyclo[3.2.1]octan-3-endo-yl (2S)-3-hydroxy-2-phenylpropanoate
For rel-(1R,10S)-1-methylbicyclo[8.3.1]tetradecane (PIN)
read rel-(1R,10R)-1-methylbicyclo[8.3.1]tetradecane (PIN)
read (1R*,10R*)-1-methylbicyclo[8.3.1]tetradecane
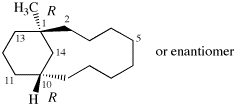
Add (1S,4E,12R)-bicyclo[10.2.2]hexadeca-4,13-diene >
For [2S,26R,1(44)E]-bicyclo[24.20.1]heptatetraconta-1(46),44,45-trien-2-ol (PIN)
read [1(44)E,2S,26R]-bicyclo[24.20.1]heptatetraconta-1(46),44,45-trien-2-ol (PIN)
For (1P)-bicyclo[23.19.1]pentatetraconta-1,2-diene (PIN)
read (1P,25R)-bicyclo[23.19.1]pentatetraconta-1,2-diene (PIN)
For [1(43)P,2R,25R)-bicyclo[23.19.1]pentatetraconta-1(44),43-diene-2-carboxylic acid (PIN)
read [1(43)P,2R,25R]-bicyclo[23.19.1]pentatetraconta-1(44),43-diene-2-carboxylic acid (PIN)
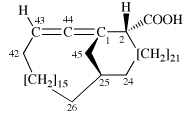
For (1R)-5′H-spiro[indene-1,2′-(1,3)oxazole] (PIN)
read (1R)-5′H-spiro[indene-1,2′-[1,3]oxazole] (PIN)
Replace the structure with:
read ...[5a,9a](azanomethano)cyclopenta[f]indolizin-10′-one (PIN)
For ... atom numbered 6; they are ranked ‘a’ and ‘b’, respectively. ...
read ... atom numbered 6; they are ranked ‘a’ and ‘a′’, respectively. ...
Replace structure with:
For (3R,4s,7S,10s)-tetraoxatetraspiro[2.0.24.0.27.0.210.03]dodecane (PIN)
read (3R,4r,7S,10s)-1,5,8,11-tetraoxatetraspiro[2.0.24.0.27.0.210.03]dodecane (PIN)
replace structure with:
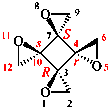
For ...using Ssequence Rule 5, ‘R’ precedes ‘S’, as determined in (b), in accordance ...
read ...using Sequence Rule 5, ‘R0’ precedes ‘S0’, in accordance ...
Add (6Z)-spiro[4.7]dodeca-2,6-diene
For (TPBY-5-12-C)-2-phenoxy-2λ5-spiro[1,3,2-dithiaphosphinane-2,2′-phenanthro[9,10-d][1,3,2]dioxaphosphole]
read (TPBY-5-12-C)-2-phenoxy-2H-2λ5-spiro[[1,3,2]dithiaphosphinane-2,2′-phenanthro[9,10-d][1,3,2]dioxaphosphole]
Replace the right-hand structure with:

For (OC-6-22′-A)-4,4′di-tert-butyl-6,6,6′,6′-tetramethyl-2H,2′H,6H,6′H-8λ6,8′-spirobi[[1,2]oxathiolo[4,3,2-hi][2,1]benzooxathiole]-2,2′-dione
read (OC-6-22′-A)-4,4′-di-tert-butyl-6,6,6′,6′-tetramethyl-2H,2′H,6H,6′H-8λ6,8′-spirobi[[1,2]oxathiolo[4,3,2-hi][2,1]benzooxathiole]-2,2′-dione
Replace the structures with:
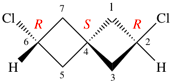
Replace the digraph with:
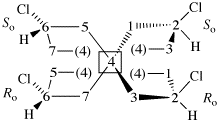
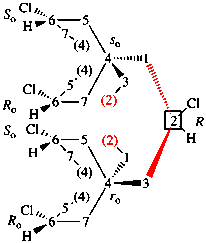
Add This is an example of an ‘Xaabb’ system, see P-93.5.3.2.
Replace with:

For (5s,11s)-1,12-dioxatrispiro[4.2.2.411.28..25]nonadecane (PIN) (spiro atoms 5 and 11 are pseudoasymmetric centers and spiro atom 8 is nonstereogenic).
read (5S,8R,11S)-1,12-dioxatrispiro[4.2.2.411.28.25]nonadecane (PIN)
read (5M)-1,12-dioxatrispiro[4.2.2.411.28.25]nonadecane
[Delete space from name and dot after 8 in both names]
For (5S,11S)-6H,12H-2,8-dimethyl-5,11-methanodibenzo[b,f][1,5]diazocine (PIN)
read (5S,11S)-2,8-dimethyl-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine (PIN)
For(1R,2r,3S,3aR,4S,7R,7aS)-1,2,3,4,5,6,7,8,8-nonachloro-2,3,3a,4,7,7a-hexahydro-4,7-methano-1H- indene (PIN)
read(1R,2r,3S,3aR,4S,7R,7aS)-1,2,3,4,5,6,7,8,8-nonachloro-2,3,3a,4,7,7a-hexahydro-1H-4,7-methanoindene (PIN) [remove space from name]
For (10aM)-1,11-dinitro-5,7-dihydrodibenzo[a,c][7]annulen-6-one (PIN)
read (11aM)-1,11-dinitro-5,7-dihydro-6H-dibenzo[a,c][7]annulen-6-one (PIN)
read (11aRa)-1,11-dinitro-5,7-dihydro-6H-dibenzo[a,c][7]annulen-6-one
For (4as,8as)-1,2,3,4,4a,5,6,7,8,8a-decahydronaphthalene (PIN)
read (4as,8as)-decahydronaphthalene (PIN)
For (4ar,8ar)-1,2,3,4,4a,5,6,7,8,8a-decahydronaphthalene (PIN)
read (4ar,8ar)-decahydronaphthalene (PIN)
For [cis-cisoid-cis-tetrahydroanthracene]
read cis-cisoid-cis-tetrahydroanthracene
Replace the right-hand structure with:
For [cis-4a-cisoid-4a,10a-trans-10a-tetradecahydroacridine]
read cis-4a-cisoid-4a,10a-trans-10a-tetradecahydroacridine
Replace the structure with:
For (14M)-15-bromo-2,13-dioxa-1(1,4)-benzenacyclotridecaphane-12-carboxylic acid (PIN)
read (14M)-15-bromo-2,13-dioxa-1(1,4)-benzenacyclotridecaphane-12-carboxylic acid
Replace the structure with:
For (2s,4s,6s,8s)-2,4,6,8-tetranonyl-1,3,5,7(1,3)-tetrabenzenacycoctaphane-14,16,34,36,54,56,74,76-octol (PIN)
read (2r,4r,6r,8r)-2,4,6,8-tetranonyl-1,3,5,7(1,3)-tetrabenzenacycoctaphane-14,16,34,36,54,56,74,76-octol (PIN)
For ...present, all descriptors are must be cited (see P-91.2.2).
read ...present, all descriptors of double bonds in rings of more than seven nodes are cited (see P-91.2.2)
For (2E)-1(2,6)-pyridina-7(1,3)-benzenacyclododecaphan-2-ene (PIN)
read (2E)-11,14,15,16-tetrahydro-1(2,6)-pyridina-7(1,3)-benzenacyclododecaphan-2-ene (PIN)
For Is the resulting molecule chiral
read Is the resulting molecule chiral ?
For Noninherently chiral substitution pattern
read Type 3 Noninherently chiral substitution pattern
For Achiral substitution pattern; substitution units are only in substituents
read Type 4 Achiral substitution pattern; stereogenic units are only in substituents
For ...(C76-D2)[5,6}fullerene shown below...
read ...(C76-D2)[5,6]fullerene shown below...
For ...(C60-Ih)[5,6]-fullerene (PIN)
read ...(C60-Ih)[5,6]-fullerene (PIN)
For ...[5,6]fullerene-3′,3′,3′′,3′′-tetracarboxylate
read ...[5,6]fullerene-3′,3′,3′′,3′′-tetracarboxylate (PIN)
For tetrakis[(1R)-1-phenylbutyl](f,sA-3′H,3′′H-di...
read tetrakis[(1R)-1-phenylbutyl] (f,sA-3′H,3′′H-di... [add space in name]
For (1M)-6,6′-dinitro-[1,1′-biphenyl]-2,2′-dicarboxylic acid (PIN)
read (1M)-6,6′-dinitro[1,1′-biphenyl]-2,2′-dicarboxylic acid (PIN)
read (1Ra)-6,6′-dinitro[1,1′-biphenyl]-2,2′-dicarboxylic acid
For (1P)-2′,5′-dimethyl-6-nitro-[1,1′-biphenyl]-2-carboxylic acid (PIN)
read (1P)-2′,5′-dimethyl-6-nitro[1,1′-biphenyl]-2-carboxylic acid (PIN)
read (1Sa)-2′,5′-dimethyl-6-nitro[1,1′-biphenyl]-2-carboxylic acid
For (1M)-2′,6-diamino-6′-methoxy-[1,1′-biphenyl]-2-carboxylic acid (PIN)
read (1M)-2′,6-diamino-6′-methoxy[1,1′-biphenyl]-2-carboxylic acid (PIN)
read (1Ra)-2′,6-diamino-6′-methoxy[1,1′-biphenyl]-2-carboxylic acid
For (2P)-2-[2′-(hydroxymethyl)naphthalen-1-yl]-3,5-dimethylphenol (PIN)
read (2P)-2-[2-(hydroxymethyl)naphthalen-1-yl]-3,5-dimethylphenol (PIN)
read (2Sa)-2-[2-(hydroxymethyl)naphthalen-1-yl]-3,5-dimethylphenol
For [1S,1′s,2S,4S,4′R]-4,4′-dimethyl-1,1′-bi(cyclohexan)-2-ol (PIN)
read (1S,1′s,2S,4S,4′R)-4,4′-dimethyl[1,1′-bi(cyclohexan)]-2-ol (PIN)
For (a) [1s,1′s,4s,4s′]-4,4′-dimethyl-1,1′-bi(cyclohexane) (PIN)
read (a) (1s,1′s,4s,4′s)-4,4′-dimethyl-1,1′-bi(cyclohexane) (PIN)
read (b) [1(4)-cis,1′(4′)-cis]-4,4′-dimethyl-1,1′-bi(cyclohexane)
For [11s,14s,21r,24r,31s,34s]-14,34-dimethyl-11,21:24,31-tercyclohexane (PIN)
read (11s,14s,21r,24r,31s,34s)-14,34-dimethyl-11,21:24,31-tercyclohexane (PIN)
read [11(14)-cis,21(24)-trans,31(34)-cis]-14,34-dimethyl-11,21:24,31-tercyclohexane
For (1s,1′r,4s,4′r)-4-bromo-4′-butyl-4-(4-ethylphenyl)-1,1′-bi(cyclohexane) (PIN)
read (1s,1′s,4s,4′r)-4-bromo-4′-butyl-4-(4-ethylphenyl)-1,1′-bi(cyclohexane)
For (1E)-1-[(1′S,1r,4S)-[1,1′-bi(cyclohexan)-3′-en-4-yl]-N-[(1r,4r)-4-phenylcyclohexyl]methan-1-imine (PIN)
read (1E)-1-{(1r,1′S,4S)-[1,1′-bi(cyclohexan)]-3′-en-4-yl}-N-[(1r,4S)-4-phenylcyclohexyl]methanimine (PIN)
read (E)-1-{1(4)-trans-(1′S)-[1,1′-bi(cyclohexan)]-3′-en-4-yl}-N-(trans-4-phenylcyclohexyl)methanimine
For (3Z)-1,1′-bicyclooct-3-ene (PIN)
read (3Z)-[1,1′-bi(cyclooctan)]-3-ene (PIN)
For (1Z,2′Z,3Z,5Z,7Z)-bicycloocta-1,2′,3,5,7-pentaene (PIN)
read (1Z,2′Z,3Z,5Z,7Z)-[1,1′-bi(cyclooctane)]-1,2′,3,5,7-pentaene (PIN)
For (2R)-1-[(1r,4R)-4-methylcyclohexyl]-3-[(1s,4S)-4-methylcyclohexyl]propan-2-ol (PIN)
read (2R)-1-[(1r,4S)-4-methylcyclohexyl]-3-[(1s,4S)-4-methylcyclohexyl]propan-2-ol (PIN)
Replace structure with:
for simplified digraph to for the configuration ‘R’ at C-1.
read simplified digraph for the configuration ‘S’ at C-1 (sequence rule 4b like > unlike).
For (11R,12S,14S,4E,7E,111S,112R,114R)-12,112-bis{[(1E)-2-phenyleth-1-en-1-yl]oxy}-2,10-dioxa-3,9(1,4)-dibenzena-1,11(1,4)-dicyclohexanaundecaphane-4,7-diene-14,114-dicarboxylic acid (PIN: a phane name)
read (2E,51S,52R,55S,8E,11E,151S,152R,154R,17E)-4,6,10,14,16-pentaoxa-1,19(1),7,13(1,4)-tetrabenzena-5,15(1,2)-dicyclohexananonadecaphane-2,8,11,17-tetraene-55,154-dicarboxylic acid (PIN: a phane name)
For (11S,12R,14R,4Z,7E,111R,112S,114S)-12,112-bis{[(1E)-2-phenyleth-1-en-1-yl]oxy}-2,10-dioxa-3,9(1,4)-dibenzena-1,11(1,4)-dicyclohexanaundecaphane-4,7-diene-14,114-dicarboxylic acid (PIN: a phane name)
read (2E,51R,52S,55R,8Z,11E,151R,152S,154S,17E)-4,6,10,14,16-pentaoxa-1,19(1),7,13(1,4)-tetrabenzena-5,15(1,2)-dicyclohexananonadecaphane-2,8,11,17-tetraene-55,154-dicarboxylic acid (PIN: a phane name)
For (2S,2′S)-2,2′-oxybis[(1E)-ethene-2,1-diyl-4,1-phenylene]dipropanoic acid (PIN)
read (2S,2′S)-2,2′-{oxybis[(1E)-ethene-2,1-diyl-4,1-phenylene]}dipropanoic acid (PIN)
For (2R)-2-bromo-2-(4-{(1E)-2-[(1E)-2-{4-[(1S)-1-carboxyethyl]phenyl}eth-1-en-1-yloxy]eth-1-en-1-yl}phenyl]propanoic acid
read (2R)-2-bromo-2-{4-[(1E)-2-{[(1E)-2-{4-[(1S)-1-carboxyethyl]phenyl}ethen-1-yl]oxy}ethen-1-yl]phenyl}propanoic acid
For (2S)-2-(4-{(1Z)-2-[(1E)-2-{4-[(1S)-1-carboxyethyl]phenyl}ethenyloxy]ethenyl}phenyl)propanoic acid (PIN;
read (2S)-2-{4-[(E)-2-{(Z)-2-{4-[(1S)-1-carboxyethyl]phenyl}ethen-1-yl]oxy}ethen-1-yl]phenyl}propanoic acid (PIN;
Replace diagram with:
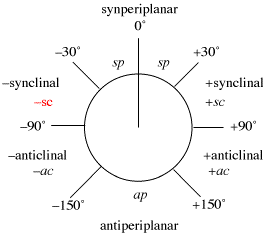
Replace the structures with:
Replace the structures with:
Replace the structure with:
For ...further specification, for example the ‘5α’ position in steroids is usually not defined but it must, however, be defined in preferred IUPAC names. When a planar...
read ...further specification. All chirality must be defined so that for example with a steroid the stereochemistry at ‘C-5’, when relevant, is indicated by α, β or ξ. When a planar...
Replace the structures with:
For orynan read corynan
for formosana read formosanan
for lythrancine read lythran
for oxyacathan read oxyacanthan
for solanidine read solanidane
for strychinidine read strychnidine
for vincaleuckoblastine read vincaleukoblastine
For pimerane read pimarane
for himalachane read himachalane
For prostantane read protostane
for ritinal read retinal
For Φ (phi) read ![]() (phi)
(phi)
for Ψ (psi) read ψ (psi)
Replace structure 1 with:
Replace the structure with:
For (1R,4s,7S)-4-ethyl-4,7-dimethylcyclodecane
read (1R,4s,7S)-4-ethyl-1,7-dimethylcyclodecane (position 1 is position 10 for germacrane)
For 19a-homo-5β-pregnane (PIN)
read 19a-homo-5β-pregnane
For 16a-homo-5α-pregnane (PIN)
read 16a-homo-5α-pregnane
Replace structure with:
Replace structure with:
Replace the structure with:
For (3αH)-5(4→3)-abeo-podocarpane
read (3αH)-5(4→3)-abeopodocarpane
Replace the structure with:
Replace the structure with:
For ...prefixes ‘cyclo’, ‘seco’, ‘homo’, and ‘nor’. In general...
read ...prefixes ‘cyclo’, ‘seco’, ‘apo’, ‘homo’, and ‘nor’. In general...
For 3α,5-cyclo-9,10-seco-5α-androstane
read 3α,5α-cyclo-9,10-seco-5α-androstane
Replace the structure with:
Replace the structure with:
Replace structure with:
For (7α,8α,8′β,9′α)-7,9a′:8′,9-diepoxy-7-oxa-9a′-homo-8,9′-neolignane
read (7α,8α,8′β,9′α)-7,9a′:8′,9-diepoxy-7′-oxa-9a′-homo-8,9′-neolignane
Replace the structure with:
For 1-[(1E,3S)-2,3-dimethyl-4-phenylbut-1-en-1-yl]benzene
read [(1E,3S)-2,3-dimethyl-4-phenylbut-1-en-1-yl]benzene
read 1,1′-[(1E,3S)-2,3-dimethylbut-1-ene-1,4-diyl]dibenzene
For (all-trans)-retinal
read all-trans-retinal
Both structures are wrong, they should be:
Replace the structure with:
For 8α-ethyl-5α-eudesmane
read 8α-ethyleudesmane
Replace the structure with:
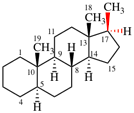
Replace the structure with:
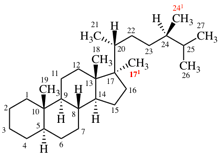
For [not (21-nor-5α-pregnan-17α-yl))methanol]
read [not (21-nor-5α-pregnan-17α-yl)methanol]
Replace the structure with:
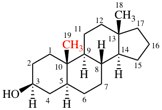
For 7β-amino-3-methyl-2,3-didehydrocepham-2-carboxylic acid
read 7β-amino-3-methyl-3,4-didehydrocepham-4-carboxylic acid
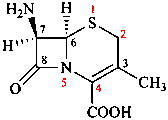
Replace the structure with:
For 19,21-epoxyspermidin-21-one
read 19,21-epoxyaspidospermidin-21-one
For (3αH,4αH)-2′,2′-dimethyl-3,4-dihydro-[1,3]-dioxolo[4′,5′:3,4]matridine
read (3αH,4αH)-2′,2′-dimethyl-3,4-dihydro[1,3]dioxolo[4′,5′:3,4]matridine
read propane-2-one matridine-3β,4β-diyl ketal
read acetone matridine-3β,4β-diyl ketal
Replace the structure with:
Replace the structures with:
Replace the structure with:
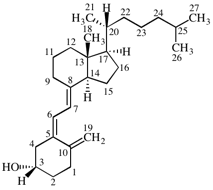 [no hydrogen at C-8]
[no hydrogen at C-8]
For Retained and systematic carbohydrate names (with recommended three-letter abbreviations in parantheses) and structures...
read Retained and systematic carbohydrate names and structures...
For 2,3-dihydroxypropanal
read (2R)-2,3-dihydroxypropanal
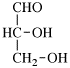
Replace the structure with:
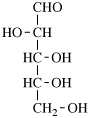
Replace the structure with:
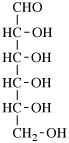
For D-alto-hexose
read D-altro-hexose
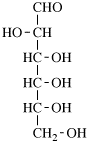
Replace the structure with:
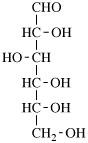
Replace the structure with:
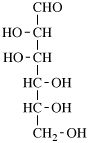
Replace the structure with:
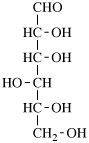
Replace the structure with:
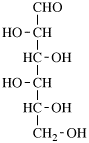
Replace the structure with:
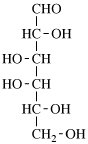
Replace the structure with:
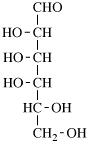
For D-ethryulose
read D-erythrulose
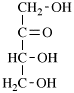
Replace the structure with:
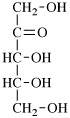
Replace the structure with:
Replace the structure with:
For Various representations ‘b’, ‘c’, ‘d’, ‘e’ and ‘f’
read Various representations ‘b’, ‘c’, ‘d’ and ‘e’
Replace structure with:
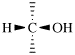
Replace structure with:
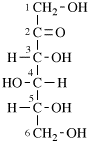
For ...the second one: step (c) is the reorientation of carbon C-5...
read ...the second reorientation, step (c), is the rotation at carbon C-5...
replace the structure with:
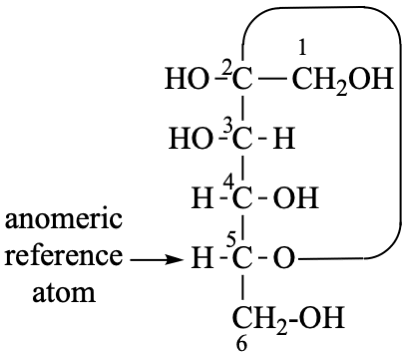
Replace structure with:
For ...Table 10.3, configuration being designated by the appropriate CIP stereodescriptors ‘R’, ‘S’, ‘r’, etc.
read ...Table 10.3; configuration is specified by a ‘D’ or ‘L’ stereodescriptor, as defined in P-102.3.2.
Delete this example as it is a duplicate of example 3.
For [not D-threo-D-allo-non-5-ulose; ...
read [not D-threo-D-allo-non-5-ulose; ...
For (1ξ,4S,5R)-5-(hydroxymethyl)oxolane-2,4-diol
read (2ξ,4S,5R)-5-(hydroxymethyl)oxolane-2,4-diol
For (2S,3R,4S,5S,6R)-3-bromo-3-chloro-6-(hydroxymethyl)oxane-2,3,4-triol
read (2S,3R,4S,5S,6R)-3-bromo-3-chloro-6-(hydroxymethyl)oxane-2,4,5-triol
For (2S,3S,4S,5R,6R)-3-acetamido-6-[(acetyloxy)methyl]-2-fluorooxane-2,4,5-triyl triacetate
read (2S,3S,4S,5R,6R)-3-acetamido-6-[(acetyloxy)methyl]-2-fluorooxane-3,4,5-triyl triacetate
For The prefix ‘meso’ must be included in the preferred name of erythritol....
read The prefix ‘meso’ may be included in the name of erythritol....
For
(2R,3S,4r,5R,6S)-heptane-1,2,3,4,5,6,7-aldito
read (2R,3S,4r,5R,6S)-heptane-1,2,3,4,5,6,7-heptol
For (2R,3S,4R,5R,6R)-3,4,5,6-tetra(acetyloxy)-2-bromooxane-2-carboxylic acid
read (2R,3S,4R,5R,6R)-3,4,5,6-tetrakis(acetyloxy)-2-bromooxane-2-carboxylic acid
For ...The descriptor ‘meso’ must be added for sake of clarity...
read ...The descriptor ‘meso’ may be added for the sake of clarity...
For D-glucar-1-amic acid
read D-glucar-6-amic acid
For (2R,3R,4R,5S)-2-(hydroxymethyl)oxane-3,4,5-triol
read (2R,3R,4R,5S)-2-(hydroxymethyl)oxane-3,4,5-triol
For P-102.6.2 Monosaccharides as substituent groups
read P-102.6.2 Substituent groups other than glycosyl groups
Delete P-102.6.1.6 Substituent groups other than glycosyl groups
Replace the structure with:
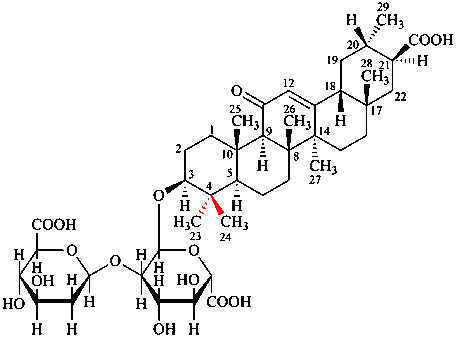
For 4-[(R)-2-amino-3-hydroxy-2-methylpropanamido]-N-{1-[(2R,5S,6R)-(5-{[4,6-dideoxy-4-(dimethylamino)-α-D-glucopyranosyl]oxy}-6-methyloxan-2-yl)]-2-oxo-1,2-dihydropyrimidin-4-yl}benzamide
read
4-[(2R)-2-amino-3-hydroxy-2-methylpropanamido]-N-{1-[(2R,5S,6R)-5-{[4,6-dideoxy-4-(dimethylamino)-α-D-glucopyranosyl]oxy}-6-methyloxan-2-yl]-2-oxo-1,2-dihydropyrimidin-4-yl}benzamide
For 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide
read 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide
For P-102.6.1.6
read P-102.6.2
For 2-(β-D-glucopyranos-2-O-yl)acetic acid
read (β-D-glucopyranos-2-O-yl)acetic acid
For ... gluco precedes fructo in the alphabetical...
read ... fructo precedes gluco in the alphabetical...
Delete line of space after arginine
read 2-amino-5-(carbamimidoylamino)pentanoic acid
For 2-aminobutanedioic acid
read aminobutanedioic acid
Replace the structure with:
H2N-CO-[CH2]2-CH(NH2)-COOH
For 2-aminoacetic acid
read aminoacetic acid
For alle
read aIle
For 2-amino-5-oxopentanoic acid
read 2-amino-6-oxohexanoic acid
For S-(2-amino-2-carboxyethyl)homosysteine
read S-(2-amino-2-carboxyethyl)homocysteine
Replace structure with:
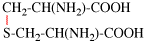
For O-(4-hydroxy-3,5-diiodophenyl)-3,5-iodotyrosine
read O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodotyrosine
For ...Ahx. The CIP stereodescriptors R and S are used...
read ...Ahx. The stereodescriptors D and L are used...
Replace the structure with:
Replace the structure with:
For (4-amino-5-carboxypentyl)amino
read (5-amino-5-carboxypentyl)amino
For The singly charged cations of asparagine, glutamine, and lysine...
read The singly charged cations of arginine, histidine, and lysine...
For ...and other nitrogeneous analogues derivatives...
read ...and other nitrogenous analogues derivatives...
For P-103.2.7 Alcohols, aldehyde, ketones, and nitriles
read P-103.2.8 Alcohols, aldehyde, ketones, and nitriles
For aminoacetamide
read 2-aminoacetamide
For aspartic 1-amide
read asparaginamide
For glutamic 1-amide
read glutaminamide
For 2-amino-1-N-phenylacetamide
read 2-amino-N1-phenylacetamide
Replace the structure with:
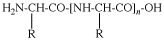
For L-leucine by L-alanine
read L-leucine by DL-alanine
For ...The retained names angiotensine II, bradikinin...
read ...The retained names angiotensin II, bradikinin...
Replace structure with:

For Ile5,Ala5]angiotensin II
read [Ile5,Ala7]angiotensin II
Replace structure with:

Replace structure with:

Replace structure with:

For [A21-glycine]insulinyl-B-arginine,arginine]insulin (human)
read [A21-glycine]insulin-B30-yl-L-arginyl-L-arginine (human)

For radykininylleucine
read bradykininylleucine
read bradykininyl-Leu
For insulintl-B-arginine.ardinine (human)
read insulin-B30-yl-L-arginyl-L-arginine (human)

For des-B1-phynylalanine-insulin (cattle)
read des-B1-phenylalanine-insulin (cattle)
read des-PheB1-insulin (cattle)

For (1R,2R,3R,5S)-cyclohexane-1,2,3,5-tetrol
read (1R,2R,3R,5R)-cyclohexane-1,2,3,5-tetrol
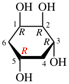
For (1R,2R,3R,5S)-cyclohexane-1,2,3,5-tetrol
read (1R,2R,3R,5R)-cyclohexane-1,2,3,5-tetrol
For 2-methyl-myo-inositol
read 2-C-methyl-myo-inositol
read (1s,2R,3S,4s,5R,6S)-1-methylcyclohexane-1,2,3,4,5,6-hexol
Delete left hand structure.
delete I and II
For 4-amino-1-[(2R,3R,4R,5R)-3-fluoro-4-hydroxy-5-(methoxymethyl)oxolan-2-yl-5-iodopyrimidin-2(1H)-one
read 4-amino-1-[(2R,3R,4R,5R)-3-fluoro-4-hydroxy-5-(methoxymethyl)oxolan-2-yl]-5-iodopyrimidin-2(1H)-one
For N-(2-hydroxyethyl)-5′-S-methyl-5′-thioguanosine
read N2-(2-hydroxyethyl)-5′-S-methyl-5′-thioguanosine
For 2′,3′,5′-tri-O-acetylguanosine
read 2′,3′,5′-tri-O-acetyladenosine
read adenosine 2′,3′,5′-triacetate
For N6-(N-propylcarbamoyl)-5′-adenylic acid
read N6-(propylcarbamoyl)-5′-adenylic acid
Replace the structure with:
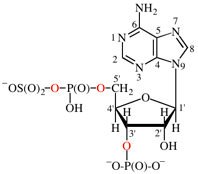
For (2S)-2-O-acetyl-1-O-hexadecanoyl-3-O-(9Z)-octadec-9-enoylglycerol
read (2S)-2-O-acetyl-1-O-hexadecanoyl-3-O-[(9Z)-octadec-9-enoyl]glycerol
For (for a discussion and examples of ‘sn’ see P-107.3.1.2)
read (for a discussion and examples of ‘sn’ see P-107.3.2)
For ...and the stereodescriptor ‘X’ may be used...
read ...and the stereodescriptor ‘Ξ’ may be used...
For 2,3-bis(octadecanoyl)-sn-glycero-3-phospho-L-serine
read 1,2-di(octadecanoyl)-sn-glycero-3-phospho-L-serine
read
O-{[(2R)-2,3-bis(octadecanoyloxy)propoxy]hydroxyphosphoryl}-L-serine
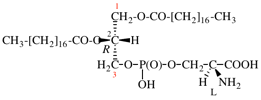
For 2,3-bis(hexadecanoyl)-sn-glycero-3-phosphoethanolamine
read 1,2-di(hexadecanoyl)-sn-glycero-3-phosphoethanolamine.
For (2R)-3-{[(2-aminoethoxy)hydroxyphosphoryl]oxy}propane-1,2-diyl dihexadecanoate
read (2R)-3-{[(2-aminoethoxy)hydroxyphosphoryl]oxy}propane-1,2-diyl di(hexadecanoate)
For L-myo-inositol 2-[(2R)-2,3-bis(hexadecanoyloxy)propyl hydrogen phosphate
read L-myo-inositol 2-[(2R)-2,3-bis(hexadecanoyloxy)propyl hydrogen phosphate]
for (2R)-3-[({[(1s,2R,3S,4s,5R,6S)-hydroxy-2,3,4,5,6-pentahydroxycyclohexyl]oxy}hydroxyphosphoryl)oxy]propane-1,2-diyl dihexadecanoate
read (2R)-3-[(hydroxy{[(1s,2R,3R,4s,5S,6S)-2,3,4,5,6-pentahydroxycyclohexyl]oxy}phosphoryl)oxy]propane-1,2-diyl di(hexadecanoate)
For (2S)-3-(O-β-D-galactopyranosyl)propane-1,2-diyl dioctadecanoate
read (2S)-3-(β-D-galactopyranosyloxy)propane-1,2-diyl di(octadecanoate)
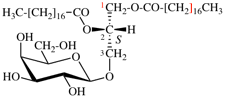
For Ru ruthenica
read Ru ruthena
For ‘Yt’
read ‘Yb’
Reverse the order of Na and K
For aminocarbonimidoyl* = carbamimidoyl
read C-aminocarbonimidoyl = carbamimidoyl*
For = aminocarbonimidoyl
read = C-aminocarbonimidoyl
For = 1 anthryl
read 1-anthryl
For arsono* = dihydroxynitroryl
read arsono* = dihydroxyarsoryl
For azinoyl* = dihydroazoryl
read azinoyl* = dihydronitroryl
For azoxy* –N(O)=N– or –N=N(O)– P-68.3.1.3.3.1
read NON-azoxy –N(O)=N– or –N=N(O)– P-68.3.1.3.3.1
For benzenecarbonyl = benzoyl* = oxo(phenyl)methyl
read benzenecarbonyl = benzoyl* = oxo(phenyl)methyl = phenylcarbonyl
For = benzenecarbothioylamino
read = (benzenecarbothioyl)amino
For benzenecarbothioamido* = benzenecarbothioylamino = thiobenzamido
read benzenecarbothioamido* = (benzenecarbothioyl)amino = thiobenzamido
For benzenecarboximidohydrazido* = 2-benzenecarboximidoylhydrazin-1-yl
read benzenecarboximidohydrazido* = 2-(benzenecarboximidoyl)hydrazin-1-yl
For 2-benzenecarboximidoylhydrazin-1-yl = benzenecarboximidohydrazido*
read 2-(benzenecarboximidoyl)hydrazin-1-yl = benzenecarboximidohydrazido*
For benzene-1,2-dicarbothioyl* = 1,2-phenylenebis(sulfanylidenemethylene) = phenylenebis(sulfanylidenemethylene) (not dithiophthaloyl)
read benzene-1,2-dicarbothioyl* = 1,2-phenylenebis(sulfanylidenemethylene) = 1,2-phenylenebis(thioxomethylene) (not dithiophthaloyl)
For benzenesulfinohydrazonamido* = benzenesulfinohydrazonoylamino
read benzenesulfinohydrazonamido* = (benzenesulfinohydrazonoyl)amino
For benzenesulfinohydrazonoylamino = benzenesulfinohydrazonamido*
read (benzenesulfinohydrazonoyl)amino = benzenesulfinohydrazonamido*
For benzhydroximoyl: see N-hydroxybenzenecarboximidoyl*
read benzohydroximoyl = N-hydroxybenzenecarboximidoyl* = N-hydroxybenzimidoyl = benzenecarbohydroximoyl C6H5-C(=N-OH)– P-65.1.7.2.2
For benzidino = [(4′-amino[1,1′-biphenyl]-4-yl)amino]*
read benzidino = (4′-amino[1,1′-biphenyl]-4-yl)amino*
For benzoyl* = benzenecarbonyl = oxo(phenyl)methyl
read benzoyl* = benzenecarbonyl = oxo(phenyl)methyl = phenylcarbonyl
For benzylidene* = phenylmethylidene
read benzylidene* = phenylmethylidene (not benzal)
Replace structures with:

For butan-1-ylidyne = butylidyne*
read butanylidyne = butylidyne*
For butan-2-yloxy* = 1-methylpropoxy (not sec-butoxy)
read (butan-2-yl)oxy* = 1-methylpropoxy (not sec-butoxy; not sec-butyloxy)
Replace structures with:
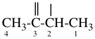
For tert-butoxy (unsubstituted*) = 2-methylpropan-2-yloxy* = 1,1-dimethylethoxy
read tert-butoxy* (unsubstituted) = (2-methylpropan-2-yl)oxy = 1,1-dimethylethoxy
For butylidyne* = butan-1-ylidyne
read butylidyne* = butanylidyne
For carbamimidoyl* = aminocarbonimidoyl
read carbamimidoyl* = C-aminocarbonimidoyl
For carbamohydrazonoyl* = hydrazinylidene(amino)methyl
read carbamohydrazonoyl* = amino(hydrazinylidene)methyl
For carbononitridoperoxy = cyanoperoxy*
read carbononitridoylperoxy = cyanoperoxy*
For (carboxycarbonothioyl)sulfanyl)
read (carboxycarbonothioyl)sulfanyl
For HOCO-CO-S–
read HOOC-CO-S–
For chloro(amido)phosphoryl = phosphoroamidochloridoyl* (H2N)ClP(O)– P-67.1.4.1.1.4
read chloroamidophosphoryl: see phosphoramidochloridoyl*
For cyano* = carbononitrodoyl
read cyano* = carbononitridoyl
For cyanocarbonyl = carbocyanatidoyl*
read cyanocarbonyl = carbocyanidoyl*
For cyanoperoxy* = carbononitridoperoxy
read cyanoperoxy* = carbononitridoylperoxy
For decanedioyl* = 1,10-dioxodecanyl
read decanedioyl* = 1,10-dioxodecane-1,10-diyl
For (NH2)P–
read (NH2)2P–
For diazenecarbohydrazido* = 2-diazenecarbonylhydrazin-1-yl (not carbazono)
read diazenecarbohydrazido* = 2-(diazenecarbonyl)hydrazin-1-yl (not carbazono)
For diazenecarbonyldiazenyl*
read (diazenecarbonyl)diazenyl*
For 2-diazenecarbonylhydrazin-1-yl = diazenecarbohydrazido* (not carbazono)
read 2-(diazenecarbonyl)hydrazin-1-yl = diazenecarbohydrazido* (not carbazono)
For HN=NN-CO-N=NH–
read HN=NN-CO-N=N–
For dihydroxycarbonimidoyl*
read C,N-dihydroxycarbonimidoyl*
For H2N=N–
read HN=N–
For dimethylammoniumylidene: see dimethylazaniumylidene*
read dimethylammoniumylidene: see N-methylmethanaminiumylidene*
For dimethylazaniumylidene* (not dimethylammoniumylidene)
read N-methylmethanamiumylidene* (not dimethylammoniumylidene; not dimethylimmonio)
For (HO)2P(O)–
read (HO)2P–
For (HO)2P(O)–
read (HO)2P–
For (CH3)3-C-O–
read (CH3)3C-O–
For dimethyliminio: see dimethylazaniumylidene*
read dimethylimmonio: see N-methylmethanaminiumylidene*
For disiloxan-1-yl*
read disiloxanyl*
For (disulfanylcarbonyl)oxy = (disulfanecarbonyl)oxy* = (dithiohydroperoxycarbonyl)oxy
read (disulfanylcarbonyl)oxy* = [(dithiohydroperoxy)carbonyl]oxy
For [(dithiohydroperoxy)carbonyl]oxy = (disulfanecarbonyl)oxy* = (disulfanylcarbonyl)oxy
read [(dithiohydroperoxy)carbonyl]oxy = (disulfanylcarbonyl)oxy*
For 1,2-dithiooxalyl = ethanebis(thioyl)* = bis(sulfanylidene)ethane-1,2-diyl
read dithiooxalyl = ethanebis(thioyl)* = bis(sulfanylidene)ethanediyl
For ethanediimidoyl* = oxalimidoyl = 1,2-diiminoethane-1,2-diyl
read ethanediimidoyl* = oxalimidoyl = diiminoethanediyl
For ethanedioyl = oxalyl* = 1,2-dioxoethane-1,2-diyl
read ethanedioyl = oxalyl* = dioxoethanediyl
For = ethanedioylbis(azanetriyl
read = ethanedioylbis(azanetriyl)
For ethane-1,2-diylbis(oxy)* = ethylenebis(oxy) (not ethane-1,2-diyldioxy)
read ethane-1,2-diylbis(oxy)* = ethylenebis(oxy) (not ethane-1,2-diyldioxy, not ethylenedioxy)
For ethanehydrazonamido* = ethanehydrazonoylamino
read ethanehydrazonamido* = (ethanehydrazonoyl)amino
For ethanehydrazonylamino = ethanehydrazonamido*
read (ethanehydrazonyl)amino = ethanehydrazonamido*
For ethanethioamido* = ethanethioylamino = thioacetamide
read ethanethioamido* = (ethanethioyl)amino = thioacetamide
For ethanethioylamino = ethanethioamido* = thioacetamido
read (ethanethioyl)amino = ethanethioamido* = thioacetamido
For ethanimidohydrazido* = 2-ethanimidoylhydrazin-1-yl
read ethanimidohydrazido* = 2-(ethanimidoyl)hydrazin-1-yl
For 2-ethanimidoylhydrazin-1-yl = ethanimidohydrazido*
read 2-(ethanimidoyl)hydrazin-1-yl = ethanimidohydrazido*
For ethylsulfinimidoyl = ethanesulfinimidoyl*
read S-ethylsulfinimidoyl = ethanesulfinimidoyl*
For ethylsulfonimidoyl = ethanesulfonimidoyl*
read S-ethylsulfonimidoyl = ethanesulfonimidoyl*
For formohydrazido = 2-formylhydrazin-1-yl*
read formohydrazido* = 2-formylhydrazin-1-yl
For 2-formylhydrazin-1-yl* = formohydrazido
read 2-formylhydrazin-1-yl = formohydrazido*
For furan-2-ylmethyl* (not furfuryl)
read (furan-2-yl)methyl* (not furfuryl)
For furfural (2-isomer only): see furan-2-ylmethyl*
read furfuryl (2-isomer only): see (furan-2-yl))methyl*
For heptylidyne* = heptan-1-ylidyne
read heptylidyne* = heptanylidyne
Delete this correction
For hexane-2,3,5-tricarbothioyl* = hexane-2,3,5-tris(sulfanylidenemethylene) = hexane-2,3,5-triyltris(thioxomethylene)
read hexane-2,3,5-tricarbothioyl* = hexane-2,3,5-triyltris(sulfanylidenemethylene) = hexane-2,3,5-triyltris(thioxomethylene)
Add
hexane-2,3,5-triyltricarbonyl = hexane-2,3,5-tricarbonyl* = hexane-2,3,5-triyltris(oxomethylene)
hexane-2,3,5-triyltris(oxomethylene) = hexane-2,3,5-tricarbonyl* = hexane-2,3,5-tryltricarbonyl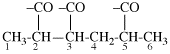
P-65.1.7.4.2
For hexane-2,3,5-tris(sulfanylidenemethylene) = hexane-2,3,5-tricarbothioyl*
read: hexane-2,3,5-triyltris(thioxomethylene) = hexane-2,3,5-tricarbothioyl* = hexane-2,3,5-triyltris(sulfanylidenemethylene)
hexane-2,3,5-triyltris(sulfanylidenemethylene) = hexane-2,3,5-tricarbothioyl* = hexane-2,3,5-triyltris(thioxomethylene) [N.B. two entries]
Delete these entries
Add hexane-2,3,5-triyltris(oxomethylene) = hexane-2,3,5-tricarbonyl* = hexane-2,3,5-triyltricarbonyl 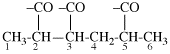 P-65.1.7.4.2
P-65.1.7.4.2
For hydrazimidophosphoryl = phosphorohydrazidimidoyl*
read hydrazidimidophosphoryl = phosphorohydrazidimidoyl*
For hydrazinecarboximidoyl* = hydrazinyl(imino)methyl = C-hydrazinylcarbonimidoyl = carbonohydrazimidoyl
read hydrazinecarboximidoyl* = hydrazinyl(imino)methyl = C-hydrazinylcarbonimidoyl = carbonohydrazidimidoyl
For P-29.
read P-29.3.2.1; P-29.3.2.2
For hydraziny(lhydrazinylidene)methyl
read hydrazinyl(hydrazinylidene)methyl
For hydrazinylidene* = diazenylidene
read hydrazinylidene* = diazanylidene
Delete hydrazinylidene(amino)methyl = carbamohydrazonyl* H2N-C(=NH-NH2)– P-66.4.2.3
For hydrazinylidenemethylidene* = diazenylidenemethylidene = hydrazonomethylidene
read hydrazinylidenemethylidene* = diazanylidenemethylidene (not hydrazonomethylidene)
For hydroperoxyphosphoryl = phosphoroperoxoyl* = peroxyphosphoryl
read (hydroperoxy)phosphoryl = phosphoroperoxoyl* = peroxyphosphoryl
For hydroselenonyl*
read hydroseleninyl* [move to above the previous entry]
For H2-N-NH-SO2–
read H2N-NH-SO2–
For (C-hydroxycarbonimidoyl)amino* = [hydroxyl(imino)methyl]amino
read (C-hydroxycarbonimidoyl)amino* = [hydroxy(imino)methyl]amino
For hydroxyl(mercapto)phosphoryl: see hydroxy(sulfanyl)phosphoryl*
read hydroxy(mercapto)phosphoryl: see hydroxy(sulfanyl)phosphoryl*
For (HO)(HS)B)–
read (HO)(HS)B–
For imino* (not azanylidene)
read imino* = azanylidene (see also azanediyl*)
For (CH3)2CH-O
read (CH3)2CH-O–
For isothiocyanatosulfonyl* = sulfurisothiocyanatidoyl
read isothiocyanatosulfonyl* = sulfur(isothiocyanatidoyl)
For 3-isouriedo: see [(aminohydroxy)methyidene]amino*
read 3-isoureido: see [amino(hydroxy)methylidene]amino*
For S-methoxycarbonimidoyl*
read C-methoxycarbonimidoyl*
For methylenebis(thio) = methylenebis(sulfanyl)*
read methylenebis(thio) = methylenebis(sulfanediyl)*
For (1-methylethyl)oxy = propan-2-yloxy* = isopropoxy
read 1-methylethoxy = (propan-2-yl)oxy* = isopropoxy
For –CH2-C(CH3)–
read –CH2-CH(CH3)–
For 2-methylphenyl* = o-tolyl
read 2-methylphenyl* = o-tolyl
For CH3-C+-(CH3)-CH2–
read CH3-C+(CH3)-CH2–
Move to before the last entry on the previous page.
read 2-naphthyl = naphthalen-2-yl*
For nitroamino: see nitramido*
read nitroamino = nitramido* O2N-NH– P-67.1.4.3.2
For 1-nitrohydrazin_1-yl*
read 1-nitrohydrazin-1-yl*
For CH3-[CH2]6-C≡
read CH3-[CH2]6-CH2–
Replace structure with:
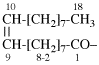
Replace structure with:
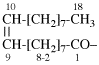
Delete this line
For oxalo* = not carboxycarbonyl; not carboxyformyl; not hydroxyl(oxo)acetyl)]
read oxalo* = carboxycarbonyl [not carboxyformyl; not hydroxy(oxo)acetyl]
For oxalooxy* = (carboxyczrbonyl)oxy
read oxalooxy* = (carboxycarbonyl)oxy [not (carboxyformyl)oxy]
For oxalylbis(azanediyl)* = ethanedioybis(azanediyl)
read oxalylbis(azanediyl)* = ethanedioylbis(azanediyl)
For oxidanyl = hydroxy* HO– P-63.1.4
read oxidanyl: see hydroxy*
For oxoacetyl* = oxaldehydoyl
read oxoacetyl*
Replace structure with:
>N-CO-CO-N<
For oxoacetyl* = oxaldehydoyl
read oxoacetyl*
Replace structure with:
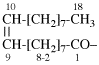
For 2-oxopropylidene* (not acetonylidene)
read 2-oxopropylidene* (not acetonylidene) CH3-CO-CH= P-64.5
Delete this line oxyl* = ylooxidanyl
Replace structure with:
CH3-CO-CH2–
For P-29.3.2.1; P-20.3.2.2
read P-29.3.2.1; P-29.3.2.2
For phenylcarbonyl: see benzoyl*
read phenylcarbonyl = benzoyl* = benzenecarbonyl = oxo(phenyl)methyl C6H5-CO– P-34.2.1.1; P-34.2.2; P-65.1.7.2.1
For = 14-phenylenebis(oxomethylene)
read = 1,4-phenylenebis(oxomethylene)
For C6H5-Se-O–
read C6H5-S-O–
For CH3-CH3-CS–
read CH3-CH2-CS–
For CH3-CH2-C=
read CH3-CH2-CH=
For selenocyanato* = carbononitridoylsylfanyl
read selenocyanato* = carbononitridoylselanyl
For silanediyldiethane-1,2-diyl* = silanediyldiethylene
read silanediyldi(ethane-2,1-diyl)* = silanediyldiethylene
For silanediyldiethylene = silanediyldiethane-1,2-diyl*
read silanediyldiethylene = silanediyldi(ethane-2,1-diyl)*
For P-29.3.1; 2; P-68.2.2
read P-29.3.1; P-68.2.2
For (HO)2Sb(O)—
read (HO)2Sb(O)–
For 2-sulfanyl-1,2-bis(sulfanylidene)ethyl* = 2-sulfanyl-2-sulfanylideneethanethioyl = trithiooxalo HS-CS-CS– P-65.1.7.2.4
read sulfanylbis(sulfanylidene)ethyl = sulfanyl(sulfanylidene)ethanethioyl* = trithiooxalo HS-CS-CS– P-65.1.7.2.4; P-65.1.7.3.3
For 2-sulfanyl-1,2-sulfanylideneethanethioyl = 2-sulfanyl-1,2-bis(sulfanylidene)ethyl* = trithiooxalo HS-CS-CS– P-65.1.7.2.4
read sulfanyl(sulfanylidene)ethanethioyl* = sulfanylbis(sulfanylidene)ethyl = trithiooxalo HS-CS-CS– P-65.1.7.2.4; P-65.1.7.3.3
For sulfurisothiocyantidoyl = isothiocyanatosulfonyl*
read sulfur(isothiocyanatidoyl) = isothiocyanatosulfonyl*
For P-65.3.2.3; P-67-1.4.4.1
read P-65.3.2.3; P-67.1.4.4.1
For tetraazanyl*
read tetraazan-1-yl*
For –S-S-S–
read –S-S-S-S–
For thioacetamido = ethanethioamido* = ethanethioylamino
read thioacetamido = ethanethioamido* = (ethanethioyl)amino
For thiobenzamido = benzenecarbothioamido* = benzenecarbothioylamino
read thiobenzamido = benzenecarbothioamido* = (benzenecarbothioyl)amino
For CH3-CO-NH–
read CH3-CS-NH–
For thioborono* = hydroxyl(sulfanyl)boranyl
read thioborono* = hydroxy(sulfanyl)boranyl
For thiocyanato* = carbonoitridoylsulfanyl
read thiocyanato* = carbononitridoylsulfanyl
For (HO)(HS)B)–
read (HO)(HS)B–
For (thioperoxy)phosphoryl = (phosphorothioperoxoyl)*
read (thioperoxy)phosphoryl = phosphoro(thioperoxoyl)*
For thiophen-2-ylmethyl* = thenyl (2- isomer only)
read (2-thiophen-2-yl)methyl* (not thenyl)
For 2-adamanyl
read 2-adamantyl
For trisiliranyl* = cyclotrisilan-1-yl
read trisiliranyl* = cyclotrisilanyl
For trithiooxalo = 2-sulfanyl-2-sulfanylideneethanethioyl* = 2-sulfanyl-1,2-bis(sulfanylidene)ethyl
read trithiooxalo = sulfanyl(sulfanylidene)ethanethioyl* = sulfanylbis(sulfanylidene)ethyl
For CH2=CH=
read CH2=C=
For ylohydroxy: see oxyl*
read ylohydroxy: see ylooxidanyl*
For ylooxidanyl = oxyl* (not ylohydroxy)
read ylooxidanyl* = ylooxy (not ylohydroxy)
Replace the structure with:
Replace the structure with:
Replace the structure with:
Replace the structure with:
For omosanine
read ormosanine**
Replace the structure with:
Replace the structure with:
For pancrasine read pancracine
For solanidine
read solanidane
replace the structure with:
For spirosolane**
read spirosolane††
For 1αH,5αH-tropane**
read tropane**
For tubocuran read tubocuraran
Replace the structure with:
Replace the structure with:
Replace structures with:
Replace structures with:
For furostane read furostan
Replace structures with:
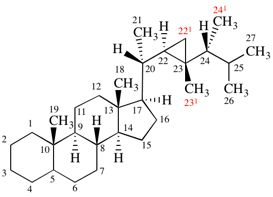
Replace structures with:
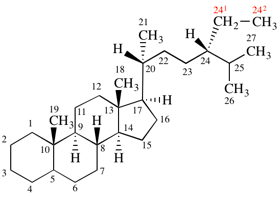
Replace the structure with:
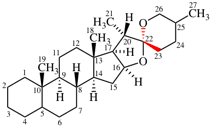
Replace structures with:
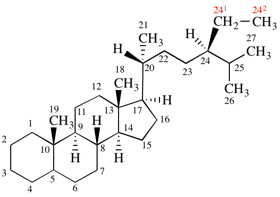
Replace structures with:
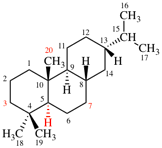
For beyerance**
read beyerane††
For β,![]() -carotene read β,
-carotene read β,![]() -carotene
-carotene
For ε,κ-xarotene
read ε,κ-carotene
For ε,χ-xarotene
read ε,χ-carotene
For The CAS name for this structure is based on gammacercane.
read The CAS name for this structure is based on gammacerane.
Replace the structure of oleanane with:
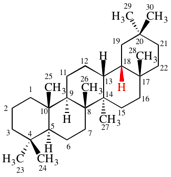
Replace the structure with:
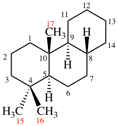
For dammerane read dammarane
Replace the structure with:
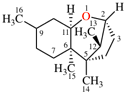
Replace the structure with:
For heterocycls
read heterocycles
22 January 2026
Return to:
Bibliographic lists home page.
IUPAC Books