Contents
The lignans comprise a class of natural plant products which are derived from cinnamic acid derivatives and which are related biochemically to phenylalanine metabolism. They fall into five major subgroups and the nomenclature in use is neither consistent between the groups or even within a subgroup.
Many lignans show physiological activity such as the tumour inhibiting podophyllotoxins. This specific activity leads to interference with cell division by two different mechanisms in animals including humans. Some are active in suppressing the central nervous system and inhibiting cyclic-AMP phosphodiesterase, while others act as fish poisons or germination inhibitors. In Chinese traditional medicine lignans are used for treatment of viral hepatitis and protection of the liver.
The growing interest in the lignans and neolignans, and the increasing number of variations of their skeletons make a rational system for naming them a necessity.
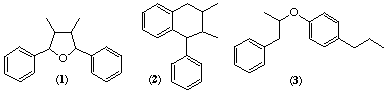
Robinson [ref 1] recognised in 1927 a common feature of many natural products was a C6C3 unit (i.e. a propylbenzene skeleton) perhaps derived from cinnamyl units. In a review of natural resins Haworth [ref 2] proposed that the class of compounds derived from two C6C3 units β,β'-linked should be called lignans (his original spelling was lignane but the 'e' was deleted in subsequent publications). The nomenclature of the diverse range of structures classified as lignans depended largely on trivial names and if necessary the appropriate numbering derived from the systematic name. This resulted sometimes in alternative numbering systems for closely related compounds with potential ambiguity in the naming of analogues. For example in the arylnaphthalenes (2) (2,7'-cyclolignane see Table 1 and LG-2.1) the aryl group might be attached to the naphthalene at C-1 or C-4 depending on the location of functional groups.
In an extensive review by Hearon and MacGregor [ref 3] the different skeletal types were consistently numbered although there was no correlation between them. Freudenberg and Weinges [ref 4] proposed in 1961 that the C6C3 unit be numbered from 1 to 9 and the second unit from 1' to 9' to provide a consistent numbering system. Thus the lignans all had an 8-8' linkage. In addition they proposed the term cyclolignans for lignans with an additional ring. This system was discussed in more detail by Weinges et al. in 1978 [ref 5].
The term neolignan was defined by Gottlieb [ref 6] as including the lignans and also related compounds where the two C6C3 units are joined by other bonds (e.g. 3-3' instead of 8-8'). In addition he included compounds where an oxygen atom provides the link between the two C6C3 units. With the arylnaphthalenes the additional ring was formed between 7 and 2' rather than 7 and 6' as proposed by Weinges [ref 4].
The inclusion of the lignans as a type of neolignan has not been generally accepted. Whiting [ref 7] in his review separated the lignans (i.e. with an 8-8' linkage) from the neolignans and highlighted their unsatisfactory nomenclature. The anomalies that have accrued in describing the stereochemistry of the podophyllotoxin group of lignans were reviewed by Dewick and Jackson [ref 8].
Some of the differences between the various proposed nomenclature systems are shown by the three skeletal types illustrated by (1) to (3). Table 1 shows some of the ways these sub-classes of lignans have been described.

| Skeleton e.g. (1) | Skeleton e.g. (2) | Skeleton e.g. (3) | Reference |
| 7,7'-epoxylignane | 2,7'-cyclolignane | 8,4'-oxyneolignane | This paper |
| 2,5-diaryltetrahydrofuran | 4-aryl-1,2,3,4-tetrahydronaphthalene | - | [ref 3] |
| 7,7'-epoxylignane | cyclolignane | - | [ref 4] |
| 7.O.7',8,8'-lignan | 8.8',7.2'-lignan | 8.O.4'-lignan | [ref 6] |
| 2,5-diaryl-3,4-dimethyltetrahydrofuran | 1-aryltetralin | (8-O-4')-neolignan | [ref 7] |
The locant columns are headed by those used in this document [see LG-2.1(a) for 2,7'-cyclolignane (5)] compared with the Chemical Abstracts Service (CAS) index heading parent numbering. Primed numbers refer to the aryl group attached to the index heading parent. An asterisk refers to a carboxy group.
| CAS Index Heading Parent | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 1' | 2' | 3' | 4' | 5' | 6' | 7' | 8' | 9' | Example |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5H-benzo[kl]bis[1,3]dioxolo- [4,5-b:4',5'-g]xanthen-5-one | 5a | 14b | 14a | 3a | 4 | 5 | 6 | 7 | - | 8b | 9 | 9a | 12a | 13 | 13a | 8a | 8 | - | carpanone |
| 1,3-benzodioxole | 4a' | 8a' | 8' | 7' | 6' | 5' | 4' | 3' | - | 5 | 4 | 3a | 7a | 7 | 6 | 1' | 2' | - | isogalcatin |
| furo[3',4':6,7]naphtho[2,3-d]- -1,3-dioxole-5,8-dione | 4a | 9a | 10 | 10a | 3a | 4 | 5 | 5a | 6 | 1' | 2' | 3' | 4' | 5' | 6' | 9 | 8a | 8 | isopicro- podophyllone |
| furo[3',4':6,7]naphtho[2,3-d]- -1,3-dioxol-6(8H)-one | 4a | 9a | 10 | 10a | 3a | 4 | 5 | 5a | 6 | 5' | 4' | 3a' | 7a' | 7' | 6' | 9 | 8a | 8 | justicidin D |
| furo[3',4':6,7]naphtho[2,3-d]- -1,3-dioxol-6(5aH)-one | 9a | 4a | 4 | 3a | 10a | 10 | 9 | 8a | 8 | 1' | 2' | 3' | 4' | 5' | 6' | 5 | 5a | 6 | podophyllotoxin |
| furo[3',4':6,7]naphtho[2,3-d]- -1,3-dioxol-6(8H)-one | 9a | 4a | 4 | 3a | 10a | 10 | 9 | 8a | 8 | 5' | 4' | 3a' | 7a' | 7' | 6' | 5 | 5a | 6 | justicidin F |
| furo[3',4':6,7]naphtho[1,2-d]- -1,3-dioxol-7(9H)-one | 5a | 10a | 10b | 3a | 4 | 5 | 6 | 6a | 7 | 5' | 4' | 3a' | 7a' | 7' | 6' | 10 | 9a | 9 | justicinol |
| naphthalene | 4a | 8a | 8 | 7 | 6 | 5 | 4 | 3 | - | 1' | 2' | 3' | 4' | 5' | 6' | 1 | 2 | - | galbulin |
| 2-naphthalenecarboxylic acid | 4a | 8a | 8 | 7 | 6 | 5 | 4 | 3 | - | 1' | 2' | 3' | 4' | 5' | 6' | 1 | 2 | * | plicatic acid |
| 2-naphthalenecarboxylic acid | 8a | 4a | 5 | 6 | 7 | 8 | 1 | 2 | * | 1' | 2' | 3' | 4' | 5' | 6' | 4 | 3 | - | thomasic acid |
| 2,3-naphthalenedicarboxylic acid | 4a | 8a | 8 | 7 | 6 | 5 | 4 | 3 | * | 1' | 2' | 3' | 4' | 5' | 6' | 1 | 2 | * | thomasidioic acid |
| 2,3-naphthalenedimethanol | 4a | 8a | 8 | 7 | 6 | 5 | 4 | 3 | α | 1' | 2' | 3' | 4' | 5' | 6' | 1 | 2 | α | isoolivil |
| 2,3-naphthalenediol | 8a | 4a | 4 | 3 | 2 | 1 | 8 | 7 | - | 1' | 2' | 3' | 4' | 5' | 6' | 5 | 6 | - | norisoguaiacin |
| 2-naphthalenol | 4a | 8a | 1 | 2 | 3 | 4 | 5 | 6 | - | 1' | 2' | 3' | 4' | 5' | 6' | 8 | 7 | - | guaiacin |
| 2-naphthalenol | 4a | 8a | 1 | 2 | 3 | 4 | 5 | 6 | - | 5' | 4' | 3a' | 7a' | 7' | 6' | 8 | 7 | - | otobaphenol |
| naphtho[1,2-d]-1,3-dioxole | 5a | 9a | 9b | 3a | 4 | 5 | 6 | 7 | - | 5' | 4' | 3a' | 7a' | 7' | 6' | 9 | 8 | - | otobain |
| naphtho[1,2-d]-1,3-dioxole | 8a | 4a | 4 | 3a | 9a | 9 | 8 | 7 | - | 1' | 2' | 3' | 4' | 5' | 6' | 5 | 6 | - | galcatin |
| naphtho[1,2-d]-1,3-dioxole | 9a | 5a | 5 | 4 | 3a | 9b | 9 | 8 | - | 1' | 2' | 3' | 4' | 5' | 6' | 6 | 7 | - | hypophyllanthin |
| naphtho[1,2-d]-1,3-dioxol-9-ol | 5a | 9a | 9b | 3a | 4 | 5 | 6 | 7 | - | 5' | 4' | 3a' | 7a' | 7' | 6' | 9 | 8 | - | hydroxyotobain |
| naphtho[1,2-d]-1,3-dioxol- -6(7H)-one | 5a | 9a | 9b | 3a | 4 | 5 | 6 | 7 | - | 5' | 4' | 3a' | 7a' | 7' | 6' | 9 | 8 | - | otobanone |
| naphtho[2,3-c]furan-1(3H)-one | 4a | 8a | 8 | 7 | 6 | 5 | 4 | 3a | 3 | 1' | 2' | 3' | 4' | 5' | 6' | 9 | 9a | 1 | sikkimotoxin |
| naphtho[2,3-c]furan-1(3H)-one | 4a | 8a | 8 | 7 | 6 | 5 | 4 | 3a | 3 | 5' | 4' | 3a' | 7a' | 7' | 6' | 9 | 9a | 1 | collinusin |
| naphtho[2,3-c]furan-1(3H)-one | 8a | 4a | 5 | 6 | 7 | 8 | 9 | 9a | 1 | 1' | 2' | 3' | 4' | 5' | 6' | 4 | 3a | 3 | conidendrin |
| naphtho[2,3-c]furan-1(3H)-one | 8a | 4a | 5 | 6 | 7 | 8 | 9 | 9a | 1 | 5' | 4' | 3a' | 7a' | 7' | 6' | 4 | 3a | 3 | justicidin C |
| phenol | 8a' | 4a' | 4' | 3a' | 9a' | 9' | 8' | 7' | - | 4 | 3 | 2 | 1 | 6 | 5 | 5' | 6' | - | attenuol |
The recommendations in this document are based on the general IUPAC recommendations for naming natural products [ref 9, ref 12]. Existing trivial names for lignans and neolignans may still be used but it is recommended that the semisystematic name for the lignan or neolignan also be quoted in a paper when first encountered. It should be noted that several different numbering systems are in use so that it must be clear with a modified trivial name which system is being used.
LG-1 Fundamental Parent Structures
The lignans and neolignans are a class of natural products derived from two C6C3 molecules (4) by coupling. The term lignan was introduced by Haworth [ref 2] for the structures where the two units are β,β'-linked. When the two are coupled in other ways (m,m'; γ,γ'; β,m'; etc) they are called neolignans. This group is also considered to include examples where the two units are joined by an ether oxygen atom which for nomenclature purposes is treated as a linking oxygen of an assembly.
Note: (2) For the purposes of numbering the various parent structures the C6C3 unit (4) is numbered 1 to 9 where the α position is 9, β is 8 and γ is 7.
If the two C6C3 units (4) are linked by a β,β'-bond the parent structure lignane (5) is used as the basis for naming the lignan.
LG-1.2 Neolignane
If the two C6C3 units (4) are linked by a bond other than a β,β'-bond the parent structure, neolignane, is used as the basis for naming the neolignan. The locants of the bond linking the two units are cited in front of the name and with the second number primed. Where there is a choice of locants preference is made in the order:
If the bond linking the two C6C3 units (4) of a neolignan involves position 1 (or 1') the corresponding phenyl ring cannot be aromatic. The maximum number of noncumulative double bonds is two and there must be one position (other than C-1 or C-1') which is saturated in the parent hydrocarbon. This position is indicated by means of the locant of this position and an uppercase italic H (or underlined type) i.e. indicated hydrogen (see rule A-21.6 [ref 10], R-1.3 [ref 13]). Where there is a choice lower locants are selected.
Examples:
If the two C6C3 units (4) are linked by an ether oxygen atom and not directly bonded together the parent structure, oxyneolignane, is used as the basis for naming the neolignan. The locants of the two positions linked by the ether oxygen are cited in front of the name with the second number primed. Where there is a choice of locants preference is made in order to:
LG-1.5 Numbering of Lignane, Neolignane and Oxyneolignane Skeletons
The parent skeletons illustrated in LG-1.1, 1.2, 1.3 and 1.4 also show the appropriate numbering. If there is still a choice of numbering preference is made in order to:
(b) lower locants required to identify indicated hydrogen (see LG-1.3)
(c) lower locants required to identify the principal functional group expressed as a suffix (see LG-4)
(d) lower locants required to identify double bonds in the side chain (see LG-3.1)
(e) lower locants required to identify substituents expressed as a prefix when the locants are considered as a set (e.g. see balanophonin in LG-5.4; the methoxy is at 3 not 5)
(f) lower locants required to identify substituents expressed as a prefix when considered in the order of citation (e.g. see second example in LG-5.4; the methoxy is at 3 not 5).
1. Robinson, R. Proc. Univ. Durham Phil. Soc. 8, 14-59 (1927-8).
2. Haworth, R. D. Ann. Rep. Prog. Chem. 33, 266 (1936).
3. Hearon, W. M. and MacGregor, W. S. Chem. Rev. 55, 957-1068 (1955).
4. Freudenberg, K. and Weinges, K. Tetrahedron 15, 115 (1961).
5. Weinges, K., Nader, F. and Kunstler, K. (1978) Chemistry of Lignans ed. by C.B.S. Rao pp.1-37.
6. Gottlieb, O. R. Fortschr. Chem. Org. Naturstoff. 35, 1-72 (1978).
7. Whiting, D. A. Nat. Prod. Rep. 2, 191-211 (1985); 4, 499-525 (1987); 7, 349-364 (1990).
8. Dewick, P. M. and Jackson, D. E. Phytochem. 20, 2277-2280 (1981).
9. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC), Nomenclature of Organic Chemistry, Section F: Natural Products and Related Compounds, Recommendations 1976, Eur. J. Biochem. 86, 1-8 (1978); also pp. 491-511 in [ref 10] and pp. 19-26 in [ref 11].
10. International Union of Pure and Applied Chemistry Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H, Pergamon Press, Oxford (1979).
11. International Union of Biochemistry and Molecular Biology Biochemical Nomenclature and Related Documents, Second edition, Portland Press, London (1992).
12. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC), Nomenclature of Organic Chemistry, Revised Section F: Natural Products and Related Compounds, Recommendations 1999, Pure App. Chem. 71, 587-643 (1999).
13. International Union of Pure and Applied Chemistry A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford (1993); corrections Pure App. Chem. 71, 1327-1330 (1999).
Note: (1) The class names lignan and neolignan are spelt in the conventional way without a terminal 'e'. The parent structures below are spelt with a terminal 'e' to indicate a saturated side chain unless modified to show unsaturation (see LG-3.1).
LG-1.1 Lignane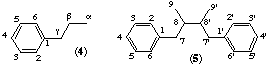
(a) position 8
Examples:
(b) lower unprimed numbers
(c)lower primed numbers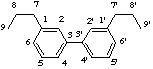
3,3'-neolignane
not 5,5'-neolignane, 3 is lower than 5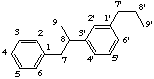
8,3'-neolignane
not 3,8'-neolignane, 8 is preferred to 8'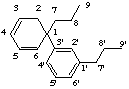
2H-1,3'-neolignane
not 6-H1,3'-neolignane, 2 is lower than 6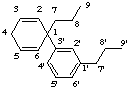
4H-1,3'-neolignane(a) position 8
Examples:
(b) lower unprimed numbers
(c) lower primed numbers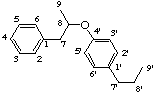
8,4'-oxyneolignane
not 4,8'-oxyneolignane, 8 is preferred to 8'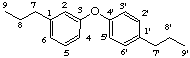
3,4'-oxyneolignane
not 5,4'- or 4,3'- or 4,5'-oxyneolignane(a) lower locants required to identify modifications of the parent structure (see LG-2)
These criteria follow the established procedures for systematic nomenclature [ref 10, ref 13].
Continue with LG-2 Modified Parent Structures
Return to Lignan home page.