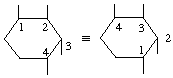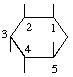Other Cyclitols
I-2. Trivial Names
The trivial name (+)-quercitol is permitted for 1L-1,3,4/2,5-cyclohexanepentol (for derivation of this name see below). The generic name 'quercitols' is abandoned.
 | (+)-Quercitol | (10) |
I-3. Description of Structure
The structure of cyclitols other than the inositols are described by use of the IUPAC 1971 Rules for the Nomenclature of Organic Chemistry, Section C [9], with the proviso that cyclitols are named as substituted cycloalkanepolyols even when some or all of the hydroxyl groups are substituted.
I-4. Assignment of Locants (Positional Numbers)
Locants (positional numbers) are assigned to the carbon atoms of the ring, and thus the direction of numbering is described, with reference to the steric relations and nature of the substituents attached to the ring. The substituents lying above the plane of the ring constitute a set, and those lying below the plane another set. Lowest locants are related to one set of the substituents according to the following criteria, which are applied successively until a decision is reached:
(i) to the substituents considered as a numerical series, without regard to configuration;
(ii) if one set of substituents is more numerous than the other, to the more numerous;
(iii) if the sets are equally numerous and one of them can be denoted by lower numbers, to that set;
(iv) to substituents other than unmodified hydroxyl groups;
(v) to the substituent first in alphabetical order (Rule C-16.3 in [9]);
(vi) for meso-compounds only - to those positions that lead to an L rather than a D designation when Recommendation I-10 is applied to the lowest-numbered asymmetric carbon atom.
Notes
a) 'Lowest numbers' are those that, when considered as a single ascending series, contain the lower number at the first point of difference, e.g. 1,2,3,6 is lower than 1,2,4,5.
b) Criterion (vi) is needed only for compounds with meso-configuration, being required only for problems involving prochirality [10]. It can be simply applied by noting that it causes numbering to be clockwise when the formula is oriented so that the substituent on the lowest-numbered asymmetric carbon atom of the ring projects upwards.
c) When two or more positions are fully equivalent it is immaterial which is chosen as the starting point.
I-5. Relative Configuration
I-5.1. The relative configurations at ring positions of a cyclitol, other than an inositol or a derivative thereof, are described by means of a fraction. The numerator of the fraction consists of the locants (assigned as described above) of the set of substituents that lies above or below the plane of the ring, these numbers being arranged in ascending order and separated by commas. The denominator contains the locants of the other set. Conventionally, the set of locants containing the lowest numbers is cited as numerator.
I-5.2. When only hydroxyl or substituted hydroxyl groups are involved, the fraction also serves as a list of locants. Its position in the name is that usually assigned to the list of locants (see examples).
Note
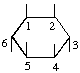
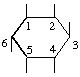
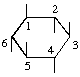
The fraction may be written with a horizontal or a sloping division line e.g. 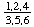 or 1,2,4/3,5,6-.
or 1,2,4/3,5,6-.
Examples (showing also which criterion of Recommendation I-4 was applied). As exceptions to general nomenclature, but in accord with carbohydrate nomenclature, ethers and esters of cyclitols may be named either by using prefixes such as 1-O-methyl, 1-O-acetyl, etc. or by adding 1-methyl ether, 1-acetate, etc., after the name of the polyol. For simplicity, only the former alternative is used in these examples.
| (i) | 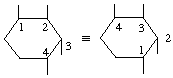 | 1L-1,2/3,4-Cyclohexanetetrol
(Note For allocation of D and L to these
and other enantiomers in the examples,
see Recommendation I-10.) | (11) |
| (i,ii) | 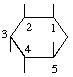 | 1D-1,2,5/3,4-Cyclohexanepentol | (12) |
| (i,iii) | 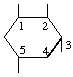 | 1L-1,2/3,5-Cyclohexanetetrol | (13) |
| (i,iv) | 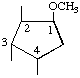 | 1D-1-O-Methyl-1,2/3,4-cyclopentanetetrol | (14) |
| (iv) | 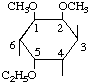 | 1L-5-O-Ethyl-1,2-di-O-methyl-neo-inositol
(Note numbers 1,2,5 for substituents
are lower than 2,3,5 or 2,4,5 or 2,5,6) | (15) |
| (iv,v) | 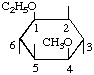 | 1L-1-O-Ethyl-4-O-methyl-muco-inositol | (16) |
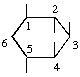
| (i,vi) | 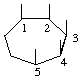 | 1,2,3,4,5/0-Cycloheptanepentol | (17) |
| (ii,vi) | 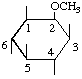 | 2-O-Methyl-myo-inositol | (18) |
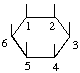
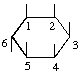
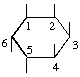
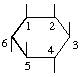
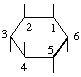
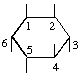

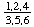 or 1,2,4/3,5,6-.
or 1,2,4/3,5,6-.