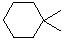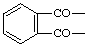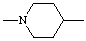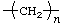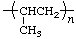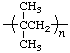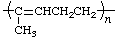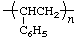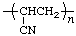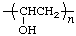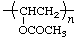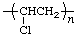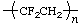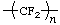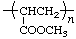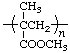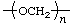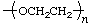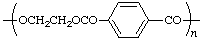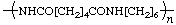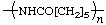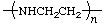Nomenclature of Regular Single-Strand Organic Polymers
10. and 11.
Continued from 9. Examples of Polymer Names 61-79
Contents of this section
10. References
1. IUPAC. "Report on nomenclature in the field of macromolecules", J. Polym. Sci. 8, 257-277 (1952).
2. IUPAC. "Report on nomenclature dealing with steric regularity in high polymers", Pure Appl. Chem. 12, 643-656 (1966).
3. IUPAC. "Nomenclature of regular single-strand organic polymers 1975", Pure Appl. Chem. 48, 373-385 (1976). Reprinted as Chapter 5 in IUPAC. Compendium of Macromolecular Nomenclature, Blackwell Scientific Publications, Oxford (1991) (PDF).
4. IUPAC. Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, Pergamon Press, Oxford (1979).
5. IUPAC. A Guide to IUPAC Nomenclature of Organic Compounds, Blackwell Scientific Publications, Oxford (1993).
6. IUPAC. "Stereochemical definitions and notations relating to polymers 1980", Pure Appl. Chem. 53, 733-752 (1981). Reprinted as Chapter 2 in IUPAC. Compendium of Macromolecular Nomenclature, Blackwell Scientific Publications, Oxford (1991) (PDF).
7. IUPAC. "Nomenclature of regular double-strand (ladder and spiro) organic polymers 1993", Pure Appl.Chem. 65, 1561-1580 (1993).
8. IUPAC. "Structure-based nomenclature for irregular single-strand organic polymers 1993", Pure Appl.Chem. 66, 873-889 (1994).
9. IUPAC. "Glossary of basic terms in polymer science 1996", Pure Appl.Chem. 68, 2287-2311 (1996).
10. IUPAC. "Nomenclature for regular single-strand and quasi-single-strand inorganic and coordination polymers 1984", Pure Appl.Chem. 57, 149-168 (1985).Reprinted as Chapter 6 in IUPAC. Compendium of Macromolecular Nomenclature, Blackwell Scientific Publications, Oxford (1991) (PDF) and as Chapter II-7 in IUPAC. Nomenclature of Inorganic Chemistry II, Royal Society of Chemistry, Cambridge (2001).
11. IUPAC. "Nomenclature of fused and bridged fused ring systems 1998", Pure Appl.Chem. 70, 143 -216 (1998).
11. Appendix
11.1 List of names of common subunits
(The use of subunits denoted with asterisk is not recommended [ref 5].)
| Adipoyl | -CO(CH2)4CO- |
| Azo* | see Diazenediyl |
| Azoimino* | see Triazene-1,3-diyl |
| Azoxy | -N(O)=N -or -N=N(O)- |
| Benzoylimino | C6H5CON< |
| Benzylidene* | see Phenylmethylene |
| Biphenyl-3,5-diyl | see 5-Phenyl-1,3-phenylene |
| Biphenyl-4,4'-diyl | 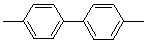 |
| Butanedioyl | -COCH2CH2CO- |
| Butane-1,1-diyl | see Propylmethylene |
| Butane-1,4-diyl | -(CH2)4- |
| Butylidene* | see Propylmethylene |
| But-1-ene-1,4-diyl | -CH=CHCH2CH2- |
| Carbonimidoyl | -C(=NH)- |
| Carbonothioyl | -CS- |
| Carbonyl | -CO- |
| Cyclohexane-1,1-diyl | 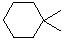 |
| Cyclohexane-1,4-diyl | 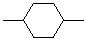 |
| Cyclohexylidene* | see Cyclohexane-1,1-diyl |
| Decanedioyl | -CO(CH2)8CO- |
| Diazenediyl | -N=N- |
| Dimethylmethylene | (CH3)2C< |
| Dioxy* | see Peroxy |
| Diphenylmethylene | (C6H5)2C< |
| Disulfanediyl | -SS- |
| Dithio* | see Disulfanediyl |
| Ethanedioyl | see Oxalyl |
| Ethane-1,1-diyl | see Methylmethylene |
| Ethane-1,2-diyl* | see Ethylene |
| Ethanediylidene | =CHCH= |
| Ethene-1,2-diyl | -CH=CH- |
| Ethylene | -CH2CH2- |
| Ethylidene* | see Methylmethylene |
| Glutaryl | -COCH2CH2CH2CO- |
| Hexamethylene* | see Hexane-1,6-diyl |
| Hexanedioyl | see Adipoyl |
| Hexane-1,6-diyl | -(CH2)6- |
| Hydrazine-1,2-diyl | -NHNH- |
| Hydrazo* | see Hydrazine-1,2-diyl |
| Hydroxyimino | HO-N< |
| Imino | -NH- |
| Iminio | -NH+- |
| Isophthaloyl | 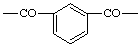 |
| Isopropylidene* | see Dimethylmethylene |
| Malonyl | -COCH2CO- |
| Methanylylidene | -CH= |
| Methylene | -CH2- |
| 1-Methylethane-1,1-diyl | see Dimethylmethylene |
| 1-Methylethylene | -CH(CH3)CH2- |
| Methylidenemethylene | CH2=C< |
| Methylidyne (-CH=) | see Methanylylidene |
| Methylmethylene | CH3CH< |
| Methylylidene | see Methanylylidene |
| Naphthalene-1,8-diyl | 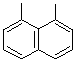 |
| Nitrilo | -N= |
| Oxalyl | -COCO- |
| Oxy | -O- |
| Pentamethylene | see Pentane-1,5-diyl |
| Pentanedioyl | see Glutaryl |
| Pentane-1,5-diyl | -(CH2)5- |
| Peroxy | -OO- |
| 1,4-Phenylene | 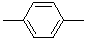 |
| Phenylmethylene | C6H5CH< |
| 5-Phenyl-1,3-phenylene | 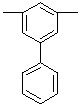 |
| Phthaloyl | 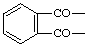 |
| Piperidine-1,4-diyl | 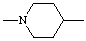 |
| Propanedioyl | see Malonyl |
| Propane-1,3-diyl | -(CH2)3- |
| Propane-2,2-diyl | see Dimethylmethylene |
| Propylene* | see 1-Methylethylene |
| Propylmethylene | CH3CH2CH2CH< |
| Silanediyl | -SiH2- |
| Silylene | see Silanediyl |
| Succinyl | -COCH2CH2CO- |
| Sulfanediyl | -S- |
| Sulfinyl | -SO- |
| Sulfonyl | -SO2- |
| Thio* | see Sulfanediyl |
| Terephthaloyl | 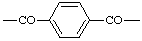 |
| Tetramethylene | see Butane-1,4-diyl |
| Thiocarbonyl* | see Carbonothioyl |
| Triazene-1,3-diyl | -N=N-NH- |
| Trimethylene* | see Propane-1,3-diyl |
| Vinylene* | see Ethene-1,2-diyl |
| Vinylidene | see Methylidenemethylene |
11.2 Structure- and source-based names for common polymers
The Commission recognized that a number of common polymers have semisystematic or trivial source-based names that are well established by usage; it is not intended that they be immediately supplanted by the structure-based names. Nevertheless, it is hoped that for scientific communication the use of semisystematic or trivial source-based names for polymers will be kept to a minimum.
For the following idealized structural representations,the semisystematic or trivial source-based names given are approved for use in scientific work; the corresponding structure-based names are given as alternative names. Equivalent names for close analogues of these polymers [e.g., other alkyl ester analogues of poly(methyl acrylate)] are also acceptable.
| Structure | Source-based name
(preferred given first) | Structure-based name |
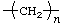 * * | polyethene
polyethylene** | poly(methylene) |
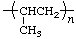 | polypropene
polypropylene | poly(1-methylethylene) |
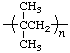 | poly(2-methylpropene)
polyisobutylene | poly(1,1-dimethylethylene) |
 | poly(buta-1,3-diene)
polybutadiene | poly(but-1-ene-1,4-diyl) |
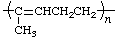 | polyisoprene | poly(1-methylbut-1-ene-1,4-diyl) |
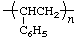 | polystyrene | poly(1-phenylethylene) |
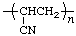 | polyacrylonitrile | poly(1-cyanoethylene) |
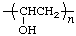 | poly(vinyl alcohol) | poly(1-hydroxyethylene) |
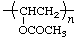 | poly(vinyl acetate) | poly(1-acetoxyethylene) |
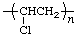 | poly(vinyl chloride) | poly(1-chloroethylene) |
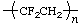 | poly(1,1-difluoroethene)
poly(vinylidene fluoride) | poly(1,1-difluoroethylene) |
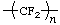 * * | poly(tetrafluoroethene)
poly(tetrafluoroethylene) | poly(difluoromethylene) |
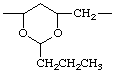 | poly(vinyl butyral)poly | [(2-propyl-1,3-dioxane-4,6-diyl)methylene] |
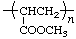 | poly(methyl acrylate) | poly[1-(methoxycarbonyl)ethylene] |
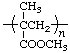 | poly(methyl methacrylate) | poly[1-(methoxycarbonyl)-1-methylethylene] |
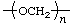 | polyformaldehyde | poly(oxymethylene) |
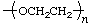 | poly(ethylene oxide) | poly(oxyethylene) |
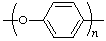 | poly(phenylene oxide) | poly(oxy-1,4-phenylene) |
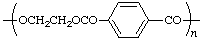 | poly(ethylene terephthalate) | poly(oxyethyleneoxyterephthaloyl) |
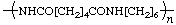 | poly(hexane-1,6-diyladipamide) | poly(iminoadipoyliminohexane-1,6-diyl) |
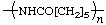 | poly(hexano-6-lactam)
poly(ε-caprolactam) | poly[imino(1-oxohexane-1,6-diyl)] |
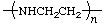 | polyaziridine
poly(ethylenimine) | poly(iminoethylene) |
* The formulae and are more often used; they are acceptable due to the past usage and an attempt to retain some similarity to the CRU formulae of homopolymers derived from other ethene derivatives.
** The name "ethylene" should be used for a divalent group, "-CH2CH2-" only and not for the monomer, "CH2=CH2".The latter is "ethene " [ref 5].
Return to home page
Return to IUPAC nomenclature home page