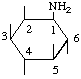
 | 1D-1-Amino-1-deoxy-myo-inositol | (19) |
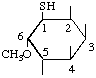 | 1L-1-Deoxy-1-mercapto-6-O-methyl-chiro-inositol 1 | (20) |
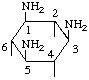 | 1,3,5-Triamino-l,3,5-trideoxy-scyllo-inositol | (21) |
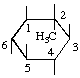 | 2-C-Methyl-myo-inositol 2 | (22) |
I-6. Trivial Names
x-Amino-x-deoxyinositols are termed generically 'inosamines': individual compounds of this group are named according to Recommendation I-7.1.
I-7. Systematic Nomenclature
I-7.1. If one, two or three hydroxyl groups of an inositol are replaced by other univalent substituents with retention of configuration, and if, according to the IUPAC 1971 Rules for the Nomenclature of Organic Chemistry, Section C [9], these substituents need not be named as suffixes, the compound is regarded as a substituted inositol and the 'deoxy' nomenclature is used. The configurational prefix and the numbering of the parent inositol are retained. (For cyclitols, the most important part of the order of decreasing priority for citation as suffix is: COOH and modified COOH, =O, OH. SH, NH2.) When this leaves alternatives criteria (iv) to (vi) of Recommendation I-4 are applied.
If a substituent that must be named as a suffix is present, the compound is named according to Recommendations I-8 and I-9.
I-7.2. Inositol derivatives in which one carbon atom carries a substituent additional to a hydroxyl are named as substituted inositols. provided that the substituent does not rank above hydroxyl for citation as suffix. (If it does, Recommendation I-9.2 applies.) For the disubstituted position in such compounds the configurational prefix refers to the hydroxyl group.
Examples:
 | 1D-1-Amino-1-deoxy-myo-inositol | (19) |
 | 1L-1-Deoxy-1-mercapto-6-O-methyl-chiro-inositol 1 | (20) |
 | 1,3,5-Triamino-l,3,5-trideoxy-scyllo-inositol | (21) |
 | 2-C-Methyl-myo-inositol 2 | (22) |
Notes
1. IUPAC Rule C-502 is compatible with a name 1L-6-O-methyl-1-thio-chiro-inositol but that name does not accord with the instruction in Recommendation I-7.1 to use the 'deoxy' nomenclature.2. The prefix C- is added to denote substitutions on carbon in accordance with carbohydrate nomenclature [11].)
Other Cyclitols
I-8. Trivial Names
The 2,3,4,5,6-pentahydroxycyclohexanones are termed generically 'inososes'; the individual compounds are named according to Recommendation I-9. The trivial name (-)-quinic or L-quinic acid is preferred for the following compound (see the last example to Recommendation I-9):
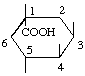 | (-)-Quinic acid; L-Quinic acid; 1L-1(OH),3,4/5-Tetrahydroxycyclohexanecarboxylic acid | (23) |
I-9. Systematic Nomenclature
I-9.1. Cyclitols containing substituents other than hydroxyl or modified hydroxyl (excepting inositols covered by Recommendation I-7) are named and numbered according to the above-mentioned IUPAC Rules, with the proviso that O-substituents are named as such (see Recommendation I-3). Substituted hydroxyl groups are not named as alkoxy, aryloxy or acyloxy groups. When these rules leave alternatives available, the criteria (ii) to (vi) of Recommendation I-4 are applied. Relative configuration is indicated as prescribed in Recommendation I-5.1. Except when it is serving as a locant set, the fraction describing the configuration is placed in parentheses, and it becomes the first element in the name, except for the configurational prefix (Recommendation I-10).
Notes
The IUPAC Rule C-10 [9] provides that one type of group be chosen as suffix, named as suffix, and given the lowest possible number(s), the remaining types prefixes. For choice of suffix, see the penultimate (parenthetical) sentence in Recommendation I-7.1.When there are substituents other than hydroxyl groups, it is usually impracticable to use the fraction describing the relative configuration as a list of locants. In stipulating that, in such cases, the fraction be placed in front of the complete name of the compound (including O-substituents), the present Recommendations differ from the Tentative Rules.
I-9.2. Cyclitol derivatives in which one carbon atom carries a substituent additional to hydroxyl are named (a) as substituted cycloalkanepolyols or (b) as hydroxy derivatives, according as a substituent (a) does not or (b) does rank above hydroxyl for citation as suffix. When the Recommendation leaves alternatives available, the criteria (ii) to (vi) of Recommendation I-4 are applied. For the disubstituted positions in such compounds, the fractional prefix refers to the hydroxyl group and this may be specified for clarity where necessary.
Note
Replacement of the hydrogen of amino, mercapto or hydroxyl groups by other atoms or groups does not change the numbering of cyclitol derivatives except when it affects criterion (iv) or (v) of Recommendation I-4. However. the IUPAC Rules [9] require that the ring carbon atom carrying as a substituent a trisubstituted ammonio (R3N+), acid, oxo, cyano or acyl group, or a derivative thereof, receive the locant 1; such cases will be relatively rare in cyclitol chemistry A convement alternative for 'onium' salts is to use the terminology exemplified by methiodide, hydrochloride, sulfate. etc.
Examples:
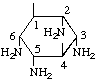 | (1,4/2,3,5,6)-2,3,5,6-Tetraamino-1,4-cyclohexanediol | (24) |
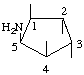 | (1,4,5/2,3)-5-Amino-1,2,3,4-cyclopentanetetrol | (25) |
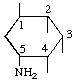 | 1L-(1/2,3,4,5)-5-Amino-1,2,3,4-cyclohexanetetrol | (26) |
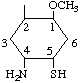 | 1D-(1,2/4,5)-4-Amino-5-mercapto-1-O-methyl-1,2-cyclohexanediol | (27) |
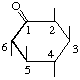 | 2L-2,3,5/4,6-Pentahydroxycyclohexanone | (28) |
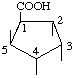 | 1L-(1,5/2,3,4)-2,3,4,5-Tetrahydroxycyclopentanecarboxylic acid | (29) |
 | 1L-1(OH),3,4/5-Tetrahydroxycyclohexanecarboxylic acid (L-quinic acid) (this numbering is opposite to former usage) | (23) |
I-10. Absolute Configuration
The absolute configuration of a cyclitol is specified by making a vertical Fischer-Tollens type of projection of the structure, with C-1 at the top and with C-2 and C-3 on the front edge of the ring. The configuration is then designated as D if the hydroxyl group at the lowest-numbered chiral center (or other substituent if no hydroxyl group is present there) projects to the right, and as L if it projects to the left (cf. Fig. 1). The prefix D or L, followed by a hyphen, is written before the name of the compound and may be preceded by the locant of the defining center. Racemic compounds are designated by the prefix DL.
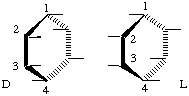
Fig. 1
Notes
a) The mere absence of a prefix D, L, or DL indicates that the compound has a meso-configuration; thus, the prefix D, L, or DL should not be omitted.b) A simple way of applying this Recommendation is as follows: when the formula is drawn in such a way that the substituent on the lowest-numbered asymmetric carbon atom is above the plane of the ring, and the numbering is clockwise, the compound is ; if anti-clockwise, it is D [see examples (8) and (9)].
1D-chiro-Inositol (8) 1L-chiro-Inositol (9) c) In a great majority of cases the lowest-numbered chiral center is position 1, so that it would be reasonable that D or L should be preceded by the locant of the defining center only when it is not 1. However, according to another nomenclature system for cyclitols [5] and also for the related carbohydrate field, the symbols D and L are assigned to the highest-numbered chiral center, which sometimes gives symbols different from those assigned according to Recommendation I-10. It is, therefore, recommended that the numeral 1 be included (as in these Recommendations).
d) Small Roman capital letters should be used in print for D and L. For compounds containing cyclitols and protein or carbohydrate residues,
Dc andLc (c for cyclitol) may be used alongsideDs ,Ls ,Dg , andLg [12]. Cyclitol nomenclature may also be combined with the use of the sequence rule [2, 3] or the stereospecific numbering (sn) system [13], where necessary.e) When many hydroxyl groups are replaced by other substituents, it may be simpler to use the sequence rule [2, 3]. Sequence-rule examples are given in Table 1, also in ref. [2, 3].
Examples:
Many examples of chiral compounds are named in the preceding examples [e.g. (11)-(16), (26)-(29)]. The following are additional.
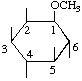 | 1D-1-O-Methyl-myo-inositol [D-(-)-Bornesitol] | (30) |
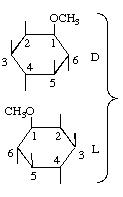 | DL-1-O-Methyl-myo-inositol | (31) |
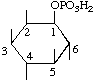 | 1D-myo-Inositol 1-(dihydrogen phosphate) | (32) |
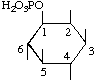 | 1L-myo-Inositol 1-(dihydrogen phosphate) | (33) |
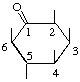 | 2L-2,3,4,6/5-Pentahydroxycyclohexanone | (34) |
l. IUPAC Commission on the Nomenclature of Organic Chemistry and IUPAC-IUB Commission on Biochemical Nomenclature (1968) IUPAC Inf. Bull. 32, 51; see also Eur. J. Biochem, 5, 1 (1968); Arch. Biochem. Biophys. 128, 269 (1968); J. Biol. Chem. 243, 5809 (1968); Biochem. J. 112, 17 (1969); Biochim. Biophys. Acta, 165, 1 (1968); German language version in Hoppe-Seyler's Z. Physiol. Chem. 350, 523 (1969); French language version in Bull. Soc. Chim. Biol. 51, 3 (1969).
2. Cahn. R. S., Ingold. C. & Prelog, V. (1966) Angew. Chem. 78, 413; Angew. Chem. Int. Ed. 5, 385.
3. International Union of Pure and Applied Chemistry (1970) J. Org. Chem. 35, 2849; see also Eur. J. Biochem. 18, 151 (1971).
4. Maquenne, L. (1900) Les sucres et leurs principaux dèrivés, Ganthiers-Villars, Paris; see also Carrè, G. & Naud, C. (1900) Paris.
5. Fletcher, H. G., Jr, Anderson, L. & Lardy, H. A. (1951) J. Org. Chem. 16, 1238.
6. Angyal, S. J. & Gilham. P. T. (1957) J. Chem. Soc. (Lond.) 3691.
7. Posternak, T. (1965) The Cyclitols, Hermann, Paris.
8. McCasland, G. E. (1965) Adv. Carbohydr. Chem. 20, 11.
9. International Union of Pure and Applied Chemistry (1971) Definitive Rules for Nomenclature of Organic Chemistry, 2nd edn, Section C, Butterworths, London. [Now in a 1979 edition]
10. Hanson, K. R. (1966) J. Am. Chem. Soc. 88, 2731.
11. IUPAC Commission on the Nomenclature of Organic Chemistry and IUPAC-IUB Commission on Biochemical Nomenclature (1970) IUPAC Inf. Bull. Append. 7; see also Eur. J. Biochem. 21, 455 (1971). [1996 version see Pure Appl. Chem. 1996, 68, 1919-2008; Adv. Carbohydr. Chem. Biochem., 1997, 52, 43-177; Carbohydr. Res., 1997, 297, 1-90; J. Carbohydr. Chem., 1997, 16, 1191-1280.]
12. International Union of Pure and Applied Chemistry (1960) J. Am. Chem. Soc. 82, 5575. [1983 version see Biochem. J., 1984, 219, 345-373; Eur. J. Biochem., 1984, 138, 9-37; 1985, 152, 1; 1993, 213, 2; Internat. J. Pept. Prot. Res., 1984, 24, following p 84; J. Biol. Chem., 1985, 260,14-42; Pure Appl. Chem., 1984, 56, 595-624; Amino Acids and Peptides, 1985, 16, 387-410; Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pages 39-69.]
13. IUPAC-IUB Commission on Biochemical Nomenclature (1967) Eur. J. Biochem. 2, 127 (1967). [1976 version see Biochem. J., 1978, 171, 21-35; Chem. Phys. Lipids, 1978, 21, 159-173; Eur. J. Biochem., 1977, 79, 11-21; Hoppe-Seyler's Z. Physiol. Chem., 1977, 358, 617-631; J. Lipid Res., 1978, 19, 114-128; Lipids, 1977, 12, 455-468; Mol. Cell. Biochem., 1977, 17, 157-171; Biochemical Nomenclature and Related Documents, 2nd edition, Portland Press, 1992, pages 180-190.
Return to main IUPAC nomenclature home page
Return to main IUBMB nomenclature home page
Return to Biochemical Nomenclature Committees home page