Numbering of Fullerenes (IUPAC Recommendations 2004)
3.1.8. Systematic numbering for (C28-D2)[5,6]fullerene
Continued from 3.1.7. Systematic numbering for (C76-D2)[5,6]fullerene
3.1.8. (C28-D2)[5,6]fullerene (Fig. 9)
(Atlas [ref 5] Ref. No. 28:1; CAS Reg. No. 145393-14-2).
This chiral fullerene has three C2 axes, shown in Figs. 9a, 9b, and 9c, respectively. The axis of Fig. 9a is preferred because it passes through the midpoint of two bonds connecting 6,6,5 atoms, whereas both the others axes pass through the midpoints of two bonds connecting lower ranking atoms (Fu-3.1.2.2). Because of symmetry, only two possible pathways must be considered, namely a to a' to b and a to a' to c (Fig. 9a) The resulting helical numberings are shown in Figs. 9d and 9e, respectively. Both numberings terminate at the same atom and application of Fu-3.1.3 is inconclusive. Application of Fu-3.1.4 (Table 3) shows that the two numberings differ at position 15, where there is a 6,5,5 atom in the numbering of Fig. 9d, but a 5,5,5 atom in the numbering of Fig. 9e. Therefore, the numbering of Fig. 9d is preferred. CAS selects the numbering of Fig. 9e [ref 3]. The stereodescriptor for the shown enantiomer of this inherently chiral fullerene is (f,sC) [ref 4].
Table 3. Rankings of atoms 1 to 15 for pathways in Figs. 9d and 9e.
| Atom | 9d | 9e |
| 1 | 6,6,5 | 6,6,5 |
| 2 | 6,6,5 | 6,6,5 |
| 3 | 6,5,5 | 6,5,5 |
| 4 | 6,5,5 | 6,5,5 |
| 5 | 6,5,5 | 6,5,5 |
| 6 | 6,5,5 | 6,5,5 |
| 7 | 5,5,5 | 5,5,5 |
| 8 | 5,5,5 | 5,5,5 |
| 9 | 6,5,5 | 6,5,5 |
| 10 | 6,5,5 | 6,5,5 |
| 11 | 6,5,5 | 6,5,5 |
| 12 | 6,5,5 | 6,5,5 |
| 13 | 5,5,5 | 5,5,5 |
| 14 | 5,5,5 | 5,5,5 |
| 15 | 6,5,5 | 5,5,5 |
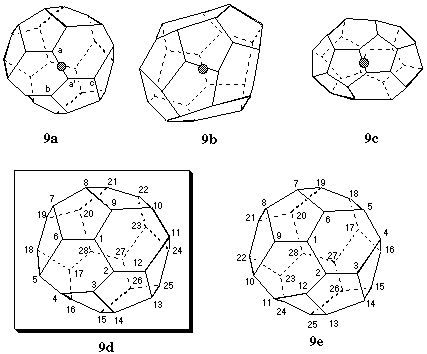
Fig. 9. Systematic numbering of (f,sC)(C28-D2)[5,6]fullerene
References for this section
3. A.L. Goodson, C.L. Gladys and D.E. Worst, J. Chem. Inf. Comp. Sci. 1995, 35, 969-978.
4. International Union of Pure and Applied Chemistry, Division of Organic Chemistry, Commission on Nomenclature of Organic Chemistry. "Nomenclature for the (C60-Ih) and (C70-D5h(6))[5,6]fullerenes (IUPAC recommendations 2002)", Pure Appl. Chem. 2002, 74, 629-695.
5. P.W. Fowler and D.E. Manolopoulos, An Atlas of Fullerenes, Clarendon Press, Oxford, 1995
Continued with 3.1.9. Systematic numbering for (C42-D3)[5,6]fullerene
Return to fullerenes (part 2) homepage
Return to IUPAC Chemical Nomenclature homepage.
