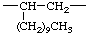 | 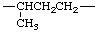 | |
| 1-decylethylene (not dodecane-2,1-diyl according to ref 5) | 1-methylpropane-1,3-diyl (not butane-3,1-diyl according to ref 5) | |
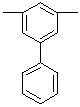 | 5-phenyl-1,3-phenylene (not biphenyl-3,5-diyl | |
Contents of this section
Rule 18
The subunits and substituted subunits are named by organic nomenclature rules [ref 4, ref 5]. Substituted subunits are parenthesized or bracketed.
Note 1: Alkyl-substituted acyclic carbon chain subunits are named as such, not as single-chain units to differentiate between the length of the acyclic carbon chain, which is part of the main chain (backbone), and an acyclic carbon chain substituent on that backbone. This is an exception from the rules in [ref 5].
Similarly, heterocyclic or carbocyclic ring assemblies, if not in the main chain (backbone), are not named as such, but as substituted rings or ring systems.
Examples:
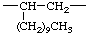 | 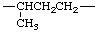 | |
| 1-decylethylene (not dodecane-2,1-diyl according to ref 5) | 1-methylpropane-1,3-diyl (not butane-3,1-diyl according to ref 5) | |
 | 5-phenyl-1,3-phenylene (not biphenyl-3,5-diyl | |
Note 2: A list of names of common subunits is in Appendix 11.1.
Rule 19
The name of the CRU is formed from the names of its subunits, including substituents (substituted subunits), and cited in order from left to right as they appear in the CRU.
Examples:
7. Naming the Polymer
-OCH2CH2- oxyethylene oxy(phenylmethylene) oxy[1-(methoxycarbonyl)ethylene] (dimethyliminio)ethylene bromide
Rule 20
Polymers (or oligomers) are named with the prefix poly (or oligo) followed in parentheses or brackets by the name of the CRU. If the name of the repeating unit is "ABC", the corresponding polymer (or oligomer) name is
Note: Where it is desired to specify the chain length in an oligomer, the appropriate Greek prefix (deca, docosa, etc.) may be used.poly(ABC) or oligo(ABC)
Example:
Rule 21deca(oxyethylene)
End-groups may be specified by prefixes placed ahead of the name of the polymer. The end-group designated by α is that attached to the left-hand side of the CRU written as described in the preceding rules, and the other end-group is designated by ω; the end-groups are cited in that order. If there is a choice, the end-group with the name starting earlier in the alphabet should be cited first.
Examples:
8. Polymer Chain as a Substituent(not α-methyl-ω-hydroxypoly(oxyethylene); alphabetical order of end-groups decides)
α-(trichloromethyl)-ω-chloropoly(1,4-phenylenemethylene) α-hydro-ω-methoxypoly(oxyethylene)
(a three-star polymer consisting of a central branch point and three single-strand chains, wherein the benzene ring is the end-group linking the three chains)
CH3O(CH2CH2O)nCO(CH2)4CO(OCH2CH2)nOCH3 α,α'-adipoylbis[ω-methoxypoly(oxyethylene)] α,α',α"-benzene-1,3,5-triyltris[poly(1-phenylethylene)]
Rule 22
If a regular single-strand chain is linked to a constitutional unit of the main chain (backbone) of a polymer molecule or to a low-molecular-weight structure, either directly or through an intervening unit, it is considered a substituent of the constitutional unit or structure. In naming the polymeric substituent, the actual bonding relations are reflected in the name of the CRU.
Examples:
[poly(oxyethylene)]imino
(a polymer-substituted imino subunit)[poly(methyleneoxy)]methylene
(a polymer-substituted methylene subunit){[poly(methyleneoxy)]methyl}methylene
(a polymer-substituted substituted methylene subunit)poly(1-{4-[poly(ethyleneoxy)]phenyl}ethylene)
(a polymer-substituted polymer)1,3,5-tris[poly(2-phenylethylene)]benzene
(a polymer-substituted low-molecular-weight compound)
4. IUPAC. Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, Pergamon Press, Oxford (1979).
5. IUPAC. A Guide to IUPAC Nomenclature of Organic Compounds, Blackwell Scientific Publications, Oxford (1993).