IUPAC-IUBMB Joint Commission on Biochemical Nomenclature (JCBN)
https://iupac.qmul.ac.uk/flavonoid/
World Wide Web version prepared by G. P. Moss
School of Physical and Chemical Sciences, Queen Mary University of London,
Mile End Road, London, E1 4NS, UK
g.p.moss@qmul.ac.uk
The recommendations in this version are identical to those in the published document prepared by Amélia P. Rauter aprauter@fc.ul.pt in Pure Appl. Chem. 2018, 90(9), 1429-1486. [Copyright IUBMB and IUPAC]. Any comments, corrections or suggestions for a future edition should be e-mailed to g.p.moss@qmul.ac.uk.
Corrections to the published document have been marked by . When the cursor points to the red dot brief details will be displayed in a tooltip window. If you click on the red dot you will go to a list of all changes to the published recommendatons.
Task Group that prepared the document
Amélia P. Rauter* (Portugal), Marcus Ennis (UK), Karl-Heinz Hellwich (Germany), Bernardo J. Herold (Portugal), Derek Horton† (USA), Gerard P. Moss (UK) and Ida Schomburg(Germany)
Abstract: Flavonoid structures, found in nature or obtained by synthesis, may be very complex. These Recommendations provide a guide for flavonoid aglycone names. This will also allow the construction of the names for their polyglycosylated species with clarity and conciseness. A joint working party of IUPAC/IUBMB members has prepared these recommendations, which establish rules for the general nomenclature of flavonoids, providing examples of acceptable trivial names, and names derived from trivial names, together with semi-systematic and fully systematic names that follow the published IUPAC recommendations.
CONTENTS
Flv-1 Introduction
Flv-2 Flavonoids — Definition and conventions
Flv-2.1 Flavonoid classes with a C6-C3-C6 carbon framework
Flv-2.1.1 Flavonoids (in restricted sense)
Flv-2.1.1.1 Flavans
Flv-2.1.1.2 Flavones and 3-hydroxyflavones (flavonols)
Flv-2.1.1.3 Anthocyanidins/anthocyanins (anthocyanidin 3-glycosides)
Flv-2.1.2 Isoflavonoids
Flv-2.1.2.1 Isoflavans
Flv-2.1.2.2 Isoflavones
Flv-2.1.3 Neoflavonoids
Flv-2.1.3.1 Neoflavans
Flv-2.1.3.2 Neoflavones
Flv-2.1.4 Chalcones and dihydrochalcones
Flv-2.1.5 Aurones
Flv-2.1.6 Pterocarpans and their 3,4-didehydro derivatives (coumestans)
Flv-2.2 Rotenoids
Flv-2.3 Flavonolignans
Flv-2.4 Biflavonoids and other flavonoid oligomers
Flv-3 Guide to the construction of semi-systematic names
Flv-3.1 Flavans, isoflavans, neoflavans and compounds derived from them
Flv-3.1.1 Flavans
Flv-3.1.1.1 Flavan aglycones
Flv-3.1.1.2 Flavan glycosides
Flv-3.1.1.3 Flavanones
Flv-3.1.2 Isoflavans
Flv-3.1.3 Neoflavans
Flv-3.2 Flavones, isoflavones, neoflavones and compounds derived from them
Flv-3.2.1 Flavones
Flv-3.2.1.1 Flavone aglycones
Flv-3.2.1.2 Flavone glycosides
Flv-3.2.1.3 C-Glycosyl substituted flavones
Flv-3.2.1.4 Flavonols and flavonol glycosides
Flv-3.2.2 Isoflavones and isoflavone glycosides
Flv-3.2.2.1 Isoflavone aglycones
Flv-3.2.2.2 Isoflavone glycosides
Flv-3.2.3 Neoflavones and neoflavone glycosides
Flv-3.2.3.1 Neoflavone aglycones
Flv-3.2.3.2 Neoflavone glycosides.
Flv-3.3 Chalcones and dihydrochalcones
Flv-3.4 Aurones
Flv-3.4.1 Aurone aglycones
Flv-3.4.2 Aurone glycosides
Flv-3.5 Anthocyanidins and anthocyanidin 3-glycosides (anthocyanins)
Flv-3.5.1 Anthocyanidins
Flv-3.5.2 Anthocyanins
Flv-3.6 Flavonoids containing additional fused/spiro rings in their structures
Flv-3.6.1 Flavones
Flv-3.6.2 Chalcones
Flv-3.6.3 Aurones
Flv-3.6.4 Anthocyanidins/Anthocyanins
Flv-3.6.5 Pterocarpans
Flv-3.6.6 Rotenoids
Flv-3.7 Flavonolignans
Flv-3.8 Biflavonoids and other flavonoid oligomers
References
Annex 1. Flavonoid class names and characteristic structures
Annex 2. InChIs and InChIKeys for the flavonoids given as examples
In IUPAC nomenclature, three levels of nomenclature are accepted in the field of natural products [6–9]: (a) the trivial name, given for convenience to a new compound, carrying no (or minimal) structural information, and often derived from the biological origin of the material; (b) the systematic name, describing the full structure that comprises the skeleton, the characteristic groups, and the substituent groups; (c) the semi-systematic name, providing a simplified alternative to the systematic name, which often becomes too cumbersome to be continually inserted into the text of a publication. The semi-systematic name is normally achieved by the creation of one of the two possible ‘semi-systematic parents’: (a) the parent hydride or parent structure, which does not have terminal hetero atoms or functional groups and therefore consists only of skeletal atoms and hydrogen; (b) the functional parent, which is a structure that has certain terminal heteroatoms or groups. When applied, the Preferred IUPAC Name (PIN) [6] is also given. The PIN is a name assigned to a chemical substance and preferred among two or more names generated from two or more IUPAC recommendations, including the many synonyms that have been coined and used over the years. These recommendations establish rules for the general nomenclature of flavonoids, providing examples of acceptable trivial, semi-systematic, and fully systematic names that follow the published IUPAC recommendations, e.g. references [6–11]. Class names for flavonoids, their parent structures, and the order of citation of primed or unprimed locants are revisited and clarified. Names of O- and C-glycosylated derivatives follow established carbohydrate nomenclature [11]. Semi-systematic names are based on the parent structures or their functional parents. These are presented and illustrative examples are provided to avoid ambiguous interpretations.
The following carbon frameworks characterize the various flavonoid classes.
Flv-2.1 Flavonoid classes with a C6-C3-C6 carbon framework
The term flavonoid is also used in a restricted sense as comprising only those compounds with a C6-C3-C6 carbon framework exhibiting the structure of a chromane or that of a 1-benzopyran (PIN) (chromene), in which the fused benzene ring is designated as ring A and the 3,4-dihydro-2H-pyran or the pyran as ring C, along with a (substituted) phenyl group (ring B) on ring C.
Depending on the position of the linkage of ring B to the chromane/1-benzopyran (chromene) moiety, three different classes can be assigned: flavonoids (Flv-2.1.1), isoflavonoids (Flv-2.1.2) and neoflavonoids (Flv-2.1.3).
The other flavonoid classes that have a structure with a C6-C3-C6 carbon framework are the chalcones (Flv-2.1.4), the aurones (Flv-2.1.5), and the pterocarpans and their 3,4-didehydro derivatives (coumestans) (Flv-2.1.6).
Flv-2.1.1 Flavonoids (in restricted sense)
Flavonoids (in restricted sense) include flavans (1) as well as compounds of type 1 with one or two additional double bonds, and carbonyl or hydroxy groups or both in ring C, used to classify flavonoids into different categories, such as flavones, flavonols, and anthocyanidins.
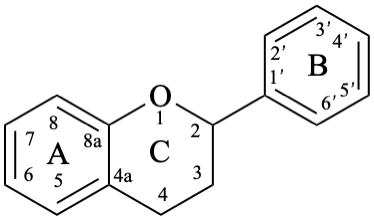
Flavans are compounds with a 2-phenyl-3,4-dihydro-2H-1-benzopyran skeleton 1, that may be substituted, and include flavan-3-ols (compounds derived from 2-phenyl-3,4-dihydro-2H-1-benzopyran-3-ol skeleton 2) and flavan-4-ones (compounds derived from 2-phenyl-2,3-dihydro-4H-1-benzopyran-4-one skeleton 3), which are generically designated as flavanols and flavanones, respectively. Compounds derived from 2-phenyl-3,4-dihydro-2H-1-benzopyran-3,4-diol skeleton 4 are flavan-3,4-diols, designated leukoanthocyanidins because these colourless compounds have their structure derived from that of an anthocyanidin (see Flv-2.1.1.3), specifically they are dehydronated 3,4-dihydroxy-1,2,3,4-tetrahydroanthocyanidins.
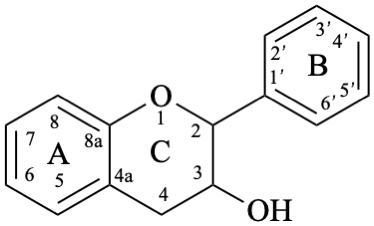
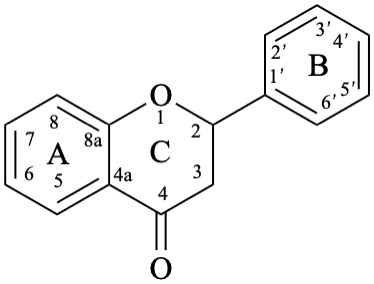
3
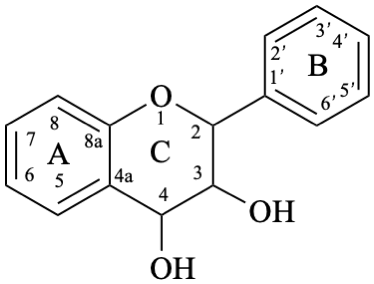
4
Flavones and 3-hydroxyflavones (flavonols) are compounds with a 2-phenyl-4H-1-benzopyran-4-one (2-phenyl-4H-chromen-4-one) skeleton 5. The term “flavonol” is used as a class name for compounds with a 3-hydroxy-2-phenyl-4H-1-benzopyran-4-one (3-hydroxy-2-phenyl-4H-chromen-4-one) skeleton 6.
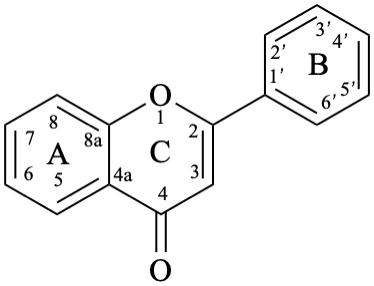
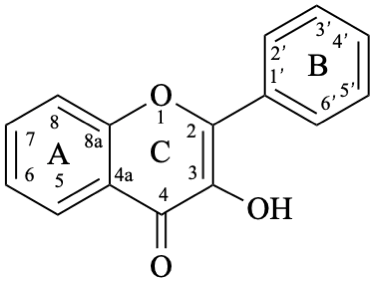
6
Anthocyanidins are compounds derived from a flavylium (2-phenyl-1λ4-benzopyran-1-ylium or 2-phenylchromenylium) ion 7. Anthocyanidins display colours from red to purple and, together with the 3-glycosides of anthocyanidin, the anthocyanins, represent a large group of plant pigments.
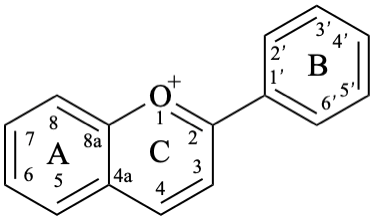
The isoflavonoids are compounds derived from the parent structure 3-phenyl-3,4-dihydro-2H-1-benzopyran (8).
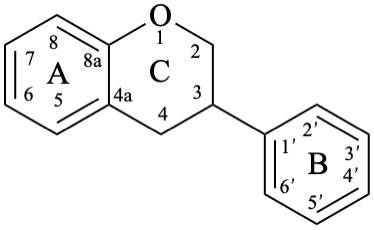
Isoflavans are compounds with a 3-phenyl-3,4-dihydro-2H-1-benzopyran skeleton 8. They may be substituted.
Flv-2.1.2.2 Isoflavones
Isoflavones are compounds with a 3-phenyl-4H-1-benzopyran-4-one (3-phenyl-4H-chromen-4-one) skeleton 9. They may be substituted.
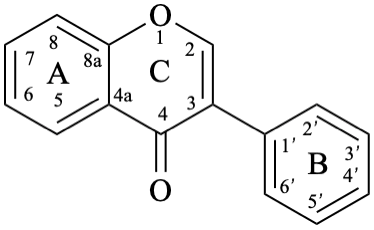
The neoflavonoids are compounds with a 4-phenyl-3,4-dihydro-2H-1-benzopyran parent structure 10.
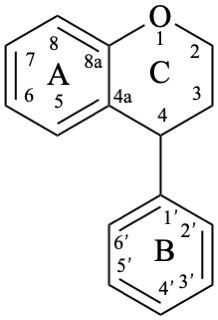
Neoflavans are compounds with a 4-phenyl-3,4-dihydro-2H-1-benzopyran skeleton 10. They may be substituted.
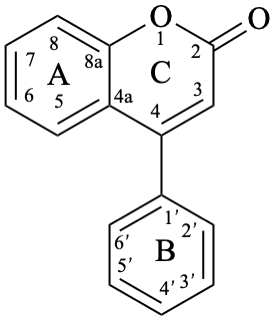
Flv-2.1.3.2 Neoflavones
Neoflavones are compounds with a 4-phenyl-2H-1-benzopyran-2-one (4-phenyl-2H-chromen-2-one) skeleton 11. They may be substituted. Neoflavones are also known as 4-phenylcoumarins.
Chalcones are compounds derived from (2E)-1,3-diphenylprop-2-en-1-one [chalcone (PIN), structure depicted as in 12a, 12b]. Some of them are intermediates in the biosynthesis of flavonoids, in which the pyran ring C has not yet been formed, and are therefore biogenetically and structurally related to them. Dihydrochalcones are compounds derived from 1,3-diphenylpropan-1-one (PIN, compound 13).
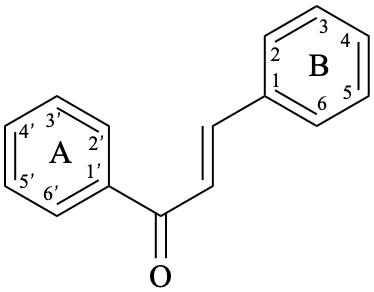
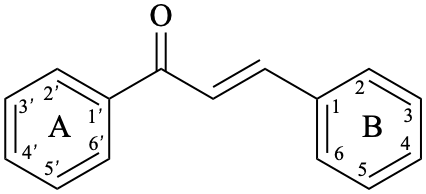
12b
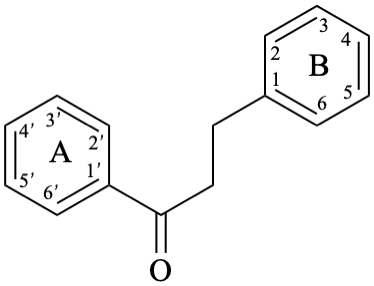
13
Flv-2.1.5 Aurones
Aurones are compounds derived from (2Z)-2-benzylidene-1-benzofuran-3(2H)-one [alternative name: (2Z)-2-(phenylmethylidene)-1-benzofuran-3(2H)-one] (aurone, compound 14).
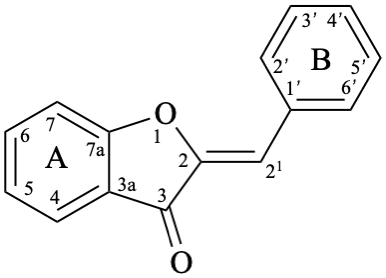
Pterocarpans are compounds derived from (6aR,11aR)-6a,11a-dihydro-6H-[1]benzofuro[3,2-c][1]benzopyran (15), and their 6a,11a-didehydro derivatives 16 are named coumestans. They are numbered systematically following the numbering rules for fused ring systems (see P-25.3.3 [6] and FR-5 [10]).
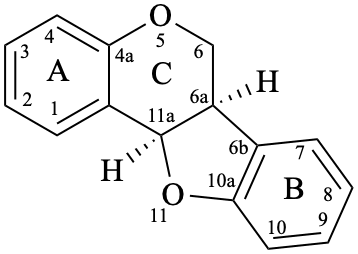
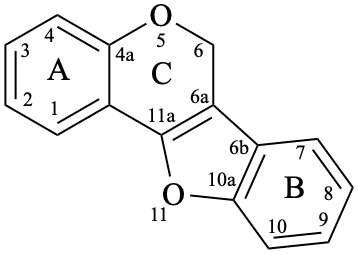
16
Rotenoids comprise rotenanes, which are compounds with a (6aS,12aS)-6,6a,12,12a-tetrahydro[1]benzopyrano[3,4-b][1]benzopyran skeleton 17b, and rotenones, with a (6aS,12aS)-6a,12a-dihydro[1]benzopyrano[3,4-b][1]benzopyran-12(6H)-one skeleton 18. They may be substituted. Since the name rotenone has been used not only for the functional parent 18, but also for a more substituted specific compound (see Example 56 in Flv-3.6.6), names based on rotenone are no longer acceptable. Rotenoids are numbered as in 17a when named as rotenane derivatives, but when named systematically, the numbering rules for fused ring systems apply, as in 17b (see P-25.3.3 [6] and FR-5 [10]).
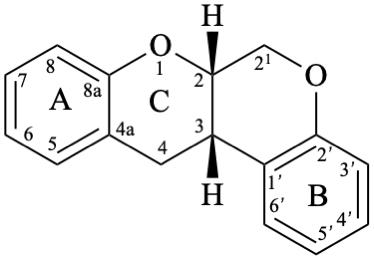
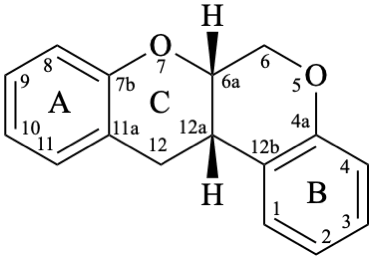
17b
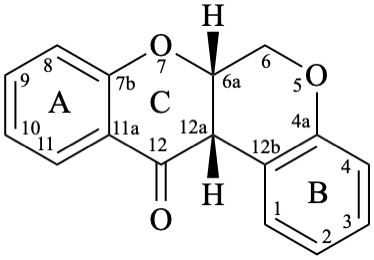
18
Flavonolignans have structures based on flavonoids condensed with C6-C3 lignan precursors (see Flv-3.7).
Flv-2.4 Biflavonoids and other flavonoid oligomers
Biflavonoid and other flavonoid oligomers, which occur widely in nature, are exemplified and named in Flv-3.8.
Locant sets are arranged in ascending order, i.e. primed locants are placed immediately after the corresponding unprimed locants (P-14.3.5 in [6] and R-0.2.4.2 in [7]). Example: 2′,3,4,4′,6,7 (not 3,4,6,7,2′,4′).
Flv-3.1 Flavans, isoflavans, neoflavans and compounds derived from them
Names of flavans, isoflavans, and neoflavans can be derived from the name of the respective parent hydride (Fig. 1). The presence of a characteristic group is denoted by a prefix or by a suffix attached to the parent name. The suffix is used for the principal characteristic group(s) according to the priority order given in R-4.1 [7], and P-33 and P-41 [6].
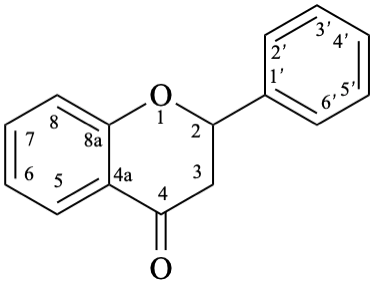
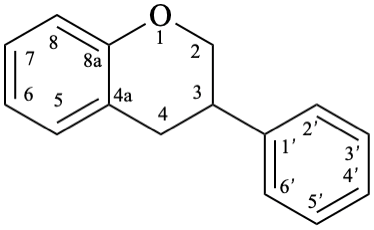
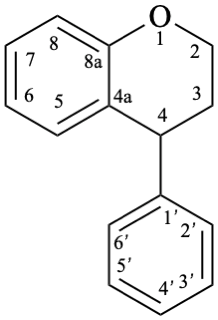
8
10
Fig. 1: Parent hydrides flavan (1), isoflavan (8), and neoflavan (10).
Flv-3.1.1.1 Flavan aglycones
Example 1:
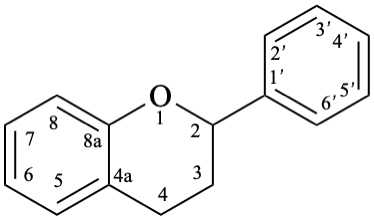
Semi-systematic name
flavan
Systematic names
2-phenyl-3,4-dihydro-2H-1-benzopyran
2-phenylchromane
2-phenyl-3,4-dihydro-2H-chromene
Example 2:
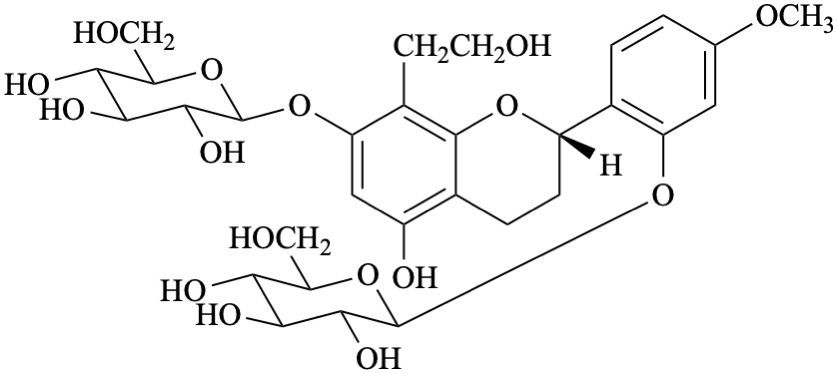
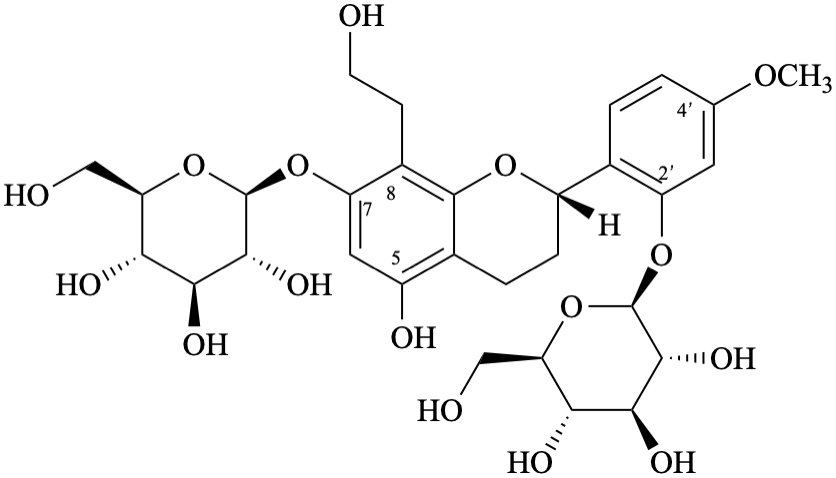
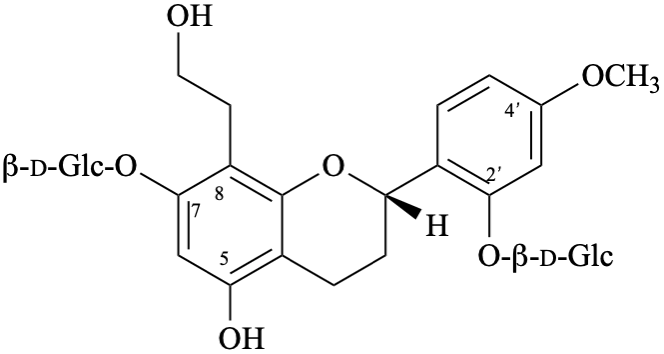
Systematic names
2-[(2S)-7-(β-D-glucopyranosyloxy)-5-hydroxy-8-(2-hydroxyethyl)-3,4-dihydro-2H-1-benzopyran-2-yl]-5-methoxyphenyl β-D-glucopyranoside
2-[(2S)-7-(β-D-glucopyranosyloxy)-5-hydroxy-8-(2-hydroxyethyl)-3,4-dihydro-2H-chromen-2-yl]-5-methoxyphenyl β-D-glucopyranoside
(2S)-7-(β-D-glucopyranosyloxy)-2-[2-(β-D-glucopyranosyloxy)-4-methoxyphenyl]-8-(2-hydroxyethyl)-3,4-dihydro-2H-1-benzopyran-5-ol
(2S)-7-(β-D-glucopyranosyloxy)-2-[2-(β-D-glucopyranosyloxy)-4-methoxyphenyl]-8-(2-hydroxyethyl)chroman-5-ol
(2S)-7-(β-D-glucopyranosyloxy)-2-[2-(β-D-glucopyranosyloxy)-4-methoxyphenyl]-8-(2-hydroxyethyl)-3,4-dihydro-2H-chromen-5-ol
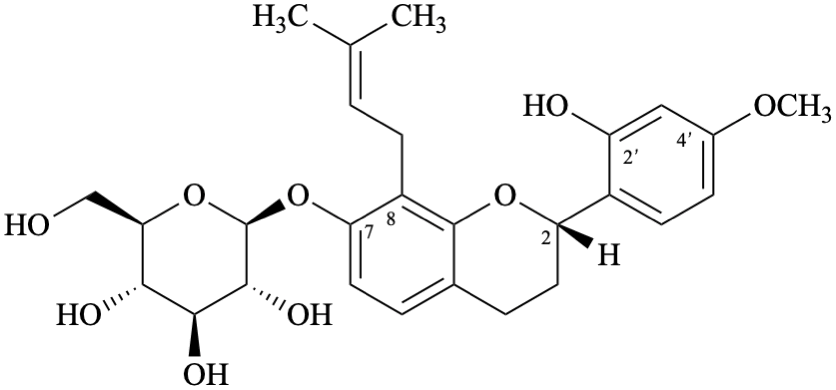
Semi-systematic name
(2S)-7-(β-D-glucopyranosyloxy)-4′-methoxy-8-(3-methylbut-2-en-1-yl)flavan-2′-ol
(2S)-2′-hydroxy-4′-methoxy-8-(3-methylbut-2-en-1-yl)flavan-7-yl β-D-glucopyranoside
Systematic names
(2S)-2-(2-hydroxy-4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-3,4-dihydro-2H-1-benzopyran-7-yl β-D-glucopyranoside
(2S)-2-(2-hydroxy-4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)chroman-7-yl β-D-glucopyranoside
(2S)-2-(2-hydroxy-4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-3,4-dihydro-2H-chromen-7-yl β-D-glucopyranoside
2-[(2S)-7-(β-D-glucopyranosyloxy)-8-(3-methylbut-2-en-1-yl)-3,4-dihydro-2H-1-benzopyran-2-yl]-5-methoxyphenol
2-[(2S)-7-(β-D-glucopyranosyloxy)-8-(3-methylbut-2-en-1-yl)chroman-2-yl]-5-methoxyphenol
2-[(2S)-7-(β-D-glucopyranosyloxy)-8-(3-methylbut-2-en-1-yl)-3,4-dihydro-2H-chromen-2-yl]-5-methoxyphenol
Example 4:
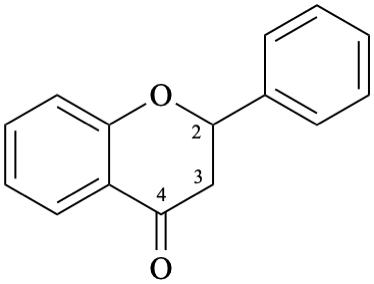
Semi-systematic name
flavan-4-one
Systematic names
2-phenyl-2,3-dihydro-4H-1-benzopyran-4-one
2-phenylchroman-4-one
2-phenyl-2,3-dihydro-4H-chromen-4-one
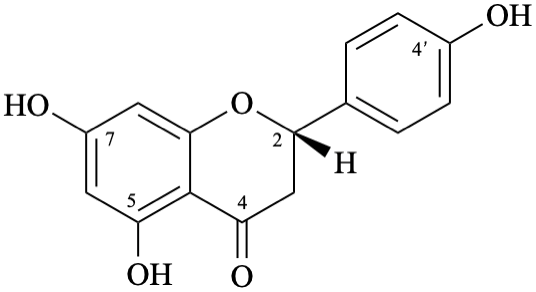
Semi-systematic name
(2S)-4′,5,7-trihydroxyflavan-4-one
Trivial name
naringenin
Systematic names
(2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-1-benzopyran-4-one
(2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)chroman-4-one
(2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-chromen-4-one
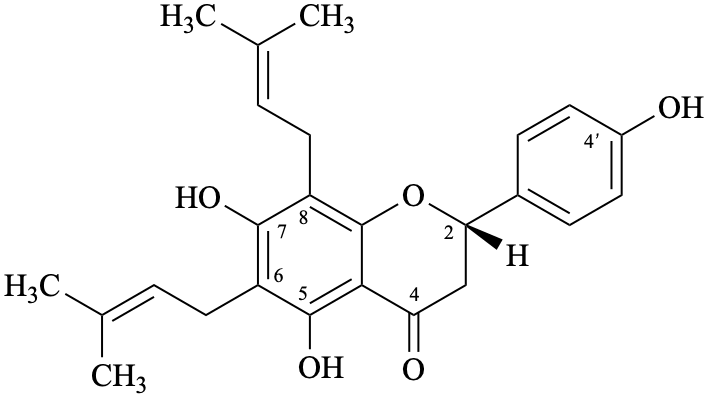
Semi-systematic name
(2S)-4′,5,7-trihydroxy-6,8-bis(3-methylbut-2-en-1-yl)flavan-4-one
Trivial names
6,8-bis(3-methylbut-2-en-1-yl)naringenin
6,8-diprenylnaringenin
lonchocarpol A
senegalensin
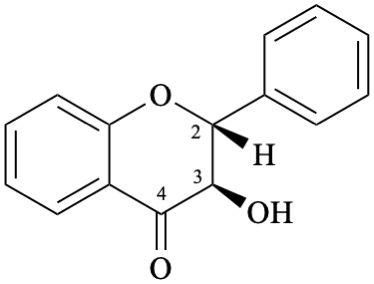
Semi-systematic name
(2R,3R)-3-hydroxyflavan-4-one
Not: flavanon-3-ol
(see P-41, table 4.1 in the 2013 IUPAC recommendations [6], R-4.1, table 10 in [7] and C-0.0 (2) (b) in the 1979 recommendations [8]).
Systematic names
(2R,3R)-3-hydroxy-2-phenyl-2,3-dihydro-4H-1-benzopyran-4-one
(2R,3R)-3-hydroxy-2-phenylchroman-4-one
(2R,3R)-3-hydroxy-2-phenyl-2,3-dihydro-4H-chromen-4-one
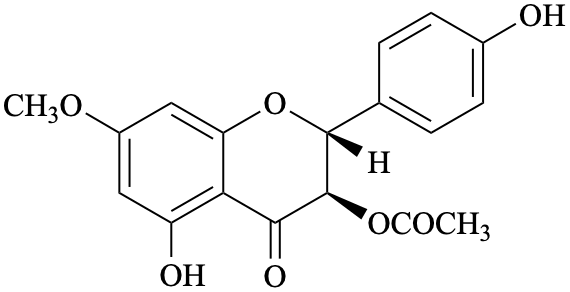
Semi-systematic name
(2R,3R)-4′,5-dihydroxy-7-methoxy-4-oxoflavan-3-yl acetate
Not: (2R,3R)-4′,5-dihydroxy-7-methoxyflavanone 3-acetate
Trivial names
7-O-methylaromadendrin 3-acetate
Not: 3-O-acetyl-7-O-methylaromadendrin
Systematic names
(2R,3R)-5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-4-oxo-3,4-dihydro-2H-1-benzopyran-3-yl acetate
(2R,3R)-5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-4-oxochroman-3-yl acetate
(2R,3R)-5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-4-oxo-3,4-dihydro-2H-chromen-3-yl acetate
Example 9:
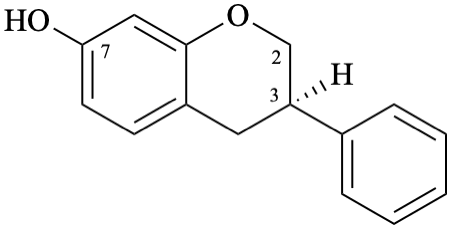
Semi-systematic name
(3R)-isoflavan-7-ol
Systematic names
(3R)-3-phenyl-3,4-dihydro-2H-1-benzopyran-7-ol
(3R)-3-phenylchroman-7-ol
(3R)-3-phenyl-3,4-dihydro-2H-chromen-7-ol
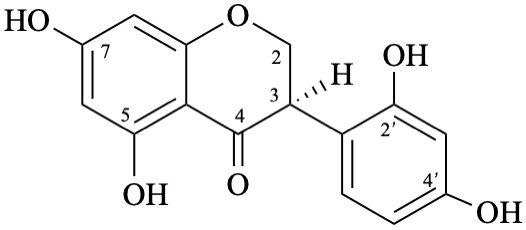 | + | 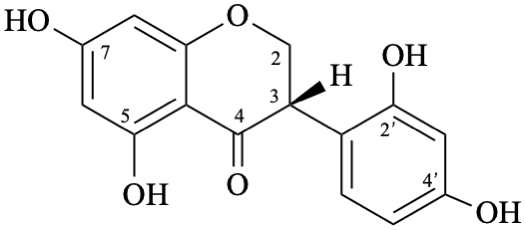 | |
| (1:1) |
Semi-systematic name
rac-2′,4′,5,7-tetrahydroxyisoflavan-4-one
Trivial name
dalbergioidin
Systematic names
rac-3-(2,4-dihydroxyphenyl)-5,7-dihydroxy-2,3-dihydro-4H-1-benzopyran-4-one
rac-3-(2,4-dihydroxyphenyl)-5,7-dihydroxychroman-4-one
rac-3-(2,4-dihydroxyphenyl)-5,7-dihydroxy-2,3-dihydro-4H-chromen-4-one
Example 11:
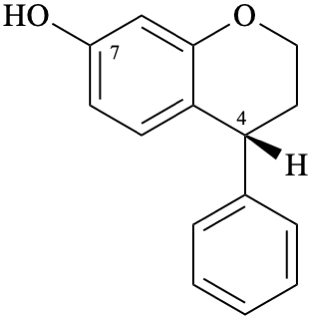
Semi-systematic name
(4R)-neoflavan-7-ol
Systematic names
(4R)-4-phenyl-3,4-dihydro-2H-1-benzopyran-7-ol
(4R)-4-phenylchroman-7-ol
(4R)-4-phenyl-3,4-dihydro-2H-chromen-7-ol
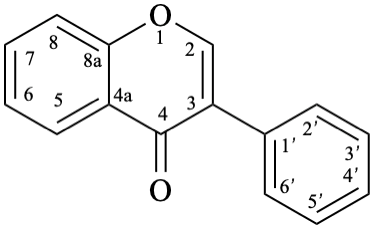
Names of flavones, isoflavones, and neoflavones can be derived from the names of the respective functional parents (Fig. 2), which already contain one carbonyl group as an implied suffix. When substituents are among those characteristic groups with a lower priority than ketones, they have to be cited as prefixes, according to table 10, Section R-4.1 [7].
When the principal characteristic group has a higher priority than a ketone, according to table 10, Section R-4.1 [7] (see also table 3.3 in P-33.2 and table 4.1 in P-41 [6]), names of flavones, isoflavones, and neoflavones are derived from the respective flavan, isoflavan, or neoflavan parent hydride. Such groups are radicals, ions, acids, acid derivatives (anhydrides, esters, acyl halides, amides), nitriles, aldehydes, and chalcogen analogues of aldehydes.
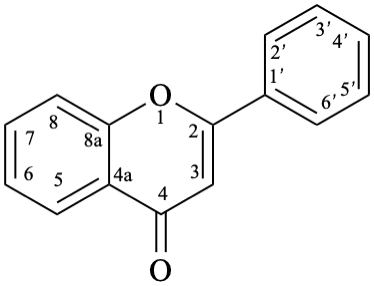
9
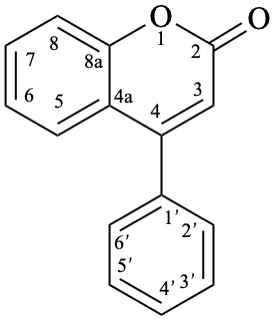
11
Fig. 2: Functional parents flavone (5), isoflavone (9), and neoflavone (11).
Flv-3.2.1 Flavones
Flv-3.2.1.1 Flavone aglycones
Example 12:
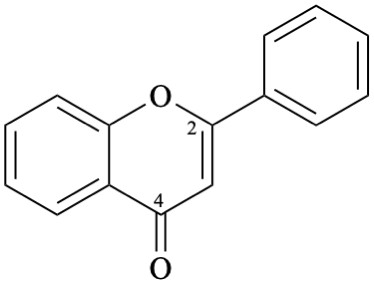
Semi-systematic name
flavone
Systematic names
2-phenyl-4H-1-benzopyran-4-one
2-phenyl-4H-chromen-4-one
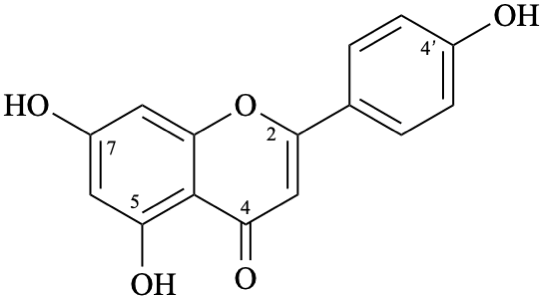
Semi-systematic name
4′,5,7-trihydroxyflavone
Trivial name
apigenin
Systematic names
5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one
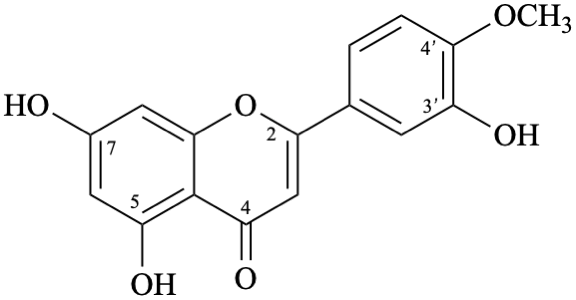
Semi-systematic name
3′,5,7-trihydroxy-4′-methoxyflavone
Trivial name
diosmetin
Systematic names
5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one
5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-chromen-4-one
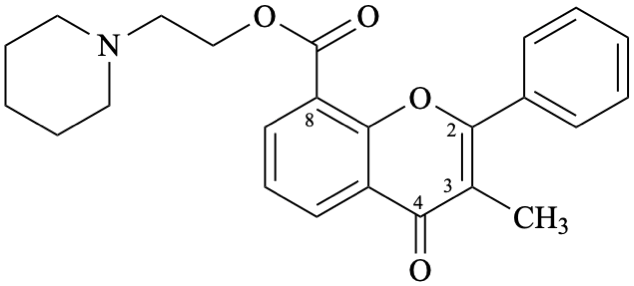
Semi-systematic name
2-(piperidin-1-yl)ethyl 3-methyl-4-oxoflav-2-ene-8-carboxylate
Not: 2-(piperidin-1-yl)ethyl 3-methyl-4-oxo-2,3-didehydroflavan-8-carboxylate (RF-8.1 [9]).
Trivial name
flavoxate
Example 16:
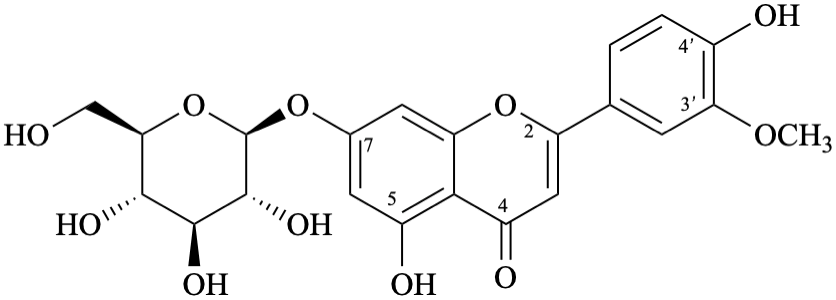
Semi-systematic name
7-(β-D-glucopyranosyloxy)-4′,5-dihydroxy-3′-methoxyflavone
Trivial name
7-O-(β-D-glucopyranosyl)chrysoeriol
Systematic names
5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-4-oxo-4H-1-benzopyran-7-yl β-D-glucopyranoside
5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-4-oxo-4H-chromen-7-yl β-D-glucopyranoside
7-(β-D-glucopyranosyloxy)-5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-4H-1-benzopyran-4-one
7-(β-D-glucopyranosyloxy)-5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-4H-chromen-4-one
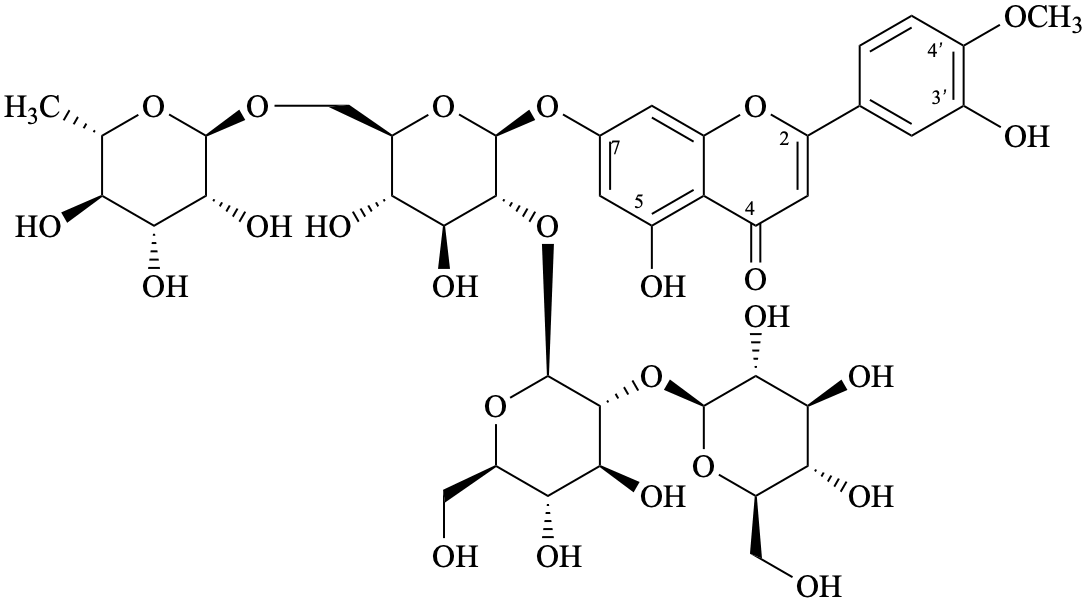
Semi-systematic name
7-{β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranosyloxy}-3′,5-dihydroxy-4′-methoxyflavone
Trivial name
7-O-{β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranosyl}diosmetin
Not: diosmetin 7-{β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranoside}
(because it specifies the oxygen atom at position 7 twice as diosmetin has a 7-OH and the term glucopyranoside includes both oxygen atoms of the anomeric acetal function.
Systematic names
5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-oxo-4H-1-benzopyran-7-yl 6-deoxy-α-L-mannopyranosyl-(1→6)-[β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl-(1→2)]-β-D-glucopyranoside
5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-oxo-4H-chromen-7-yl 6-deoxy-α-L-mannopyranosyl-(1→6)-[β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl-(1→2)]-β-D-glucopyranoside
7-{6-deoxy-α-L-mannopyranosyl-(1→6)-[β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl-(1→2)]-β-D-glucopyranosyloxy}-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one
7-{6-deoxy-α-L-mannopyranosyl-(1→6)-[β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl-(1→2)]-β-D-glucopyranosyloxy}-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-chromen-4-one
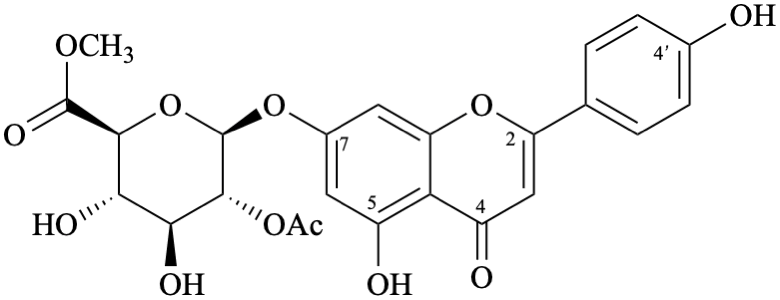
Semi-systematic name
methyl (4′,5-dihydroxy-4-oxoflav-2-en-7-yl 2-O-acetyl-β-D-glucopyranosid)uronate
Not: methyl (4′,5-dihydroxy-4-oxo-2,3-didehydroflavan-7-yl 2-O-acetyl-β-D-glucopyranosid)uronate (RF-8.1 in [9])
Not: methyl (4′,5-dihydroxyflavon-7-yl 2-O-acetyl-β-D-glucopyranosid)uronate (rule C-0.0 (2) (b) in the 1979 recommendations [8]).
Trivial name
7-O-(methyl 2-O-acetyl-β-D-glucopyranosyluronate)apigenin
Not: methyl [apigenin 7-(2-O-acetyl-β-D-glucopyranosiduronate)]
(because it specifies the oxygen atom at position 7 twice)
Compounds arising formally from the elimination of water from the anomeric hydroxy group and a hydrogen atom bound to a carbon atom (thus creating a C–C bond) are named using the appropriate glycosyl group [11]. In the past they have been frequently named as flavonoid C-glycosides, but this terminology is not recommended (2-Carb-33.7 [11]).
Example 19:
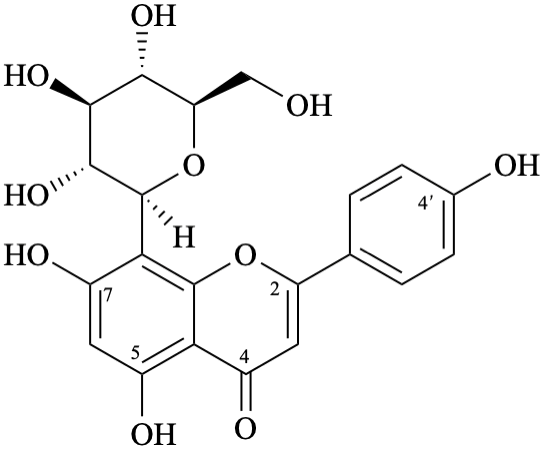
Semi-systematic name
8-(β-D-glucopyranosyl)-4′,5,7-trihydroxyflavone
Trivial names
vitexin
8-(β-D-glucopyranosyl)apigenin
Not: 8-C-(β-D-glucopyranosyl)apigenin,
because the substituent ‘β-D-glucopyranosyl’ is linked directly from its anomeric carbon to C-8 of the aglycone,
and the use of C to reinforce this C–C bond is not necessary.
Systematic names
8-(β-D-glucopyranosyl)-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
8-(β-D-glucopyranosyl)-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one
(1S)-1,5-anhydro-1-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-1-benzopyran-8-yl]-D-glucitol
(1S)-1,5-anhydro-1-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-8-yl]-D-glucitol
Example 20:
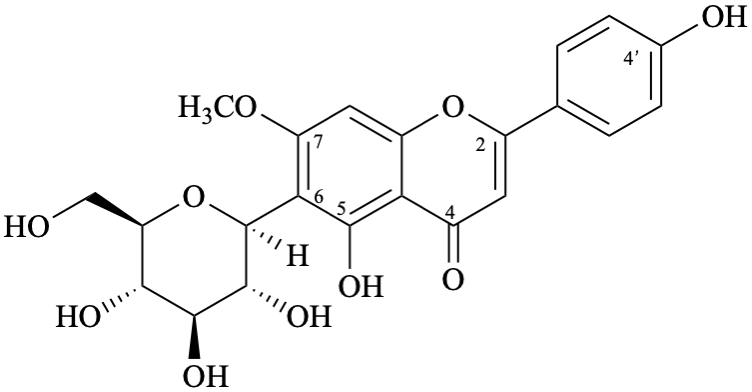
Semi-systematic name
6-(β-D-glucopyranosyl)-4′,5-dihydroxy-7-methoxyflavone
Trivial name
6-(β-D-glucopyranosyl)-7-O-methylapigenin
Systematic names
6-(β-D-glucopyranosyl)-5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-4H-1-benzopyran-4-one
6-(β-D-glucopyranosyl)-5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one
(1S)-1,5-anhydro-1-[5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-4-oxo-4H-1-benzopyran-6-yl]-D-glucitol
(1S)-1,5-anhydro-1-[5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-4-oxo-4H-chromen-6-yl]-D-glucitol
Example 21:
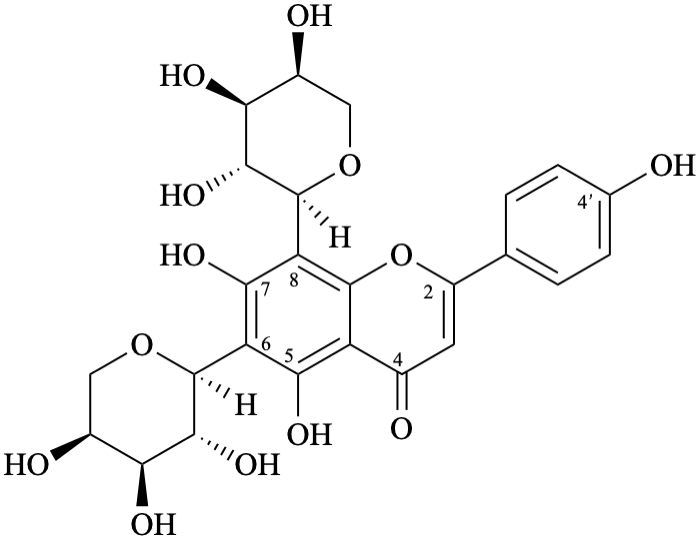
Semi-systematic name
6,8-di-(α-L-arabinopyranosyl)-4′,5,7-trihydroxyflavone
Trivial name
6,8-di-(α-L-arabinopyranosyl)apigenin
Not: apigenin 6,8-di-C-α-L-arabinopyranoside,
because an α-L-arabinopyranosyl substituent is linked by the anomeric carbon atom to C-6 and to C-8 an of apigenin.
The anomeric carbon is not linked to an oxygen atom, but to a carbon atom, and it is incorrect to name
this compound as a pyranoside that includes both oxygen atoms of the anomeric acetal function [11].
Systematic names
6,8-di-(α-L-arabinopyranosyl)-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
6,8-di-(α-L-arabinopyranosyl)-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one
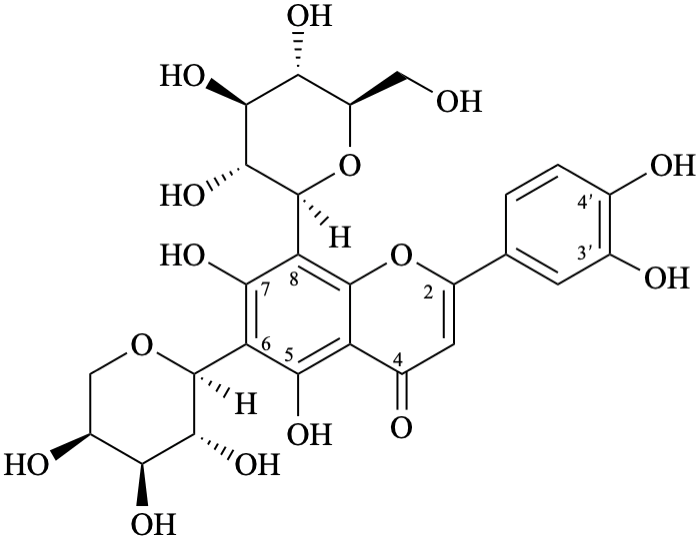
Semi-systematic name
6-(α-L-arabinopyranosyl)-8-(β-D-glucopyranosyl)-3′,4′,5,7-tetrahydroxyflavone
Trivial names
isocarlinoside
6-(α-L-arabinopyranosyl)-8-(β-D-glucopyranosyl)luteolin
Not: 6-C-(α-L-arabinopyranosyl)luteolin 8-β-D-glucopyranoside
(since it specifies an oxygen atom linked to position 8, because the term glucopyranoside includes both oxygen atoms of the anomeric acetal function).
Systematic names
6-(α-L-arabinopyranosyl)-2-(3,4-dihydroxyphenyl)-8-(β-D-glucopyranosyl)-5,7-dihydroxy-4H-1-benzopyran-4-one
6-(α-L-arabinopyranosyl)-2-(3,4-dihydroxyphenyl)-8-(β-D-glucopyranosyl)-5,7-dihydroxy-4H-chromen-4-one
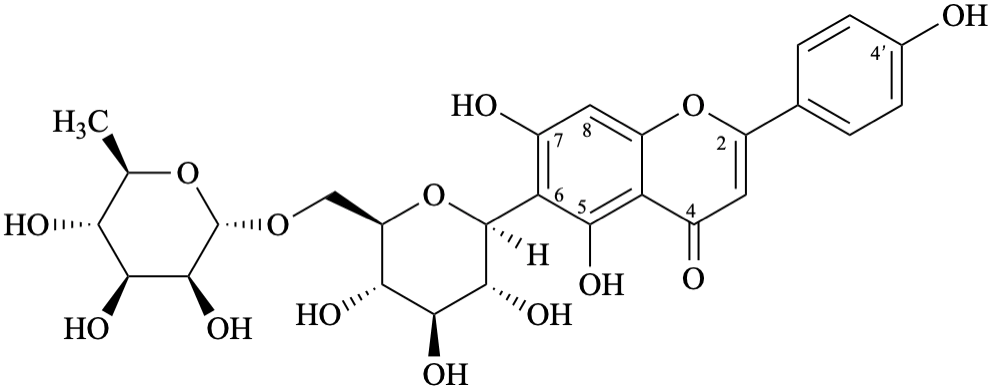
Semi-systematic name
4′,5,7-trihydroxy-6-[α-D-rhamnopyranosyl-(1→6)-β-D-glucopyranosyl]flavone
Trivial names
dulcinoside
6-[α-D-rhamnopyranosyl-(1→6)-β-D-glucopyranosyl]apigenin
Not: 6-C-[6′′-O-(α-D-rhamnopyranosyl)-β-D-glucopyranosyl]apigenin,
see the IUPAC Recommendations for Carbohydrate Nomenclature [11].
Apigenin does not have any hydroxy group at position 6, thus the anomeric carbon atom is linked to position 6 by a C–C bond and this should not be specified twice.
Systematic names
6-[6-deoxy-α-D-mannopyranosyl-(1→6)-β-D-glucopyranosyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
6-[6-deoxy-α-D-mannopyranosyl-(1→6)-β-D-glucopyranosyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one
(1S)-1,5-anhydro-6-O-(6-deoxy-α-D-mannopyranosyl)-1-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-1-benzopyran-6-yl]-D-glucitol
Example 24:
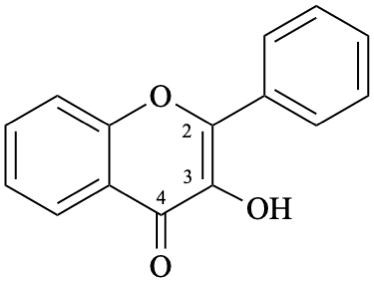
Semi-systematic name
3-hydroxyflavone
Systematic name
3-hydroxy-2-phenyl-4H-1-benzopyran-4-one
3-hydroxy-2-phenyl-4H-chromen-4-one
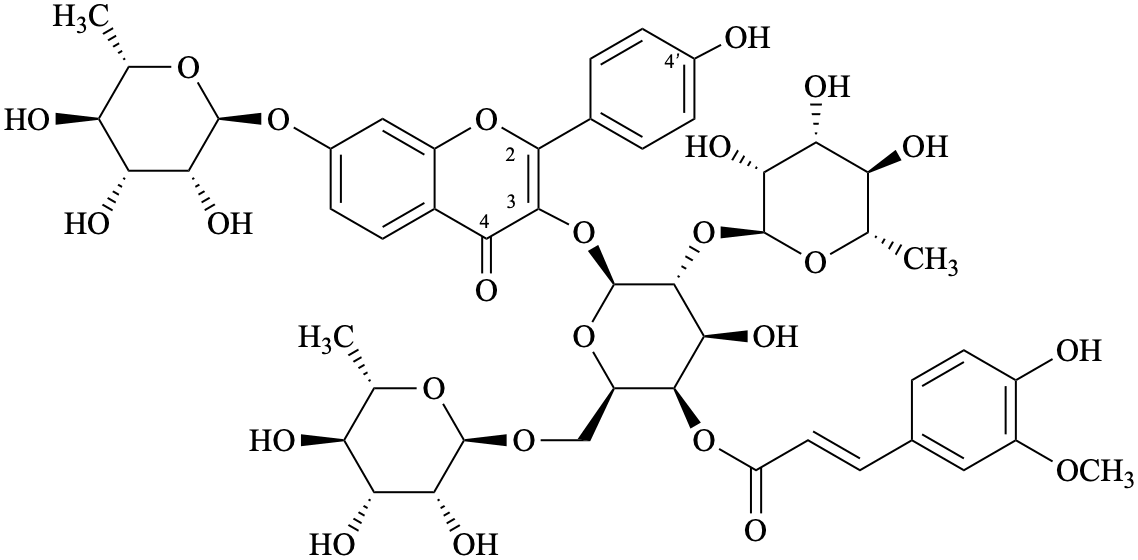
Semi-systematic name
4′-hydroxy-3-{4-O-[(2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoyl]-[α-L-rhamnopyranosyl-(1→2)]-[α-L-rhamnopyranosyl-(1→6)]-β-D-galactopyranosyloxy}-7-(α-L-rhamnopyranosyloxy)flavone
Systematic names
7-(6-deoxy-α-L-mannopyranosyloxy)-2-(4-hydroxyphenyl)-4-oxo-4H-1-benzopyran-3-yl [6-deoxy-α-L-mannopyranosyl-(1→r2)]-[6-deoxy-α-L-mannopyranosyl-(1→6)]-4-O-[(2E)-3-(4-hydroxy-3-methoxyphenyl)-prop-2-enoyl]-β-D-galactopyranoside
7-(6-deoxy-α-L-mannopyranosyloxy)-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-3-yl [6-deoxy-α-L-mannopyranosyl-(1→2)]-[6-deoxy-α-L-mannopyranosyl-(1→6)]-4-O-[(2E)-3-(4-hydroxy-3-methoxyphenyl)-prop-2-enoyl]-β-D-galactopyranoside
{7-(6-deoxy-α-L-mannopyranosyloxy)-2-(4-hydroxyphenyl)-4-oxo-4H-1-benzopyran-3-yl 4-deoxy-[6-deoxy-α-L-mannopyranosyl-(1→2)]-[6-deoxy-α-L-mannopyranosyl-(1→6)]-β-D-galactopyranosid-4-yl} (2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate
{7-(6-deoxy-α-L-mannopyranosyloxy)-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-3-yl 4-deoxy-[6-deoxy-α-L-mannopyranosyl-(1→2)]-[6-deoxy-α-L-mannopyranosyl-(1→6)]-β-D-galactopyranosid-4-yl} (2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate
3-{[6-deoxy-α-L-mannopyranosyl-(1→2)]-[6-deoxy-α-L-mannopyranosyl-(1→6)]-4-O-[(2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoyl]-β-D-galactopyranosyloxy}-7-(6-deoxy-α-L-mannopyranosyloxy)-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
3-{[6-deoxy-α-L-mannopyranosyl-(1→2)]-[6-deoxy-α-L-mannopyranosyl-(1→6)]-4-O-[(2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoyl]-β-D-galactopyranosyloxy}-7-(6-deoxy-α-L-mannopyranosyloxy)-2-(4-hydroxyphenyl)-4H-chromen-4-one
Flv-3.2.2.1 Isoflavone aglycones
Example 26:
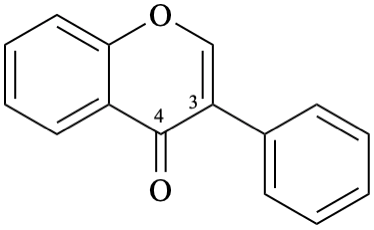
Semi-systematic name
isoflavone
Systematic name
3-phenyl-4H-1-benzopyran-4-one
3-phenyl-4H-chromen-4-one
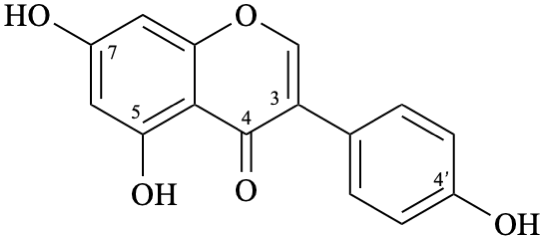
Semi-systematic name
4′,5,7-trihydroxyisoflavone
Trivial name
genistein
Systematic names
5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one
Example 28:
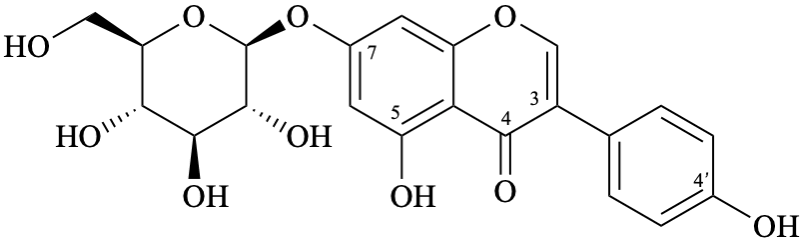
Semi-systematic name
7-(β-D-glucopyranosyloxy)-4′,5-dihydroxyisoflavone
Trivial names
genistin
7-O-(β-D-glucopyranosyl)genistein
Systematic names
5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-1-benzopyran-7-yl β-D-glucopyranoside
5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl β-D-glucopyranoside
7-(β-D-glucopyranosyloxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
7-(β-D-glucopyranosyloxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one
Flv-3.2.3.1 Neoflavone aglycones
Example 29:
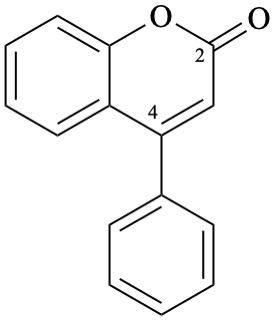
Semi-systematic name
neoflavone
Trivial name
4-phenylcoumarin
Systematic names
4-phenyl-2H-1-benzopyran-2-one
4-phenyl-2H-chromen-2-one
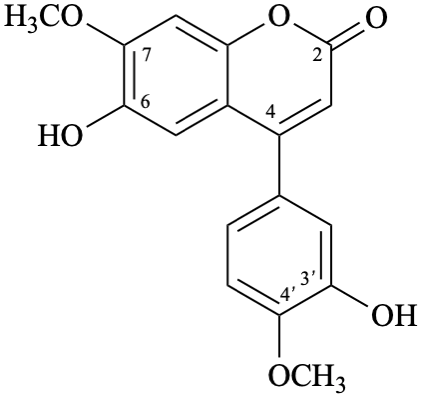
Semi-systematic name
3′,6-dihydroxy-4′,7-dimethoxyneoflavone
Trivial name
melannein
Systematic names
6-hydroxy-4-(3-hydroxy-4-methoxyphenyl)-7-methoxy-2H-1-benzopyran-2-one
6-hydroxy-4-(3-hydroxy-4-methoxyphenyl)-7-methoxy-2H-chromen-2-one
Example 31:
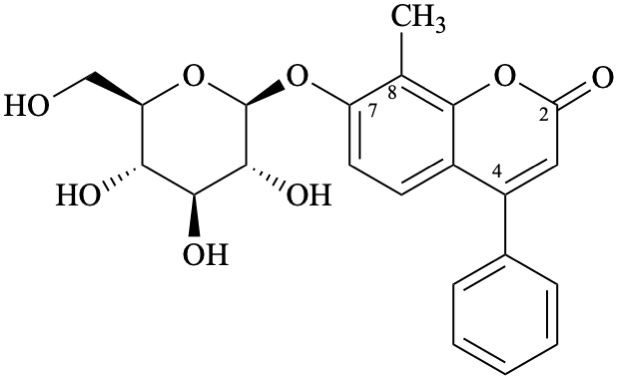
Semi-systematic name
7-(β-D-glucopyranosyloxy)-8-methylneoflavone
Trivial name
7-(β-D-glucopyranosyloxy)-8-methyl-4-phenylcoumarin
Systematic names
8-methyl-2-oxo-4-phenyl-2H-1-benzopyran-7-yl β-D-glucopyranoside
8-methyl-2-oxo-4-phenyl-2H-chromen-7-yl β-D-glucopyranoside
7-(β-D-glucopyranosyloxy)-8-methyl-4-phenyl-2H-1-benzopyran-2-one
7-(β-D-glucopyranosyloxy)-8-methyl-4-phenyl-2H-chromen-2-one
There is ambiguity in the literature concerning the numbering of the carbon atoms in chalcone. Most authors, following IUPAC rule C-313.2 [8] as applied with alkyl aryl ketones such as acetophenone, have assigned primed numbers to atoms in the phenyl ring attached to the carbonyl group, and unprimed numbers to atoms in the other phenyl group. Atoms of the ethene-1,2-diyl linker have been designated α and β. Some authors have used a reverse system, assigning unprimed numbers to the ring attached to the carbonyl group and primed numbers to the other phenyl ring. [The latter usage correlates with the numbering in the flavan parent structure (Flv-2.1.1.1) that arises biosynthetically from a chalcone].
Because of this ambiguity, the term chalcone has been restricted since 1993 to its use as a retained name of “Type 3, no substitution” (R-9.1, table 27a [7]), and individual substituted chalcones are named systematically, based usually on (2E)-prop-2-en-1-one.
The Dictionary of Flavonoids [4] and other standard sources [1], for example, adhere consistently to the numbering of chalcones according to IUPAC rule C-313.2 [8], and the alternative usage has been largely abandoned. Since the use of Greek letters as locants has been restricted, it is now proposed that the term chalcone as a functional parent be retained as a “Type 2, limited substitution” name (P-64.2.1.1 [6]). This allows substitution in the phenyl rings, except where a substituent has priority over the carbonyl group, in which situation a fully systematic name is required (Examples 34, and 35). For the same reason, dihydrochalcones are named systematically (Example 36).
Example 32:
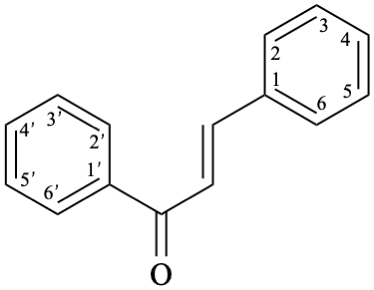
Semi-systematic name
chalcone (PIN)
Systematic name
(2E)-1,3-diphenylprop-2-en-1-one
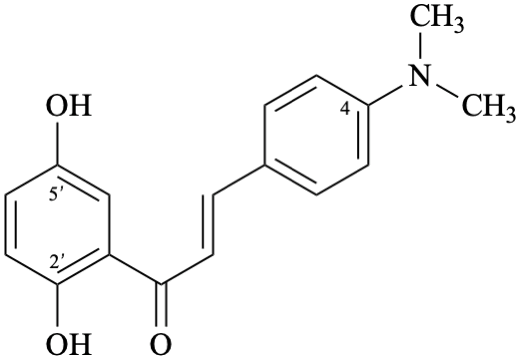
Semi-systematic name
4-(dimethylamino)-2′,5′-dihydroxychalcone (PIN)
Systematic name
(2E)-1-(2,5-dihydroxyphenyl)-3-[4-(dimethylamino)phenyl]prop-2-en-1-one
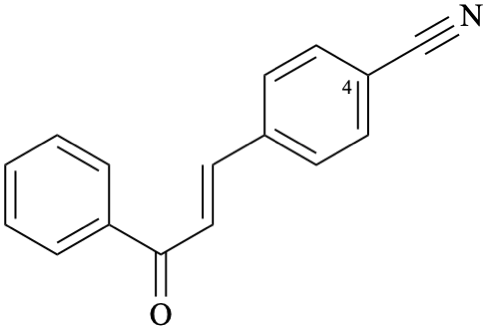
Systematic name
4-[(1E)-3-oxo-3-phenylprop-1-en-1-yl]benzonitrile (PIN)
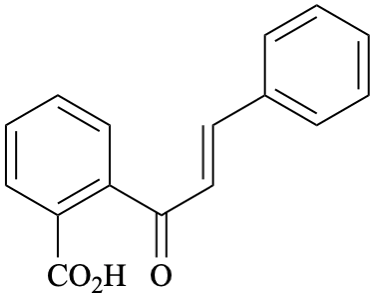
Systematic name
2-[(2E)-3-phenylprop-2-enoyl]benzoic acid (PIN)
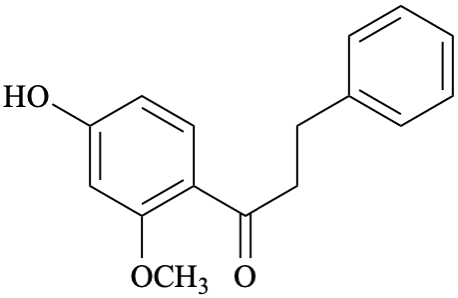
Systematic name
1-(4-hydroxy-2-methoxyphenyl)-3-phenylpropan-1-one (PIN)
Flv-3.4.1 Aurone aglycones
Example 37:
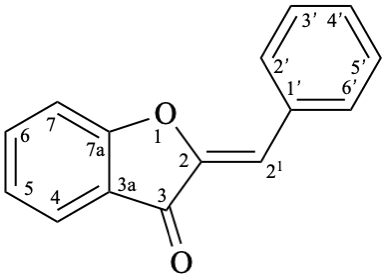
Systematic name
(2Z)-2-benzylidene-1-benzofuran-3(2H)-one
(2Z)-2-(phenylmethylidene)-1-benzofuran-3(2H)-one
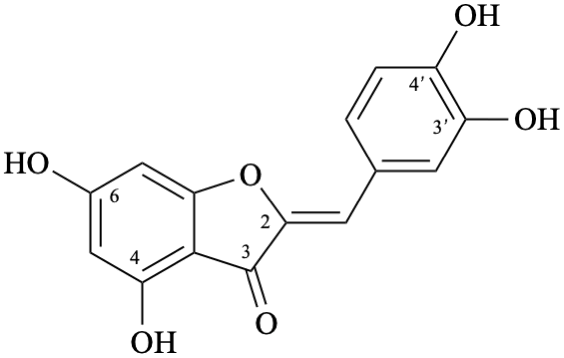
Semi-systematic name
3′,4,4′,6-tetrahydroxyaurone
Trivial name
aureusidin
Systematic name
(2Z)-2-[(3,4-dihydroxyphenyl)methylidene]-4,6-dihydroxy-1-benzofuran-3(2H)-one
(2Z)-2-(3,4-dihydroxybenzylidene)-4,6-dihydroxy-1-benzofuran-3(2H)-one
Example 39:
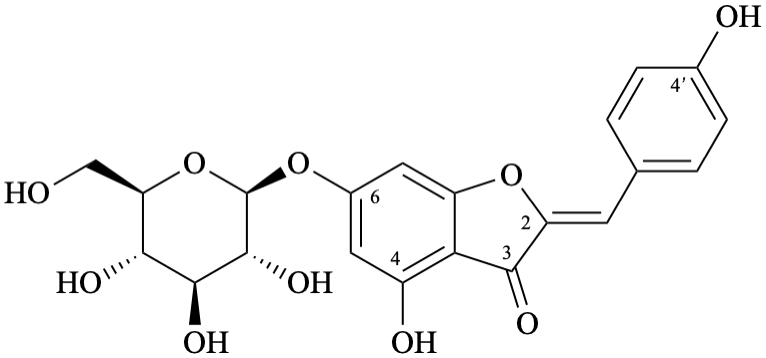
Semi-systematic name
6-(β-D-glucopyranosyloxy)-4,4′-dihydroxyaurone
Systematic name
(2Z)-6-(β-D-glucopyranosyloxy)-4-hydroxy-2-(4-hydroxybenzylidene)-1-benzofuran-3(2H)-one
(2Z)-6-(β-D-glucopyranosyloxy)-4-hydroxy-2-[(4-hydroxyphenyl)methylidene]-1-benzofuran-3(2H)-one
Flv-3.5.1 Anthocyanidins
Anthocyanidins contain a flavylium (2-phenyl-1λ4-benzopyran-1-ylium, 2-phenylchromenylium) ion as a parent structure.
Example 40:
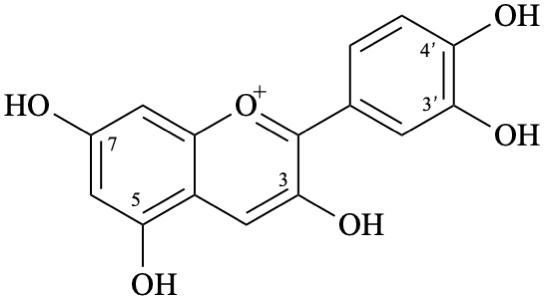
Semi-systematic name
3,3′,4′,5,7-pentahydroxyflavylium
(also accepted as a retained name — see P-73.3.2, table 7.5 in [6])
Trivial name
cyanidin
Systematic names
2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-1λ4-benzopyran-1-ylium
2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromenylium
Example 41:
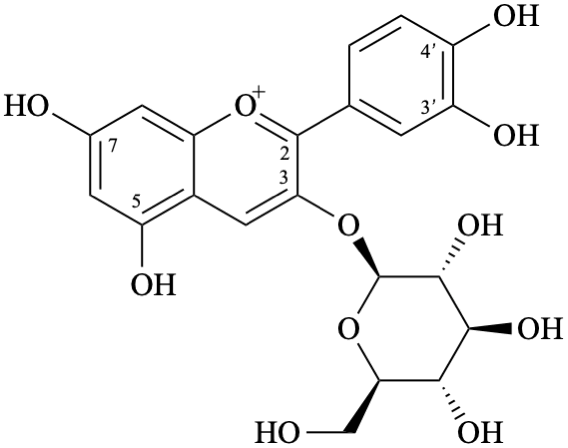
Semi-systematic name
3-(β-D-glucopyranosyloxy)-3′,4′,5,7-tetrahydroxyflavylium
(also accepted as a retained name – see P-73.3.2, table 7.5 in [6])
Trivial name
3-O-(β-D-glucopyranosyl)cyanidin
Systematic names
2-(3,4-dihydroxyphenyl)-3-(β-D-glucopyranosyloxy)-5,7-dihydroxy-1λ4-benzopyran-1-ylium
2-(3,4-dihydroxyphenyl)-3-(β-D-glucopyranosyloxy)-5,7-dihydroxychromenylium
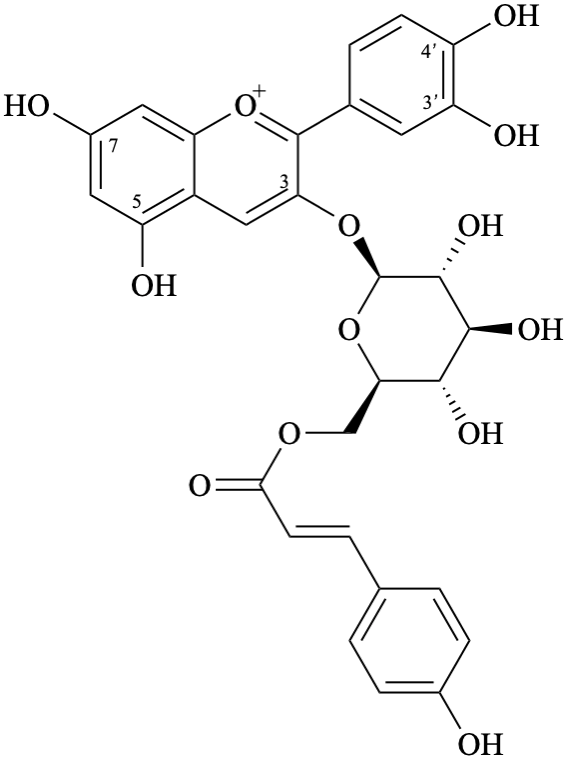
Semi-systematic name
3′,4′,5,7-tetrahydroxy-3-{6-O-[(E)-3-(4-hydroxyphenyl)prop-2-enoyl]-β-D-glucopyranosyloxy}flavylium
(also accepted as a retained name – see P-73.3.2, table 7.5 in [6])
Trivial name
3-O-{6-O-[(E)-3-(4-hydroxyphenyl)prop-2-enoyl]-β-D-glucopyranosyl}cyanidin
Systematic names
2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-{6-O-[(2E)-3-(4-hydroxyphenyl)prop-2-enoyl]-β-D-glucopyranosyloxy}-1λ4-benzopyran-1-ylium
2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-{6-O-[(2E)-3-(4-hydroxyphenyl)prop-2-enoyl]-β-D-glucopyranosyloxy}chromenylium
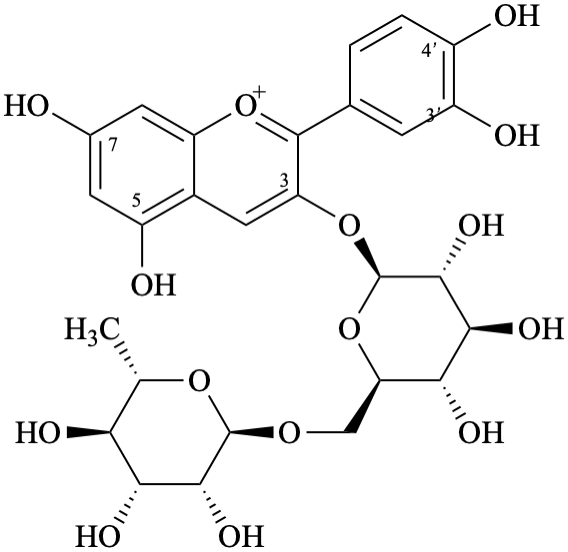
Semi-systematic name
3′,4′,5,7-tetrahydroxy-3-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyloxy]flavylium
Trivial name
3-O-β-rutinosylcyanidin
Systematic names
3-[6-deoxy-α-L-mannopyranosyl-(1→6)-β-D-glucopyranosyloxy]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-1λ4-benzopyran-1-ylium
3-[6-deoxy-α-L-mannopyranosyl-(1→6)-β-D-glucopyranosyloxy]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromenylium
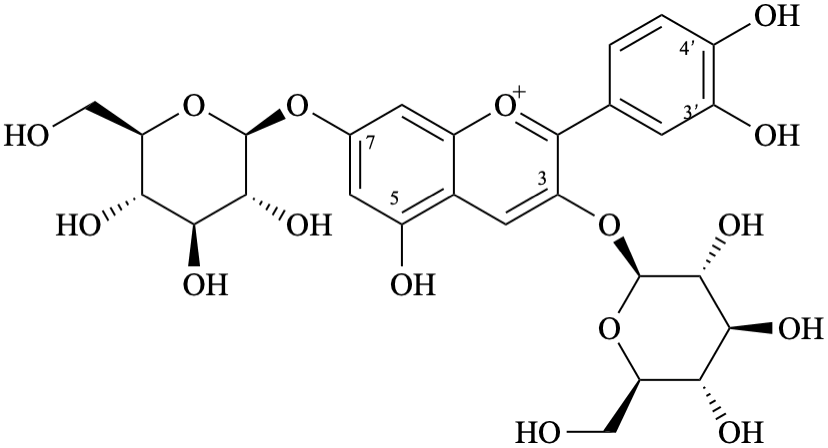
Semi-systematic name,br> 3,7-bis(β-D-glucopyranosyloxy)-3′,4′,5-trihydroxyflavylium
Trivial name
3,7-di-O-(β-D-glucopyranosyl)cyanidin
Locants for additional rings must be double primed to prevent confusion with those for the phenyl ring, which are already single primed.
Flv-3.6.1 Flavones
Example 45:
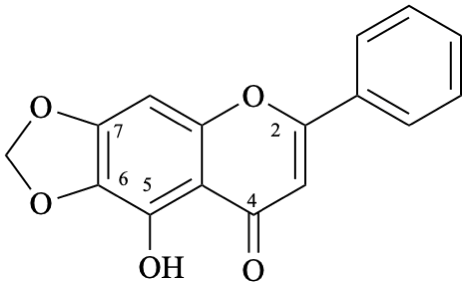
Semi-systematic name
5-hydroxy-6,7-[methylenebis(oxy)]flavone
Systematic names
9-hydroxy-6-phenyl-8H-[1,3]dioxolo[4,5-g][1]benzopyran-8-one
9-hydroxy-6-phenyl-8H-[1,3]dioxolo[4,5-g]chromen-8-one
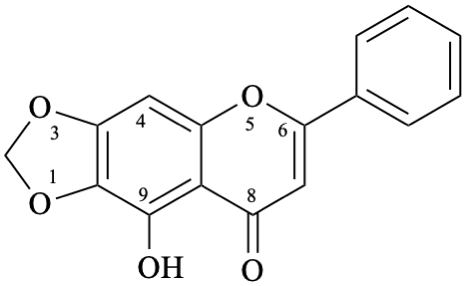
Example 46:
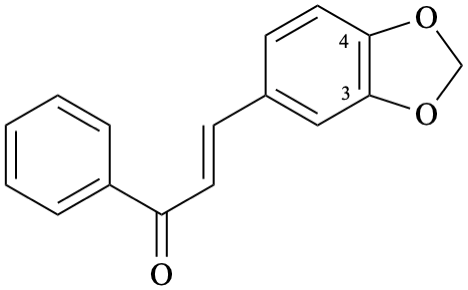
Semi-systematic name
3,4-[methylenebis(oxy)]chalcone
Example 47:
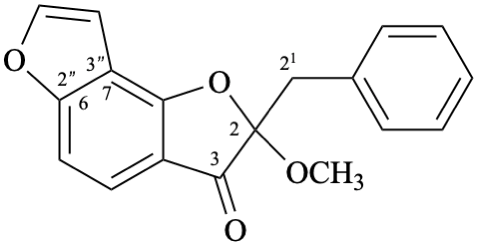
Semi-systematic name
(+)-2-methoxy-2,21-dihydrofuro[2′′,3′′:6,7]aurone
Trivial name
castillene A
Systematic name
(+)-2-benzyl-2-methoxybenzo[1,2-b:3,4-b′]difuran-3(2H)-one
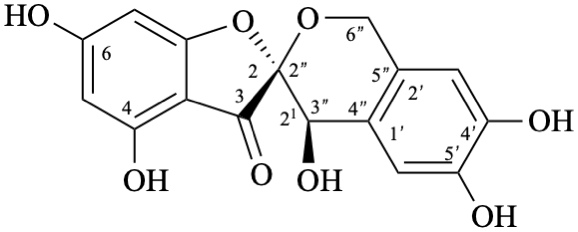 | or enantiomer |
Semi-systematic name
rel-(2R,21R)-21,4,4′,5′,6-pentahydroxy-21,6′′-dihydropyrano[2′′,3′′,4′′,5′′:2,21,1′,2′]aurone
Trivial name
crombenin
Systematic names
rel-(2R,4′R)-4,4′,6,6′,7′-pentahydroxy-1′,4′-dihydro-3H-spiro[[1]benzofuran-2,3′-[2]benzopyran]-3-one
rel-(2R,4′R)-4,4′,6,6′,7′-pentahydroxy-3H-spiro[[1]benzofuran-2,3′-isochroman]-3-one
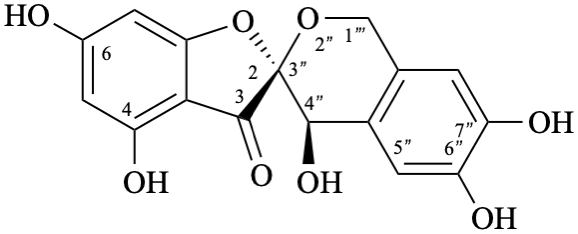
Example 49:
Semi-systematic name
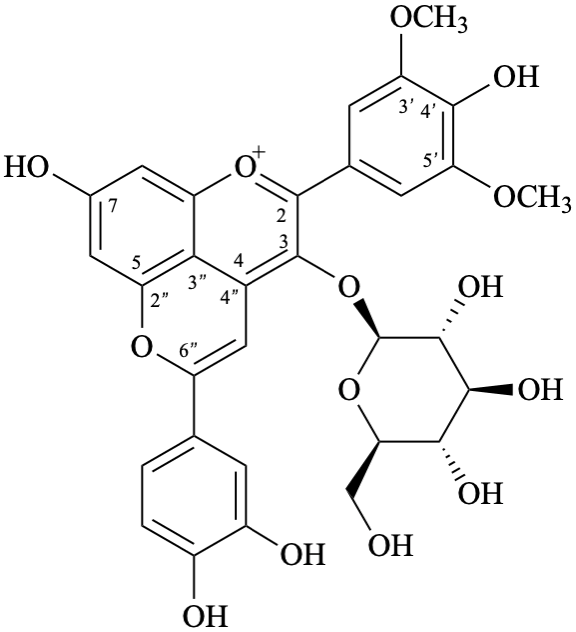
6′′-(3,4-dihydroxyphenyl)-3-(β-D-glucopyranosyloxy)-4′,7-dihydroxy-3′,5′-dimethoxypyrano[4′′,3′′,2′′:4,5]flavylium
Systematic names
5-(3,4-dihydroxyphenyl)-3-(β-D-glucopyranosyloxy)-8-hydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)-1λ4-pyrano[4,3,2-de][1]benzopyran-1-ylium
5-(3,4-dihydroxyphenyl)-3-(β-D-glucopyranosyloxy)-8-hydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)pyrano[4,3,2-de]chromenylium
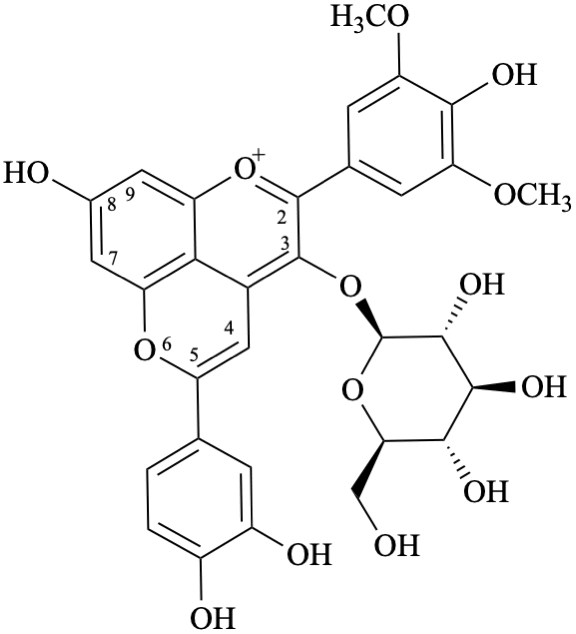
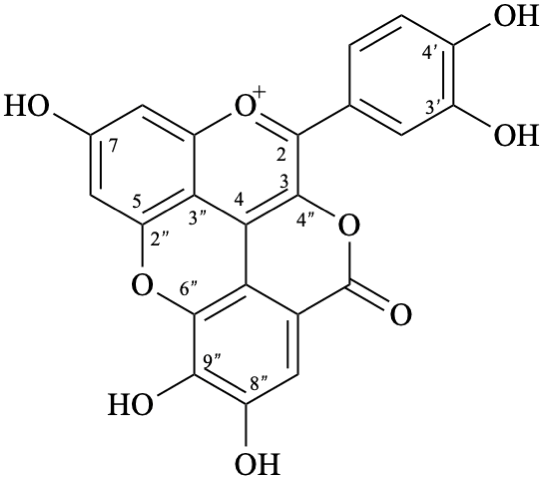
Semi-systematic name
3′,4′,7,8′′,9′′-pentahydroxy-6′′-oxo-6′′H-1′′,5′′-dioxaphenaleno[4′′,3′′′2′′:3,4,5]flavylium
Trivial name
rosacyanin B
Systematic name
11-(3,4-dihydroxyphenyl)-4,5,8-trihydroxy-2H-1,6-dioxa-10-oxoniabenzo[cd]pyren-2-one
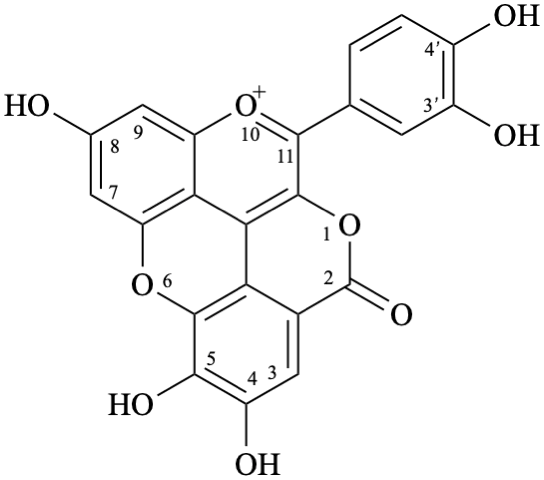
Although simple pterocarpans are ring-closure products of isoflavonoids, systematic numbering is used [6,10].
Example 51:
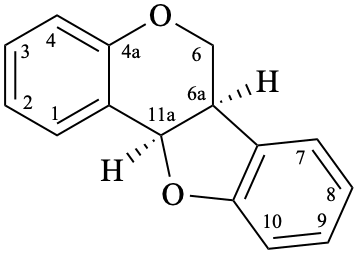
Semi-systematic name
pterocarpan
Systematic names
(6aR,11aR)-6a,11a-dihydro-6H-[1]benzofuro[3,2-c][1]benzopyran
(6aR,11aR)-6a,11a-dihydro-6H-[1]benzofuro[3,2-c]chromene
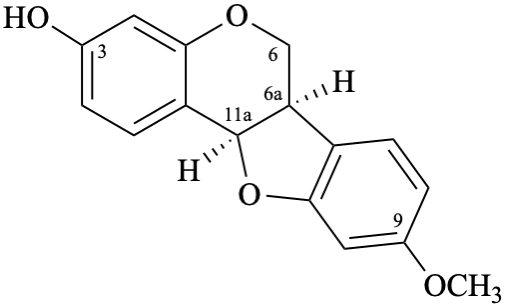
Semi-systematic name 9-methoxypterocarpan-3-ol
Trivial name
(–)-medicarpin
Systematic names
(6aR,11aR)-9-methoxy-6a,11a-dihydro-6H-[1]benzofuro[3,2-c][1]benzopyran-3-ol
(6aR,11aR)-9-methoxy-6a,11a-dihydro-6H-[1]benzofuro[3,2-c]chromen-3-ol
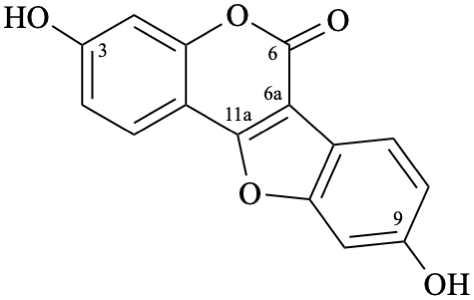
Semi-systematic names
3,9-dihydroxypterocarp-6a(11a)-en-6-one
3,9-dihydroxycoumestan-6-one
Not: 3,9-dihydroxy-6a,11a-didehydropterocarpan-6-one,
because of rule RF-8.1 on introduction of unsaturation in parent names ending with ‘an’ [9].
Trivial name
coumestrol
Systematic names
3,9-dihydroxy-6H-[1]benzofuro[3,2-c][1]benzopyran-6-one
3,9-dihydroxy-6H-[1]benzofuro[3,2-c]chromen-6-one
Example 54:
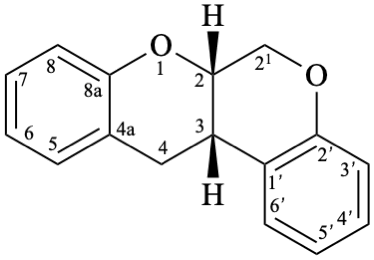
Semi-systematic name
Rotenane
Systematic names
(6aS,12aS)-6,6a,12,12a-tetrahydro[1]benzopyrano[3,4-b][1]benzopyran
(6aS,12aS)-6,6a,12,12a-tetrahydrochromeno[3,4-b]chromene
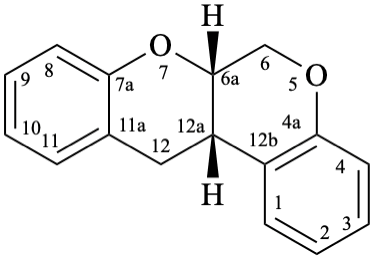
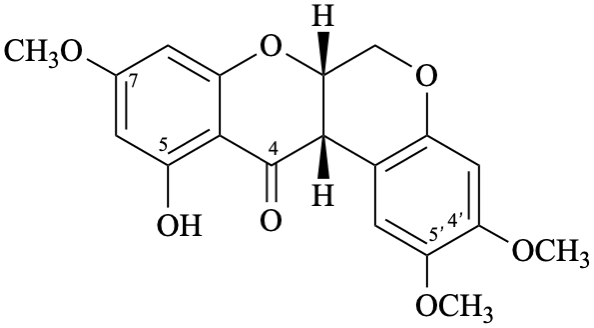
Semi-systematic name
5-hydroxy-4′,5′,7-trimethoxyrotenan-4-one
Trivial name
sermundone
Systematic names
(6aS,12aS)-11-hydroxy-2,3,9-trimethoxy-6a,12a-dihydro[1]benzopyrano[3,4-b][1]benzopyran-12(6H)-one
(6aS,12aS)-11-hydroxy-2,3,9-trimethoxy-6a,12a-dihydrochromeno[3,4-b]chromen-12(6H)-one
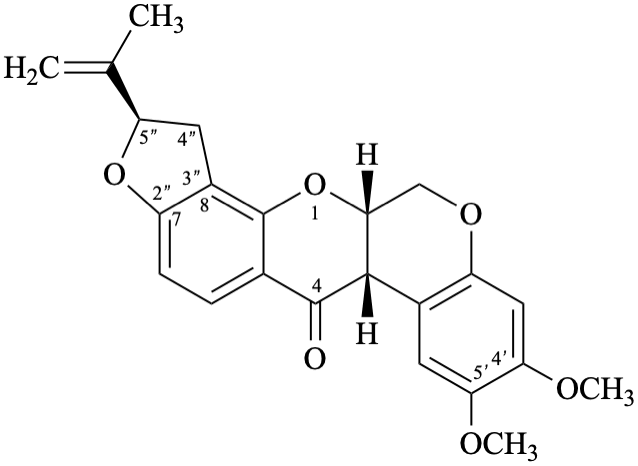
Semi-systematic name
(5′′R)-4′,5′-dimethoxy-5′′-(prop-1-en-2-yl)-4′′,5′′-dihydrofuro[2′′,3′′:7,8]rotenan-4-one
Trivial name
Not: rotenone
Note – Since the name rotenone is identical to that of the functional parent 18 (see Flv-2.2) its use is no longer recommended.
Systematic names
(2R,6aS,12aS)-8,9-dimethoxy-2-(prop-1-en-2-yl)-1,2,12,12a-tetrahydro[1]benzopyrano[3,4-b]furo[2,3-h][1]benzopyran-6(6aH)-one
(2R,6aS,12aS)-8,9-dimethoxy-2-(prop-1-en-2-yl)-1,2,12,12a-tetrahydrochromeno[3,4-b]furo[2,3-h]chromen-6(6aH)-one
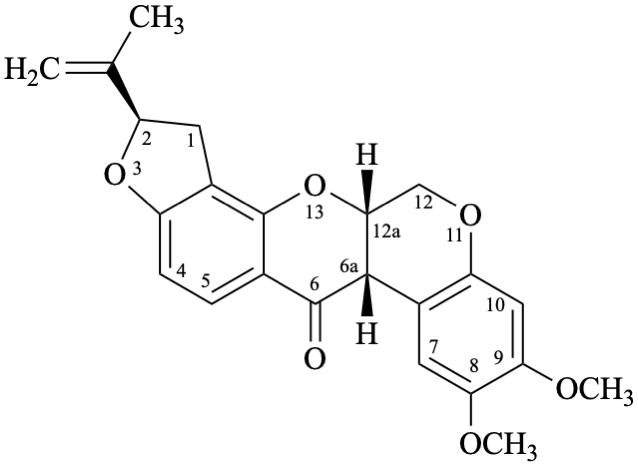
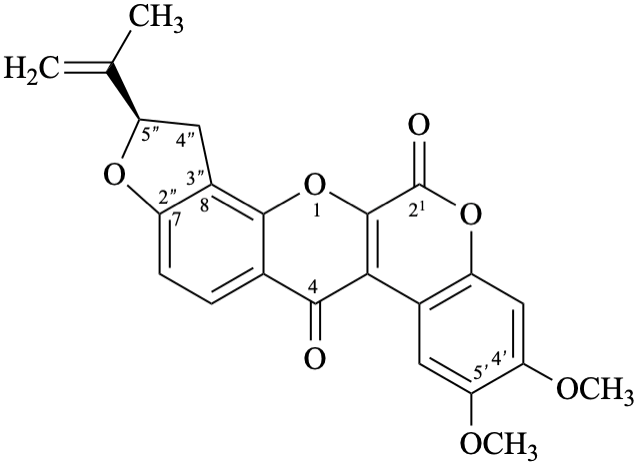
Semi-systematic name
(5′′R)-4′,5′-dimethoxy-5′′-(prop-1-en-2-yl)-4′′,5′′-dihydrofuro[2′′,3′′:7,8]roten-2-ene-21,4-dione
Not: (5′′R)-4′,5′-dimethoxy-5′′-(prop-1-en-2-yl)-2,3-didehydro-4′′,5′′-dihydrofuro[2′′,3′′:7,8]rotenane-21,4-dione,
because of rule RF-8.1 on introduction of unsaturation in parent names ending with ‘an’ [9].
Systematic names
(2R)-8,9-dimethoxy-2-(prop-1-en-2-yl)-1,2-dihydro[1]benzopyrano[3,4-b]furo[2,3-h][1]benzopyran-6,12-dione
(2R)-8,9-dimethoxy-2-(prop-1-en-2-yl)-1,2-dihydrochromeno[3,4-b]furo[2,3-h]chromene-6,12-dione
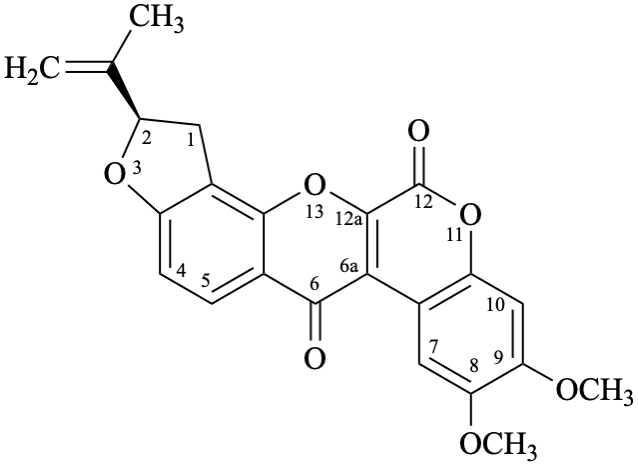
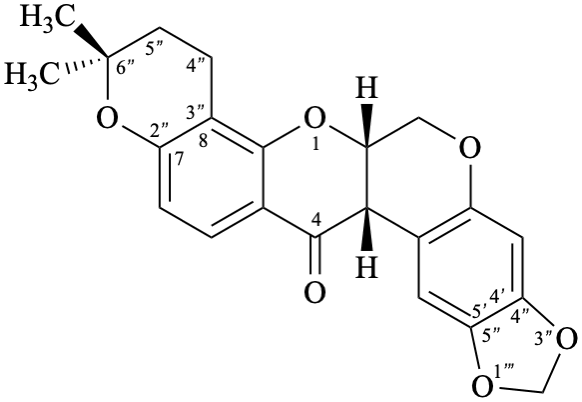
Semi-systematic name
6′′′,6′′′-dimethyl-5′′′,6′′′-dihydro-4′′′H-[1,3]dioxolo[4′′,5′′:4′,5′]pyrano[2′′′,3′′′:7,8]rotenan-4-one
Systematic names
(5aS,12bS)-2,2-dimethyl-3,4,5a,12b-tetrahydro-2H-[1,3]dioxolo[4,5-g]pyrano[2,3-c:6,5-f′]bis([1]benzopyran)-13(6H)-one
(5aS,12bS)-2,2-dimethyl-3,4,5a,12b-tetrahydro-2H-[1,3]dioxolo[4,5-g]pyrano[2,3-c:6,5-f′]dichromen-13(6H)-one
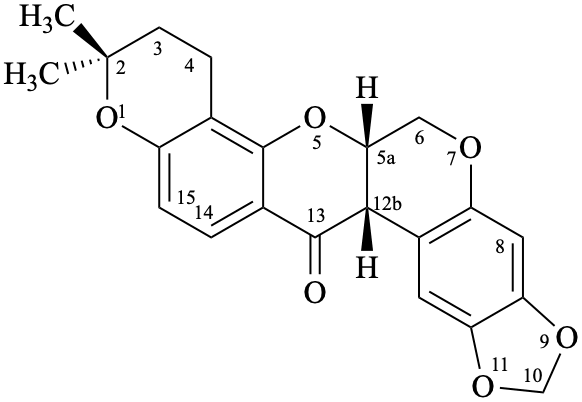
Example 59:
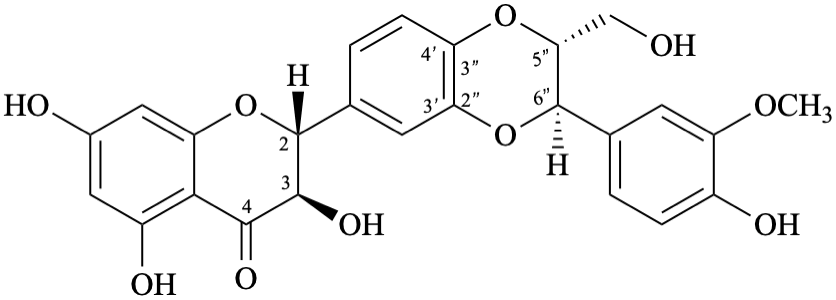
Semi-systematic name
(2R,3R,5′′R,6′′R)-3,5,7-trihydroxy-6′′-(4-hydroxy-3-methoxyphenyl)-5′′-(hydroxymethyl)-5′′,6′′-dihydro[1,4]dioxino[2′′,3′′:3′,4′]flavan-4-one
Trivial names
silibinin, also called silybin
Systematic names
(2R,3R)-3,5,7-trihydroxy-2-[(2R,3R,)-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-2,3-dihydro-4H-1-benzopyran-4-one
(2R,3R)-3,5,7-trihydroxy-2-[(2R,3R,)-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]chroman-4-one
(2R,3R)-3,5,7-trihydroxy-2-[(2R,3R,)-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-2,3-dihydro-4H-chromen-4-one
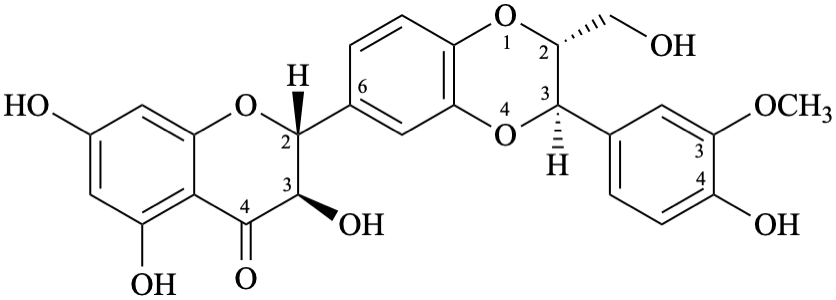
Biflavonoids and other flavonoid oligomers have been widely reported in the flavonoid literature [13–19] and are also included in the present recommendations. The recommended semi-systematic names consider the terminal flavonoid first in alphabetical order as the preferred one, that is substituted by the other components. If the terminal flavonoid components are identical, the one with the lower attachment locant is cited first in the name.
Example 60:
Semi-systematic name
[(3S,4R)-4′-methoxyisoflavan-2′,7-diol]-(4→5′)-[(3R)-4′-methoxyisoflavan-2′,7-diol]
Systematic names
(3S,4R)-4-{4-hydroxy-5-[(3R)-7-hydroxy-3,4-dihydro-2H-1-benzopyran-3-yl]-2-methoxyphenyl}-3-(2-hydroxy-4-methoxyphenyl)-3,4-dihydro-2H-1-benzopyran-7-ol
(3S,4R)-4-{4-hydroxy-5-[(3R)-7-hydroxychroman-3-yl]-2-methoxyphenyl}-3-(2-hydroxy-4-methoxyphenyl)chroman-7-ol
(3S,4R)-4-{4-hydroxy-5-[(3R)-7-hydroxy-3,4-dihydro-2H-chromen-3-yl]-2-methoxyphenyl}-3-(2-hydroxy-4-methoxyphenyl)-3,4-dihydro-2H-chromen-7-ol
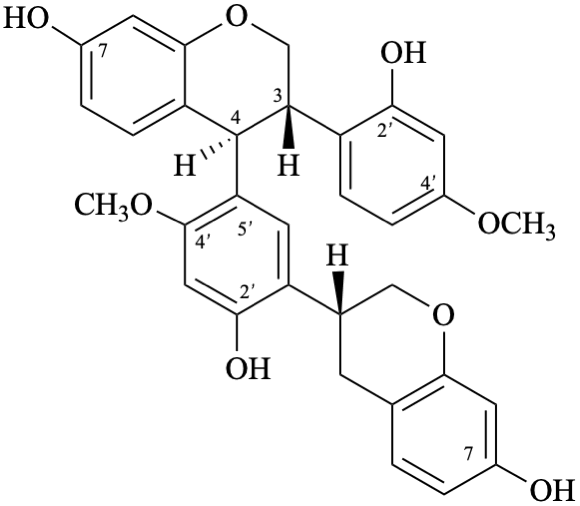
Semi-systematic name
[(2S)-5-hydroxy-4′,7-dimethoxyflavan-4-one]-(2′→6)-[(2S)-3′,4′,5,7-tetrahydroxyflavan-4-one]
Trivial name
[(2S)-4′,7-di-O-methylnaringenin]-(2′→6)-[(2S)-3′-hydroxynaringenin]
Systematic names
(2S)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-6-{2-[(2S)-5-hydroxy-7-methoxy-4-oxo-3,4-dihydro-2H-1-benzopyran-2-yl]-5-methoxyphenyl}-2,3-dihydro-4H-1-benzopyran-4-one
(2S)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-6-{2-[(2S)-5-hydroxy-7-methoxy-4-oxochroman-2-yl]-5-methoxyphenyl}chroman-4-one
(2S)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-6-{2-[(2S)-5-hydroxy-7-methoxy-4-oxo-3,4-dihydro-2H-chromen-2-yl]-5-methoxyphenyl}-2,3-dihydro-4H-chromen-4-one
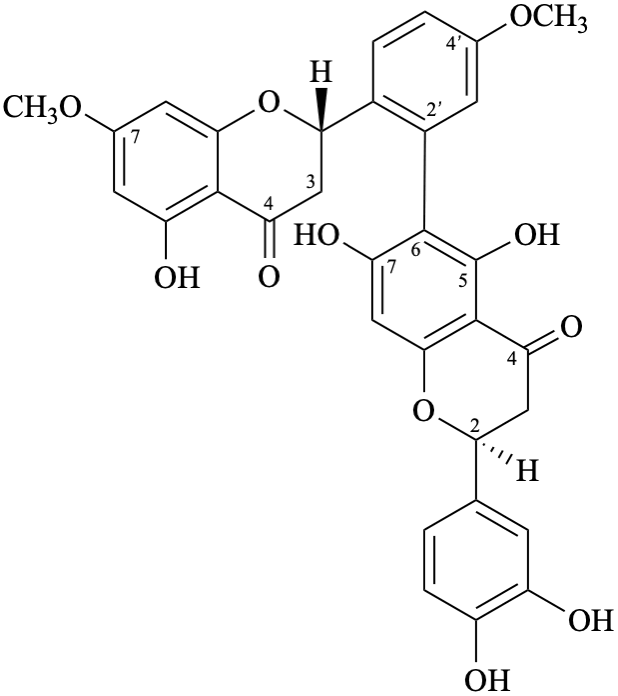
Semi-systematic name
(3′,4′,5,7-tetrahydroxyflavone)-(2′→6)-(3′,4′,5,7-tetrahydroxyflavone)-(2′→6)-(3′,4′,5,7-tetrahydroxyflavone)
Systematic names
6-(6-{6-[6-(5,7-dihydroxy-4-oxo-4H-1-benzopyran-2-yl)-2,3-dihydroxyphenyl]-5,7-dihydroxy-4-oxo-4H-1-benzopyran-2-yl}-2,3-dihydroxyphenyl)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-1-benzopyran-4-one
6-(6-{6-[6-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)-2,3-dihydroxyphenyl]-5,7-dihydroxy-4-oxo-4H-chromen-2-yl}-2,3-dihydroxyphenyl)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4-one
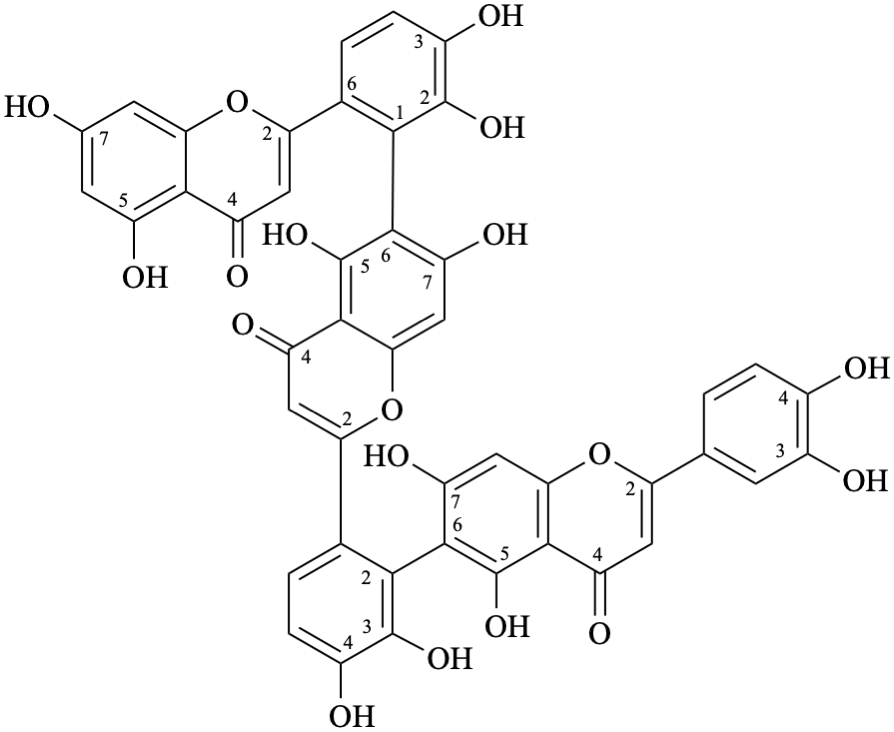
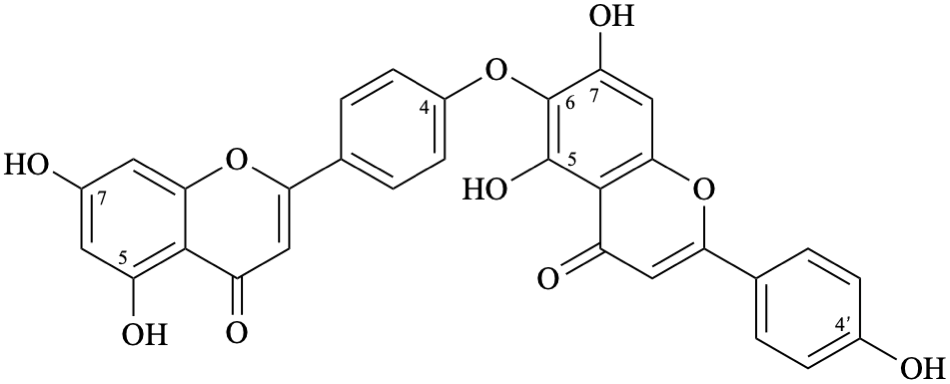
Semi-systematic name
(5,7-dihydroxyflavone)-(4′-oxy-6)-(4′,5,7-trihydroxyflavone)
Trivial name
hinokiflavone
Systematic names
6-[4-(5,7-dihydroxy-4-oxo-4H-1-benzopyran-2-yl)phenoxy]-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
6-[4-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)phenoxy]-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one
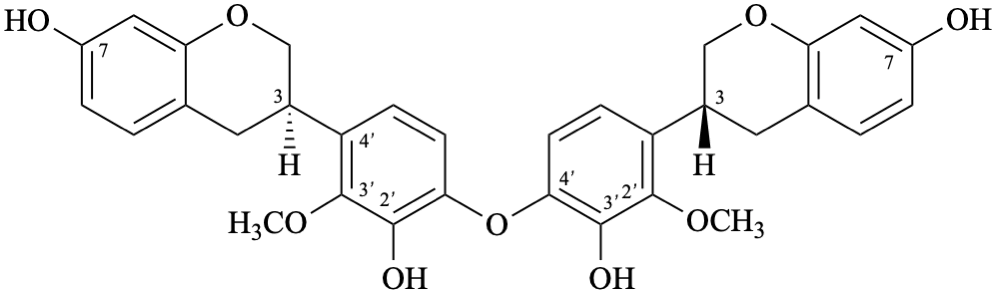
Semi-systematic name
[(3R)-2′-methoxyisoflavan-3′,7-diol]-(4′-oxy-4′)-[(3R)-2′-methoxyisoflavan-3′,7-diol]
Trivial name
biscyclolobin
Systematic names
3,3′-[oxybis(3-hydroxy-2-methoxy-4,1-phenylene)]bis[(3R)-3,4-dihydro-2H-1-benzopyran-7-ol]
3,3′-[oxybis(3-hydroxy-2-methoxy-4,1-phenylene)]bis[(3R)-chroman-7-ol]
3,3′-[oxybis(3-hydroxy-2-methoxy-4,1-phenylene)]bis[(3R)-3,4-dihydro-2H-chromen-7-ol]
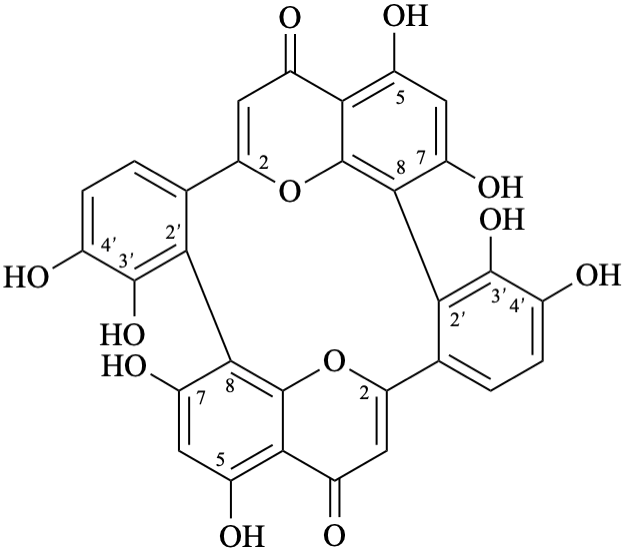
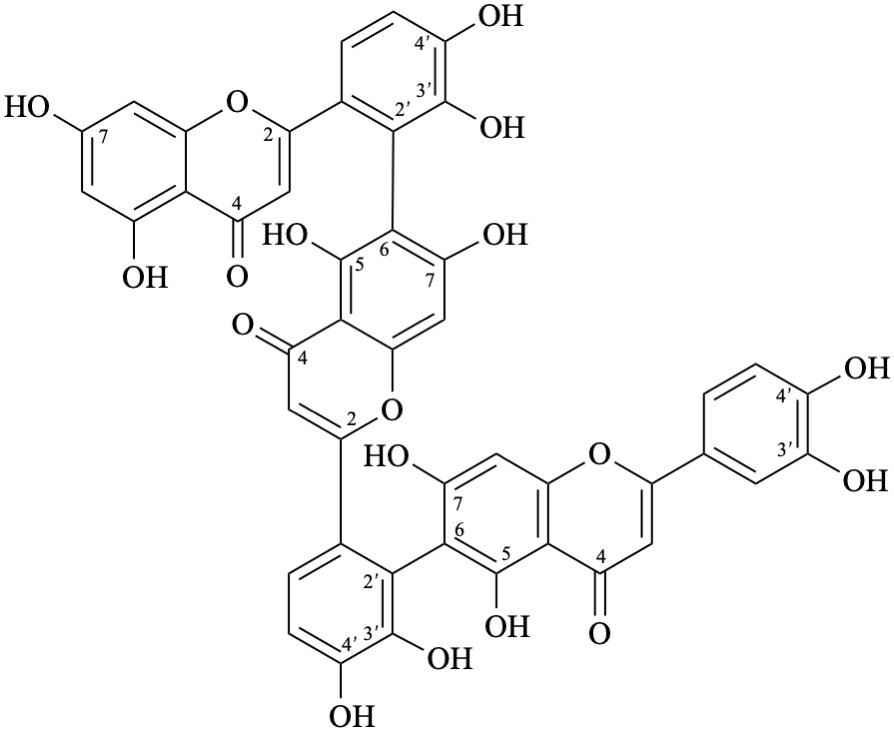
Semi-systematic name
(3′,4′,5,7-tetrahydroxyflavone)-(2′→8,8→2′)-(3′,4′,5,7-tetrahydroxyflavone)
Trivial name
anhydrobartramiaflavone
Systematic names
15,17,23,24,35,37,45,46-octahydroxy-14H,34H-1(2,8),3(8,2)-bis([1]benzopyrana)-2,4(1,2)-dibenzenacyclotetraphane-14,34-dione
Note – Structure numbering [6] is given below:
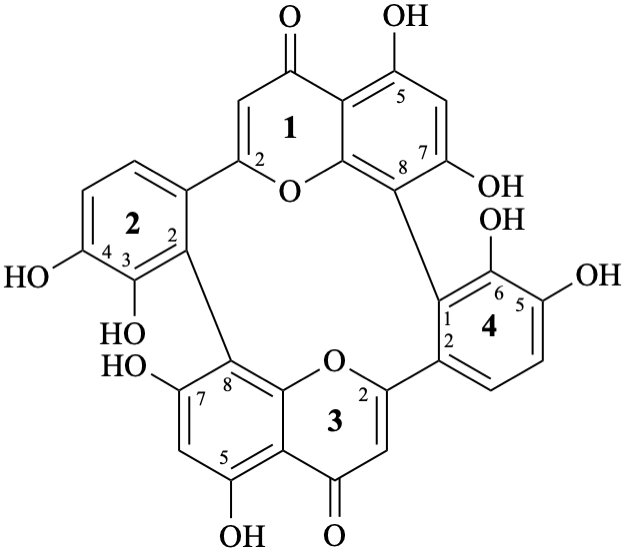
3,4,5,7,13,14,15,17-octahydroxy-8,10:18,20-di(ethanylylidene)tetrabenzo[b,d,h,j][1,7]dioxacyclododecine-22,24-dione
Note – Systematic numbering [6,10] is given below:
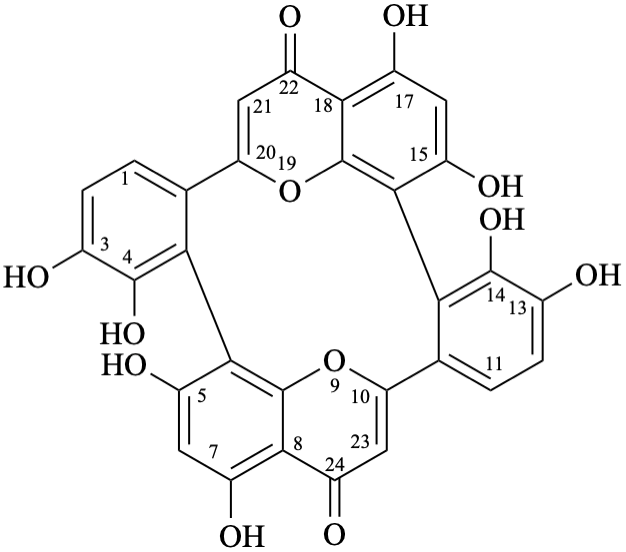
[1] Flavonoids – Chemistry, Biochemistry and Applications, Ø. M. Andersen, K. R. Markham (Eds.), CRC Press, Taylor and Francis Group, Boca Raton (2006).
[2] The Science of Flavonoids, E. Grotewold (Ed.), Springer, New York (2006).
[3] Flavonoids and Related Compounds: Bioavailability and Function, J. P. E. Spencer, A. Crozier (Eds.), Oxidative Stress and Disease Series, Taylor and Francis, Boca Raton (2012).
[4] Dictionary of Flavonoids, J. Buckingham, V. R. N. Munasinghe (Eds.), CRC Press, Taylor and Francis Group, Boca Raton (2015).
[5] The Handbook of Natural Flavonoids, J. B. Harborne, H. Baxter (Eds.), Vols. 1 and 2, John Wiley, New York (1999).
[6] H. A. Favre, W. H. Powell. Nomenclature of Organic Chemistry. IUPAC Recommendations and Preferred Names 2013 (The ‘Blue Book’), The Royal Society of Chemistry, Cambridge (2014).
[7] A Guide to IUPAC Nomenclature of Organic Compounds, R. Panico, W. H. Powell, J.-C. Richer (Eds.), Blackwell Scientific Publications, Oxford (1993). Corrections: H. A. Favre, K.-H. Hellwich, G. P. Moss, W. H. Powell, J. G. Traynham, Corrections to A Guide to IUPAC Nomenclature of Organic Compounds. Pure Appl. Chem. 71, 1327 (1999).
[8] Nomenclature of Organic Chemistry. Sections A, B, C, D, E, F and H. 1979 Edition (the ‘Blue Book’), J. Rigaudy, S. P. Klesney (Eds.), Pergamon Press, Oxford (1979).
[9] P. M. Giles, Jr. Pure Appl. Chem. 71, 587 (1999); Corrections and modifications: H. A. Favre, P. M. Giles Jr., K.-H. Hellwich, A. D. McNaught, G. P. Moss, W. H. Powell. Pure Appl. Chem. 76, 1283 (2004).
[10] G. P. Moss. Pure Appl. Chem. 70, 143 (1998).
[11] A. D. McNaught. Pure Appl. Chem. 68, 1919 (1996).
[12] G. P. Moss. Pure Appl. Chem. 72, 1493 (2000).
[13] H. Geiger. Biflavonoids and triflavonoids, in The Flavonoids: Advances in Research since 1986, J. B. Harborne (Ed.), pp. 95–115, Chapman & Hall, London (1994).
[14] H. Geiger, C. Quinn. Biflavonoids, in The Flavonoids: Advances in Research since 1980, J. B. Harborne, T. J. Mabry (Eds.), pp. 99–124, Chapman & Hall, London (1988).
[15] H. Geiger, C. Quinn. Biflavonoids, in The Flavonoids, J. B. Harborne, T. J. Mabry (Eds.), pp. 505–534. Chapman & Hall, London (1982).
[16] H. Geiger, C. Quinn. Biflavonoids, in The Flavonoids, J. B. Harborne, T. J. Mabry, H. Mabry, (Eds.), pp. 692–742, Chapman & Hall, London (1975).
[17] H. P. Kim, H. Park, K. H. Son, H. W. Chang, S. S. Kang. Arch. Pharmacol. Res. 31, 265 (2008).
[18] Biflavonoids: Occurrence, Structural Features and Bioactivity (Chemistry Research and Applications). A. G. Mercader, A. B. Pomilio (Eds.), Nova Science Publishers, New York (2011).
[19] A. Thapa, E. Y. Chi. Adv. Exp. Med. Biol. 863, 55 (2015).
Annex 1 Flavonoid class names and characteristic structures
and
Annex 2. InChIs and InChIKeys for the flavonoids given as examples
Return to main IUPAC Chemical Nomenclature home page
Return to main IUBMB Biochemical Nomenclature home page