 | |
| 3-D Representation | Schlegel Representation |
Contents of this section
The classical definition of a fullerene is a compound composed solely of an even number of carbon atoms which form a cage-like fused-ring polycyclic system with twelve five-membered rings and the rest six-membered rings; the archetypal example is the sixty atom structure, where the atoms and bonds delineate a truncated icosahedron (refs. 4,5). However, the term has been broadened to include any closed-cage structure having more than twenty carbon atoms consisting entirely of three coordinate carbon atoms (ref. 3).
Fulleranes are fully saturated fullerenes, for example, the hydrocarbon C60H60.
Heterofullerenes, norfullerenes, homofullerenes, and secofullerenes have been called fulleroids (fullerene-like) because they resemble fullerenes in structure but do not conform to the definition of a fullerene as given above. It is convenient to refer to them as fulleroids and name them as modified fullerenes.
The recommended systematic names for the icosahedral C60 and the D5h(6) C70 fullerenes are (C60-Ih)[5,6]fullerene and (C70-D5h(6))[5,6]fullerene. The parenthetical prefix gives the carbon content and the point group symbol; and the bracketed numbers indicate the ring sizes in the fullerene. The latter is important in fullerenes with rings other than five- and six-membered. The subscript parenthetical (6) following the point group symbol D5h in the latter name indicates that the five-membered ring on the five-fold symmetry axis is surrounded by six-membered rings. This differentiates this fullerene from an isomeric (C70-D5h)[5,6]fullerene which has five-membered rings surrounding the five-membered ring on the five-fold symmetry axis which would have the name (C70-D5h(5))[5,6]fullerene. This technique is being further evaluated to determine its value for differentiating among other fullerenes with degenerate point group symbols. The recommended names have the same information as the corresponding CAS names, but in a different format.
The names [60-Ih]fullerene and [70-D5h]fullerene given in the IUPAC Preliminary Survey (ref. 1) are names first introduced into the literature for the C60-Ih and C70-D5h(6) fullerenes. Since important structural information is omitted from these names, they are to be considered as trivial names to be used only for these specific compounds.
Systematic numbering for (C60-Ih)[5,6]fullerene and (C70-D5h(6))[5,6]fullerene is based on the following rules adapted from the CAS publication (ref. 3). These rules are sufficient to systematically number (C60-Ih)[5,6]fullerene and (C70-D5h(6))[5,6]fullerene. More rules are needed for other fullerenes. The CAS rules (ref. 3) are being evaluated and, if found appropriate, will be included in a subsequent report.
Proper rotation axes, (Cn), are examined in sequence, from the highest-order to the lowest-order axis, until at least one contiguous spiral pathway is found that begins in a ring through which a proper rotation axis passes, at the end of a bond bisected by a proper rotation axis, or at an atom through which a proper rotation axis passes. Numbering begins at the end of such a contiguous spiral pathway.
If there is a choice for the selection of a reference axis or for the end of a reference axis to begin the numbering, a ring is preferred to a bond which, in turn is preferred to an atom.
When there is a choice between rings, a larger ring is preferred to a smaller one. Where there is still a choice, the preferred ring contains the preferred atom (see Fu-3.1.2.3) at the first point of difference.
When there is a choice between bonds, the preferred bond is the common bond between the set of largest rings, for example, 66 > 65 > 55. Where there is still a choice, the preferred bond contains the preferred atom (see Fu-3.1.2.3) at the first point of difference.
When there is a choice among atoms, the preferred atom is the common atom in the set of largest rings, for example, 666 > 665 > 655 > 555.
Where there is a choice of spiral pathways for numbering, the preferred pathway terminates as close as possible, in terms of the number of bonds, to the reference axis of the spiral.
Fu-3.2. Systematic numbering for (C60-Ih)[5,6]fullerene
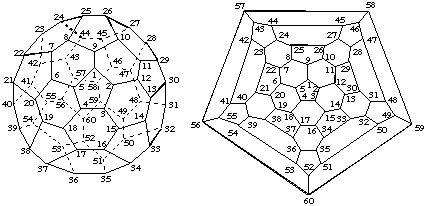
This fullerene (see Fig. 1) has six equivalent C5 axes passing through opposite pentagons, each of which gives identical contiguous spiral pathways in either direction from any atom of any pentagon. Thus, each of these C5 axes can be the reference axis. According to Fu-3.1.1, it is not necessary to investigate any of the ten equivalent C3 axes passing through opposite hexagons nor the fifteen equivalent C2 axes passing through opposite bonds, all of which give contiguous spiral pathways. The systematic numbering is given in Fig. 1 in both 3-D and Schlegel format.
Note. In this document, the convention for Schelegel diagrams is that the central polygon is considered to be closest to the viewer.
 | |
| 3-D Representation | Schlegel Representation |
Fig. 1. Systematic Numbering for (C60-Ih)[5,6]Fullerene
Note. For clarity, double bonds are not shown in fullerene structures throughout this report.Fu-3.3. Systematic numbering for (C70-D5h(6))[5,6]fullerene
The principal axis for this fullerene (see Fig. 3) is the C5 axis through opposite pentagons. There are no contiguous spiral pathways from any atom in either pentagon and thus the C5 axis cannot be a reference axis. Therefore, possible contiguous spiral pathways must be investigated from any of the five equivalent C2 axes that pass through the center of a six-membered ring at one end and bisect the bond between the two six-membered rings at the other end (see Fig. 2a). Since, according to Fu-3.1.2, a ring is preferred to a bond, the search for a contiguous spiral pathway must start in the six-membered ring. There are twelve possible pathways, one in each direction, clockwise and anticlockwise from each atom of the six-membered ring. However, because of symmetry, atoms marked 'a' in Fig. 2a are equivalent, as are atoms marked 'b' Therefore, there are only three possible pathways to explore, i.e., 'bab', clockwise (Fig. 2b); 'abb', clockwise (Fig. 2c) and 'bba' clockwise (Fig. 2d). The clockwise 'bab' pathway (Fig. 2b) terminates at atom 'c' at the end of the bond bisected by the reference axis. The clockwise 'abb' pathway (Fig. 2c) and the clockwise 'bba' pathway (Fig. 2d) terminate at 'c*', an atom at the end of a bond one removed from the bond bisected by the reference axis, and 'c**', an atom at the end of a bond two removed from the bond bisected by the the reference axis, respectively. Thus, according to Fu-3.1.3, the preferred contiguous spiral pathway for numbering is the clockwise 'bab' pathway (Fig. 2b) and thus the systematic numbering for (C70-D5h(6))[5,6]fullerene is that shown in Fig. 3.
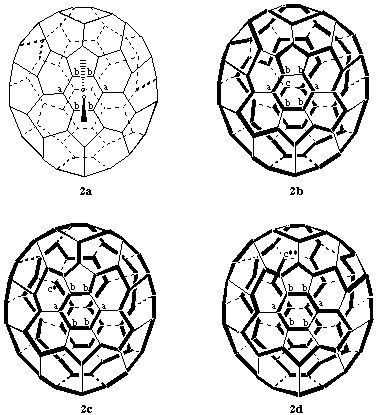
Fig. 2. Procedural Drawings for Numbering (C70-D5h(6))[5,6]Fullerene
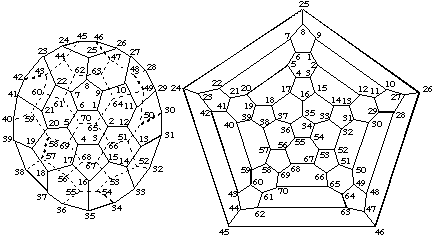 | |
| 3-D Representation | Schlegel Representation (viewed along the C5 axis) |
Fig. 3. Systematic Numbering for (C70-D5h(6))[5,6]Fullerene
Note. For clarity, double bonds are not shown in fullerene structures throughout this report.Fu-3.4. Trivial numbering.
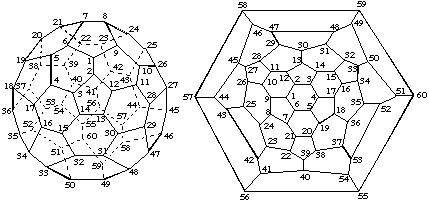
This numbering, shown in Figs. 4 and 5 is to be used when the trivial names [60-Ih]fullerene and [70-D5h]fullerene given in Fu-2.2 are used; they are based on the reported 'most reactive bond' (ref. 2).
Note: Such a criterion does not lend itself to the establishment of a systematic method for numbering other fullerenes, especially ones that haven't yet been characterized.
 | |
| 3-D Representation | Schlegel Representation |
Fig. 4. Trivial Numbering for [60-Ih]Fullerene
Note. For clarity, double bonds are not shown in fullerene structures throughout this report.
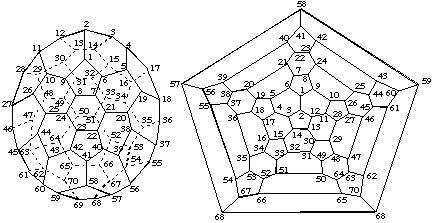 | |
| 3-D Representation | Schlegel Representation |
Fig. 5. Trivial Numbering for [70-D5h]Fullerene2
Note. For clarity, double bonds are not shown in fullerene structures throughout this report.
1. International Union of Pure and Applied Chemistry. "Nomenclature and Terminology of Fullerenes: A Preliminary Survey". Pure Appl. Chem. 1997, 69, 1411-1434.
2. R. Taylor, "C60, C70, C76, C78 and C84: Numbering, π-Bond Order Calculations and Addition Pattern Considerations". J. Chem. Soc., Perkin Trans. II 1993, 813-824.
3. A. L. Goodson, C. L. Gladys, and D. E. Worst, "Numbering and Naming of Fullerenes by Chemical Abstracts Service". J. Chem. Inf. Comp. Sci. 1995, 35, 969-978.
4. International Union of Pure and Applied Chemistry. Division of Organic Chemistry. Commission on Nomenclature of Organic Chemistry, Commission on Physical Organic Chemistry. "Glossary of Class Names of Organic Compounds and Reactive Intermediates Based on Structure (IUPAC Recommendations 1995)". Pure Appl. Chem. 1995, 67, 1307-1375.
5. International Union of Pure and Applied Chemistry, Compendium of Chemical Terminology, IUPAC Recommendations. 2nd Edition, Compiled by A. D. McNaught and A. Wilkinson, Blackwell Science, Oxford, 1997, 459 pp.