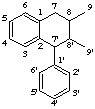
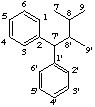
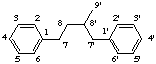
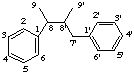
2,7'-cyclolignane
not 2',7-, 6,7'-, or 6',7-cyclolignane
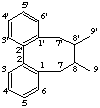
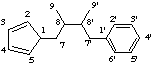
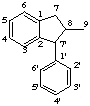
2,2'-cyclolignane
not 6,6'-cyclolignane
Continued from LG-0 and LG1 Introduction and Parent Structures
Contents
Modification of the fundamental parent structure (see LG-1) which results in additional rings and/or addition or removal of carbon atoms to that structure (i.e. not treated as a substituent) are indicated by the prefixes described below. These prefixes are always cited next to the parent structure rather than mixed with the other substituent prefixes (see LG-4) i.e. they are nondetachable.
LG-2.1 Additional Carbocyclic Rings (the prefix cyclo-)
(a) Cyclolignane If the carbon skeleton of a lignan has an additional carbocyclic ring which is formed by direct bonding between two atoms of the lignane skeleton this is indicated by the prefix cyclo- and the two locants which identify the relevant bond (see rule F-4.1 [ref 9], rule RF-4.3 [ref 12], rule R-1.2.6.1 [ref 13]). If there is a choice of locants lower numbers are preferred in the order:
(i) unprimed numbersExamples:
(ii) primed numbers


2,2'-cyclolignane
not 6,6'-cyclolignane
(b) Cycloneolignane If the carbon skeleton of a neolignan with the two C6C3 units (4) directly bonded has an additional carbocyclic ring which is formed by direct bonding between two atoms of the neolignane skeleton this is indicated by the prefix cyclo- and the two locants which identify the relevant bond (see rule F-4.1 [ref 9], rule RF-4.3 [ref 12], rule R-1.2.6.1 [ref 13]). If there is a choice lower numbers are preferred in the order:
(i) unprimed numbers for cycloExamples:
(ii) primed numbers for cyclo
(iii) indicated hydrogen
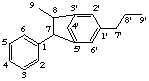
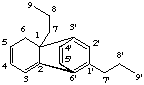
6H-2,6'-cyclo-1,3'-neolignane
not 6H-2,2'-cyclo-1,5'-neolignane
1,3'-neolignane is the preferred parent
Note: These parent structures do not exist but are required for naming hydrogenated or partially hydrogenated compounds (see for example LG-4.4 example 2).LG-2.2 Ring Cleavage (the prefix seco-)
Cleavage of a ring bond with addition of the appropriate number of hydrogen atoms is indicated by the prefix seco and the locants of the bond cleaved (see rule F-4.7 [ref 9], rule RF-4.4.1 [ref 12], rule R-1.2.6.2 [ref 13]). The original numbering of the system is retained. If there is a choice of locants lower numbers are preferred in the order:
(a) unprimed numbersExample:
(b) primed numbers
Note that strict application of the rules for this example gives 1,7-seco-1,7'-cyclolignane which would result in a different numbering of ring C1-C6. However the probable relationship with 2,7'-cyclolignane is best illustrated by the name shown. Another possible name is 1(77')-abeo-lignane (see LG-2.7).
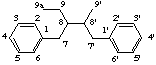
LG-2.3 Removal of Skeletal Atoms (the prefix nor-)
If the parent structure is derived from lignane or neolignane or oxyneolignane by the loss of one or more carbon atoms this is expressed by the prefix nor- (or dinor-, etc) (see rule F-4.2 and F-4.4 [ref 9], rule RF-4.1.1 [ref 12]). If there is a choice of locations for the removal of the carbon atom preference is made in the order:
(a) unprimed numberThis number is cited with the prefix nor- while the numbers of all other atoms are unchanged. If necessary double bond locations are identified by indicated hydrogen (see LG-1.3).
(b) higher number
Examples:
8',9'-dinor-2,7'-cyclo-8,7'-neolignane
(8,7' preferred to 7,8')
Note: In this example the two modifying prefixes are cited in alphabetical order (see rule F-4.7 [ref 9], rule RF-4.7 [ref 12])
7-norlignane
not 9'-nor-8,7'-neolignane
(lignane is the preferred parent)
LG-2.4 Addition of Skeletal Atoms (the prefix homo-)
If the parent structure is derived from lignane, neolignane or oxyneolignane by the addition of one or more carbon atoms this is expressed by the prefix homo- (or dihomo-, etc) (see rule F-4.5 [ref 9], rule RF-4.2 [ref 12]). The additional carbon atom(s) are numbered by adding a letter "a" (then "b", "c", etc) to the locant of the appropriate atom. If there is a choice for the location of the extra carbon atom preference is made in the order:
(a) unprimed numbersIf necessary double bond locations are identified by indicated hydrogen (see LG-1.3).
(b) higher number
Examples:
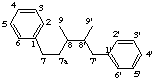
7a-homolignane
not 9a'-homo-8,9'-neolignane (lignane preferred)
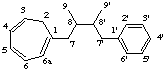
2H-6a-homolignane
not 2'H-6a'-homolignane (6a preferred to 6a')
LG-2.5 Replacement of Skeletal Atoms (the prefix oxa, etc)
When a carbon atom of a lignane, neolignane or oxyneolignane is replaced by a heteroatom the replacement ("oxa-aza") system of nomenclature is used (see rule B-4 [ref 10], rule F-4.11 [ref 9], rule RF-5 [ref 12], rule R-1.2.2 [ref 13]). If there is a choice of locants preference is made in order to:
(b) lower numbers(a) unprimed numbers
If necessary indicated hydrogen (see LG-1.3) should be used to identify double bond positions.
Examples:
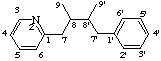
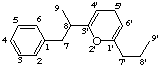
5'H-2'-oxa-8,3'-neolignane
LG-2.6 Bridged Parent Structure (the prefix epoxy, etc)
Additional rings created by bridging two positions of a lignane, neolignane or oxyneolignane by an atom or appropriate group are indicated by the name of the bridging atom or group as a prefix with the appropriate locants. The most common examples of such additional rings are the furanoid or furanofuranoid lignanes. In these cases the bridging oxygen atom is indicated by the prefix epoxy. If there is a choice of locants preference is made in the order to:
(a) unprimed numbersIf there is more than one identical bridge each pair of locants is cited in order, separated by colons. If necessary double bond locations are identified by indicated hydrogen (see LG-1.3).
(b) lower numbers.
Examples:
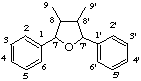
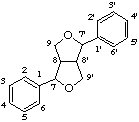
7,9':7',9-diepoxylignane
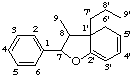
2',7-epoxy-6'H-8,1'-neolignane
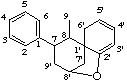
2',8'-epoxy-7,9'-cyclo-6'H-8,1'-neolignane
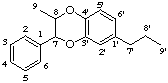
3',7-epoxy-8,4'-oxyneolignane
not 4',8-epoxy-7,3'-oxyneolignane (8,4'-oxyneolignane is the preferred parent)
Composite bridges are named by a combination of simple bridges and are cited within round brackets. If the bridge is further substituted the bridge is numbered starting from the highest numbered attachment position. Each atom in the bridge is numbered starting with 1" as it is necessary to distinguish between it and the unprimed and single primed numbers of the main skeleton.
Example:
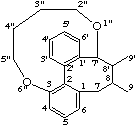
LG-2.7 Bond Migration (the prefix abeo-)
A parent structure which does not possess an accepted skeleton but may be considered in a formal sense to arise from one by migration of one or more bonds may be named by the prefix x(yz)abeo- (see rule F-4.9 [ref 9], rule RF-4.5.1 [ref 12], rule R-1.2.7.1 [ref 13]). This designates the migration of the group terminating in the atom numbered x from position y to position z.
Example:
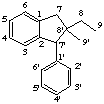
The above example might also be called 7'(8'8)abeo-2,7'-cyclolignane, 8'(7'
8)abeo-2,7'-cyclo-8,7'-neolignane or 8'(7'
8)abeo-2,7'-cyclo-8,7'-neolignane if it was required to show the relationship to the corresponding unrearranged neolignane skeletons. A more systematic name for this example is 8-ethyl-2,7'-cyclo-8',9'-dinor-8,7'-neolignane (see LG-2.3 example 4). The prefix abeo- may also be used to show the relationship between two lignans, neolignans or oxyneolignans.
Example:
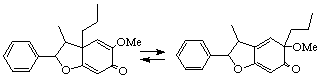
 5'-methoxy-7'(1'
5'-methoxy-7'(1'
1'-methoxy-6'H-7'(1'3')abeo-4',7-epoxy-
 1'-methoxy-4',7-epoxy-
1'-methoxy-4',7-epoxy-
-8,3'-neolignan-6'-one -8,3'-neolignan-6'(1'H)-one
Note: In the rearrangement above the double bond locations are identified by indicated hydrogen (LG-1.3) with the names of the left hand structure and by added hydrogen (LG-4.4) with the names of the right hand structure. This is because of the difference between the hypothetical parents.LG-2.8 Combination of Prefixes
If more than one modifying prefixes is used the effect on the parent is applied sequentially from right to left 'advancing backwards' (see rule F-4.7). When there is a choice of operations the minimum number of changes should be used. Prefixes are cited as far as possible in alphatetical order from left to right. The order must avoid improper use of the prefixes or impossible situations. For example LG-2.2 is illustrated with 1,7-seco-2,7'-cyclolignane. The alternative name 2,7'-cyclo-1,7-secolignane is not permitted as it would require "1,7-secolignane" to form it and this is not possible.
9. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC), Nomenclature of Organic Chemistry, Section F: Natural Products and Related Compounds, Recommendations 1976, Eur. J. Biochem. 86, 1-8 (1978); also pp. 491-511 in [ref 10] and pp. 19-26 in [ref 11].
10. International Union of Pure and Applied Chemistry Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H, Pergamon Press, Oxford (1979).
11. International Union of Biochemistry and Molecular Biology Biochemical Nomenclature and Related Documents, Second edition, Portland Press, London (1992).
12. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC), Nomenclature of Organic Chemistry, Revised Section F: Natural Products and Related Compounds, Recommendations 1999, Pure App. Chem. 71, 587-643 (1999).
13. International Union of Pure and Applied Chemistry A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford (1993); corrections Pure App. Chem. 71, 1327-1330 (1999).