The choice of orientation is made in the order:
(a) unprimed numbers on the leftIt is conventional to draw the 2,7'-cyclolignane skeleton with the tetrahydro-naphthalene ring horizontal and the phenyl group below, and the 2,2'-cyclolignane skeleton with one benzene ring above the other as exceptions to this recommendation.
(b) unprimed numbers 1-6 are placed in the lower left corner
(c) primed numbers 1'-6' are placed in the lower right corner
(d) lowest numbered chiral centre in the α form.
Stereochemistry associated with a ring system which includes position 8 is shown by means of α (indicating below the plane) or β (indicating above the plane) for a bridgehead hydrogen (or substituent) or for a substituent attached to the ring system (see rule RF-10.1 [ref 12]). If there are two substituents at one position preference is given to:
(a) the substituent which includes a skeletal atom
(b) the group preferred by the sequence rules (see appendix to section E [ref 14], and ref 15)
If α or β are not applicable then R or S should be used (see rule R-7.2.1 [ref 13], rule E-4.9 [ref 14]). With bridged bicyclic systems the largest ring is considered to be the plane and the stereochemistry of the shortest bridge is indicated by α or β. If there are two bridges of equal length it is recommended to use R or S for each bridgehead configuration. Also the stereochemistry due to substituents on a bridge should be indicated by R or S. In the rare cases where double bond stereochemistry is relevant this should be indicated by the italic letters E or Z (see rule R-7.1.2 [ref 13], rule E-2.2 [ref 14]). Each group of stereodescriptors is placed in brackets in front of the name. If the stereochemistry is unknown this is indicated by ξ (xi).
Unless indicated to the contrary (see LG-5.4) it is assumed that absolute configuration is implied by the configuration symbols (LG-5.2).
Examples:
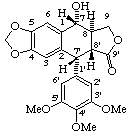
(7α,7'α,8α,8'β)-7-hydroxy-3',4',5'-trimethoxy-4,5-methylenedioxy-2,7'-cyclolignano-9',9-lactone
(trivial name podophyllotoxin)
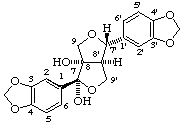
(7β,7'α,8α,8'α)-3,4:3',4'-bis(methylenedioxy)-7,9':7',9-diepoxylignane-7,8-diol
(trivial name arboreol)
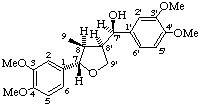
(7'R)-(7α,8β,8'β)-3,3',4,4'-tetramethoxy-7,9'-epoxylignan-7'-ol
(trivial name magnostellin A)
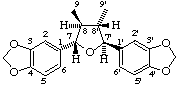
(7α,7'β,8β,8'α)-3,4:3',4'-bis(methylenedioxy)-7,7'-epoxylignane
(trivial name (-)-galbacin)
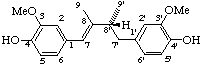
(7E)-(8'R)-3,3'-dimethoxylign-7-ene-4,4'-diol
(trivial name (-)-guaiaretic acid)
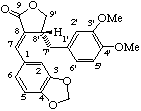
(7E)-(8'α)-3',4'-dimethoxy-3,4-methylenedioxylign-7-eno-9,9'-lactone
Note: The two structures above are both 7E due to the priority rules [ref 14, ref 15].
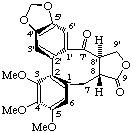
(2Ra)-(8β,8'α)-3,4,5-trimethoxy-4',5'-methylenedioxy-7'-oxo-2,2'-cyclolignano-9,9'-lactone
(trivial name (-)-steganone)
Note: 2Ra refers to the axial chiral biphenyl unit (see ref 15 for details).
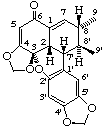
(3R)-(2β,7'β,8α,8'β)-1,7-didehydro-1,2,3,6-tetrahydro-2',3-epoxy-3,4:4',5'-bis(methylenedioxy)-2,7'-cyclolignan-6-one
(trivial name carpanone)
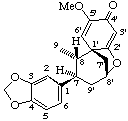
(1'β,7β,8α,8'β)-5'-methoxy-3,4-methylenedioxy-2',8'-epoxy-7,9'-cyclo-4'H-8,1'-neolignan-4'-one
(trivial name futoenone)
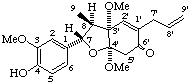
(3'α,4'α,7α,8β)-4',5'-dihydro-4-hydroxy-3,3',4'-trimethoxy-4',7-epoxy-8,3'-neolign-8'-en-6'(3'H)-one
(trivial name liliflone)
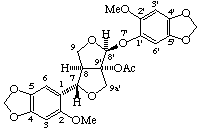
(7α,8α,8'β,9'α)-2,2'-dimethoxy-4,5:4',5'-bis(methylenedioxy)-7,9a':8',9-diepoxy-7'-oxa-9a'-homo--8,9'-neolignan-9'-yl acetate
(trivial name phrymarolin I)
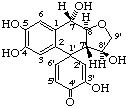
(1'ξ,7α,7'β,8α,8'β)-3',4,5,7,8'-pentahydroxy-8,9'-epoxy-1',2-cyclo-9-nor-8,7'-neolignan-4'-one
(trivial name hydroxyathrotaxin)
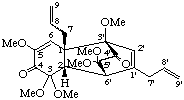
(3'R,6'S)-(1β,2β)-2,3,5',6'-tetrahydro-3,3,3',5,5',5'-hexamethoxy-2,6'-cyclo-1,3'-neoligna-8,8'-diene--4,4'(3'H)-dione
(trivial name asatone)
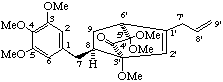
(3'S,6'S,8R)-5',6'-dihydro-3,3',4,5,5',5'-hexamethoxy-6',9-cyclo-8,3'-neolign-8'-en-4'(3'H)-one
(trivial name heterotropanone)
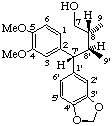
(7'S,8R,8'S)-4,5-dimethoxy-3',4'-methylenedioxy-1,7-seco-2,7'-cyclolignan-7-ol
Note: Due to the acyclic nature of 7', 8 and 8' the R/S-system is used rather than α/β. Also see note to LG-2.2.
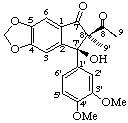
(8'R)-(7'α)-7'-hydroxy-3',4'-dimethoxy-4,5-methylenedioxy-7(8
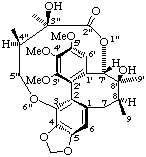
(2Sa,3"R,4"R)-(7'α,8β,8'α)-3",8'-dihydroxy-3',4',5'-trimethoxy-3",4"-dimethyl-4,5-methylenedioxy--3,7'-(epoxybutanoxy)-2,2'-cyclolignan-2"-one
(trivial name gomisin D)
Note that although the principal functional group is a lactone one of the oxygen atoms is included in the bridge so that the oxygen atom of the carbonyl group is treated as a "ketone".
When the relative, but not the absolute, configuration is known this is shown by prefixing the name of one enantiomer with rel- (see rule E-4.10(b) [ref 14], rule RF-10.6 [ref 12]). If known the sign of rotation of polarised light (+) or (-) may be added. If R or S is needed to describe the stereochemistry then all chiral centres will need to be described using R* or S* (see rule E-4.10 (a) [ref 14], rule R-7.2.2 [ref 13], F-6.7 [ref 9], rule RF-10.6 [ref 12]).
Examples:
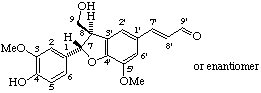
rel-(7'E)-(7α,8β)-4,9-dihydroxy-3,5'-dimethoxy-4',7-epoxy-8,3'-neolign-7'-en-9'-al
(trivial name balanophonin)
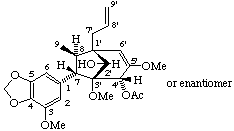
rel-(2'R)-(1'β,3'β,4'α,7α,8β)-3',4'-dihydro-2'-hydroxy-3,3',5'-trimethoxy-4,5-methylenedioxy-
-2'H-3',7-cyclo-8,1'-neolign-8'-en-4'-yl acetate
The relative configuration of an achiral lignan, neolignan or oxyneolignan is specified by the configuration symbols (LG-5.2) in the same way as before (LG-5.3).
Example:
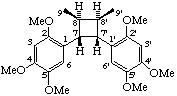
(7α,7'α,8β,8'β)-2,2',4,4',5,5'-hexamethoxy-7,7'-cyclolignane
(trivial name heterotropan)
LG-5.5 Racemate
Racemates are named by citing the italicised prefix rac- in front of the whole name for the enantiomer with the lowest numbered chiral centre in the α form (see rule F-6.6 [ref 9], rule RF-10.5 [ref 12]).
Examples:
The stereochemistry of enantiomers are in general clearly indicated by the procedure of LG-5.3. If it is necessary to indicate an enantiomeric relationship between two lignans or neolignans where only the relative configuration is known, or if not even the relative configuration is known then the italicised prefix ent- may be used to show this relationship (see rule F-6.5 [ref 9], rule RF-10.3 [ref 12]). The prefix ent- is more commonly used to show the relationship using trivial names.
Examples:
9. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC), Nomenclature of Organic Chemistry, Section F: Natural Products and Related Compounds, Recommendations 1976, Eur. J. Biochem. 86, 1-8 (1978); also pp. 491-511 in [ref 10] and pp. 19-26 in [ref 11].
10. International Union of Pure and Applied Chemistry Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H, Pergamon Press, Oxford (1979).
11. International Union of Biochemistry and Molecular Biology Biochemical Nomenclature and Related Documents, Second edition, Portland Press, London (1992).
12. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC), Nomenclature of Organic Chemistry, Revised Section F: Natural Products and Related Compounds, Recommendations 1999, Pure App. Chem. 71, 587-643 (1999).
13. International Union of Pure and Applied Chemistry A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford (1993); corrections Pure App. Chem. 71, 1327-1330 (1999).
14. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC), Nomenclature of Organic Chemistry, Section E: Stereochemistry, Recommendations 1974, Pure Appl. Chem. 45, 11-30 (1976) also on pp. 473-490 in [ref 10] and pp. 1-18 in [ref 11]. See [ref 15] for a more complete treatment of the R,S-system.
15. Cahn, R. S., Ingold, C. K. and Prelog, V. Angew. Chem. 78, 413-447 (1966); Angew. Chem. Int. Ed. Engl. 5, 385-415, 511 (1966); Prelog, V. and Helmchen, G., Angew. Chem. 94, 614-631 (1982); Angew. Chem. Int. Ed. Engl. 21, 567-583 (1982).
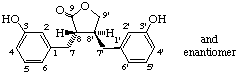
rac-(8α,8'β)-3,3'-dihydroxylignano-9,9'-lactone
(trivial name enterolactone)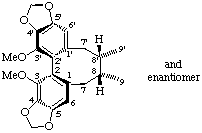
rac-(8α,8'α)-3,3'-dimethoxy-4,5:4',5'-bis(methylenedioxy)-2,2'-cyclolignane
(trivial name (±)-wuweizisu C)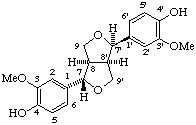
(7α,7'β,8α,8'α)-3,3'-dimethoxy-7,9':7',9-diepoxylignane-4,4'-diol
(trivial name (+)-epipinoresinol or ent-symplocosigenol)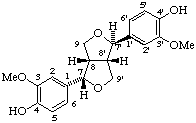
(7α,7'β,8β,8'β)-3,3'-dimethoxy-7,9':7',9-diepoxylignane-4,4'-diol
(trivial name (-)-epipinoresinol or symplocosigenol)
Continue with LG-6 and LG-7 Trimers and Higher Analogues, Summary and References
Return to Lignan home page.