Nomenclature for the C60-Ih and C70-D5h(6) Fullerenes
(IUPAC Recommendations 2002)
Fu-4. Structurally Modified Fullerenes
Continued from Fu-1 to Fu-3
Contents of this section
Modification of fullerene structures by the addition or deletion of atoms, or by the breaking or formation of bonds is described by the prefixes 'homo', 'nor', 'seco', and 'cyclo' in a manner similar to their use in organic stereoparent nomenclature, including their combinations (ref. 6).
Fu-4.1. Homofullerenes
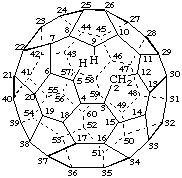
The replacement of a carbon-carbon bond of a fullerene by a methylene group is described by attaching the nondetachable prefix 'homo' to the name of the parent fullerene. The original numbering of the parent fullerene is retained. The location of the homo operation is described by a complex locant formed according to the method devised for insertion of a methylene group into a bond sector of a stereoparent (ref. 6). The complex locant is formed by adding the letters 'a', 'b', etc. to the pair of locants for the replaced bond which are the lowest locants consistent with the numbering of the fullerene, and enclosing the higher number in parentheses, for example, '1(9)a'; such complex locants must be used in parent names, but where there is no ambiguity, simple locants formed by adding the letter 'a' to the lower locant, such as '1a' for the case above, may be used in structural formulas and for citation of substituents, etc.
Because of the possibility of rearrangement of the double bonds in homofullerene structures, indicated hydrogen is used to designate the 'extra' hydrogen on the homo atom, even for di-, tri-, tetra-, etc. homofullerenes that could be considered to be hydro derivatives of fully unsaturated structures (see second and third examples below).
Note 1: A complex locant format is used by CAS (ref. 3) in which the higher-numbered locant and the letter 'a' are enclosed in parentheses following the locants of the replaced bond, as 1,2(2a); the corresponding simple locant for the added methylene group would be 2a. This was done in an attempt to remove the ambiguity in dihomofullerenes where two of the homo bonds originate from the same atom (see third example below), where, according to the preliminary fullerene report (ref. 1), both homo atoms would be assigned the same locant. Although this would remove ambiguity in a large number of cases, it would not do so in cases like the following (CA index name): 3,15(15a):14,15(15a)-dihomo-1,9-dinor[5,6]fullerene-C60-Ih , where the simple locants for the added methylene groups would both be 15a; the IUPAC name recommended herein for this modified fullerene would be 3(15)a,14(15)a-dihomo-1,9-dinor(C60-Ih)[5,6]fullerene with the corresponding simple locants 3a and 14a, respectively.
Note 2: In CAS index names the complete point group symbol is in italic type in contrast to IUPAC names in which only the on-line capital letter is in italic type, the subscript letters or numbers being given in normal type.
Examples:
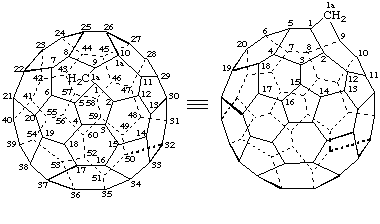
1aH-1(9)a-Homo(C60-Ih)[5,6]fullerene
(locant for structure, 1a)
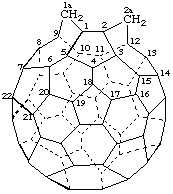
1aH,2aH-1(9)a,2(12)a-Dihomo(C60-Ih)[5,6]fullerene
[not la,2a-Dihydro-1(9)a,2(12)a-dihomo(C60-Ih)[5,6]fullerene;
locants for the structure, 1a and 2a]
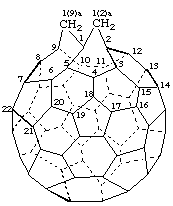
1(2)aH,1(9)aH-1(2)a,1(9)a-Dihomo(C60-Ih)[5,6]fullerene
[not l(2)a,1(9)a-Dihydro-1(2)a,1(9)a-dihomo(C60-Ih)[5,6]fullerene;
locants for the structure must be 1(2)a and 1(9)a, because both simple locants would be 1a] 
Fu-4.2. Norfullerenes.
The nondetachable prefix 'nor' describes the deletion of carbon atoms from a fullerene structure; however, bonds attached to the atom removed are not reconnected as is the case in stereoparent nomenclature (ref. 6). As a result, hydrogen atoms may be required to retain the connectivity of the remaining atoms. If after applying the nor procedure there are an odd number of carbon atoms bearing hydrogen atoms one will have also changed from sp2 to sp3 hybridization. This position is shown by use of indicated hydrogen in front of the name. A connectivity of three may also be satisfied by a heteroatom, such as nitrogen or boron and a connectivity of two by a heteroatom such as oxygen or sulfur. Where there is a choice, locants for the atoms removed and indicated hydrogen are as low as possible. 
Note 1: In organic stereoparent nomenclature atoms removed by the 'nor' operation are those having the highest possible number of the ring or chain segment (ref. 6).
Note 2: The main problem with the 'nor' operation is the limit to the number of carbon atoms that can be removed from a particular fullerene structure. Eventually, systematic ring nomenclature will provide a name that may be easier to understand than a polynorfullerene name. It would not be helpful to set a precise number of carbon atoms that can be removed by the nor operation; a realistic figure would depend a lot on whether blocks of carbon atoms or isolated carbon atoms are being removed. For CAS, more than twenty percent of the carbon atoms of a fullerene cannot be removed by the nor operation unless it can be shown to be a special situation. For (C60-Ih)[5,6]fullerene and (C70-D5h(6))[5,6]fullerene it is recommended that at least two 'belts' of rings or at least one-half of the atoms of the parent fullerene must remain intact after the 'nor' operation.
Examples:
2H-1-Nor(C60-Ih)[5,6]fullerene 
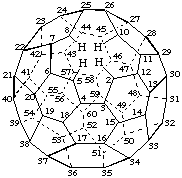
1,9-Dinor(C60-Ih)[5,6]fullerene
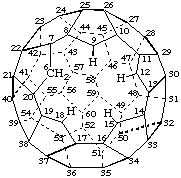
6H-1,2,3,4,5-Pentanor(C60-Ih)[5,6]fullerene
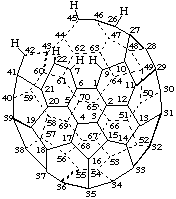
8,23,24,25-Tetranor(C70-D5h(6))[5,6]fullerene
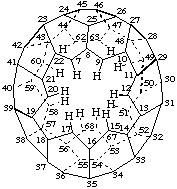
1,2,3,4,5,6,61,64,65,66,69,70-Dodecanor(C70-D5h(6))[5,6]fullerene
Fu-4.3. Secofullerenes. The nondetachable prefix 'seco' indicates the removal of fullerene bonds. Numbering of the parent fullerene is retained; where there is a choice, lowest possible locants are used to describe the seco positions. A connectivity of three for the carbon atoms involved in the 'seco' operation is retained by the addition of hydrogen atoms following appropriate rearangement of the double bonds of the system; these hydrogen atoms are implied in the name. 
Note: As for the 'nor' operation, the main problem with the 'seco' operation is the limit to the number of fullerene bonds that can be removed from a particular fullerene structure. Eventually, systematic ring nomenclature will provide a name that is easier to understand than a polysecofullerene name. It would not be helpful to set a precise number of fullerene bonds that can be removed by the 'seco' operation; a realistic figure would depend a lot on which fullerene bonds are removed. Obviously, a parent fullerene should not be split into separate fragments.
Examples:
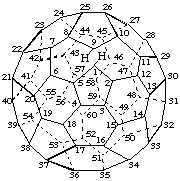
1,9-Seco(C60-Ih)[5,6]fullerene
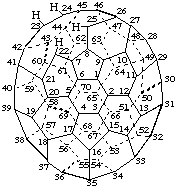
22,23:24,25-Diseco(C70-D5h(6))[5,6]fullerene
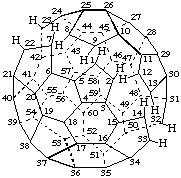
1,9:2,12:7,8:13,14:22,23:32,33-Hexaseco(C60-Ih)[5,6]fullerene
Fu-4.4. Cyclofullerenes.
The nondetachable prefix 'cyclo' indicates the formation of a bond between two atoms of a modified fullerene or a multicomponent fullerene (see Fu-11). In single component fullerenes, it almost always occurs in combination with one or more of the structure-modifying prefixes described above.
Fu-4.5. Combinations of structure-modifying prefixes
Combinations of structure-modifying prefixes described above may be used and are cited in the order nor, homo, seco, cyclo 'advancing backwards' from the parent fullerene name, i.e., proceeding from right to left away from the parent fullerene name. This is also the order that the operation indicated by each prefix takes for assignment of lowest locants. Nor prefixes are preferred for lowest locants and homo prefixes are preferred to seco or cyclo for lowest locants since homo locants may be needed for the latter operations. Locants for cyclo and seco prefixes are determined by the lowest set of locants, then by the order of citation of the locants in the name.
Note: This order of citation is not the same as given in the Revised Section F: Natural Products and Related Compounds (ref. 6) where alphabetical order is preferred reading left to right unless such an order would not be possible given the construction of the structure by 'advancing backwards' from the parent structure. CAS uses alphabetical order reading from left to right.
Examples:
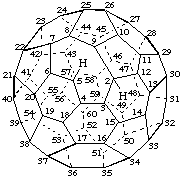
2H-2,9-Cyclo-1-nor(C60-Ih)[5,6]fullerene
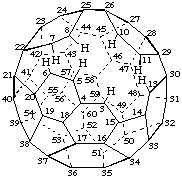
6,7-Seco-1,2,9,12-tetranor(C60-Ih)[5,6]fullerene
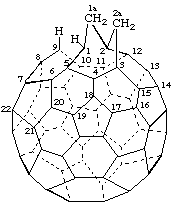
1aH,2aH-1,9-Seco-1(2)a,2(3)a-dihomo(C60-Ih)[5,6]fullerene
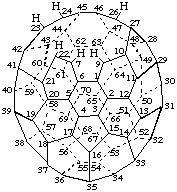
22,23-Seco-8,25-dinor(C70-D5h(6))[5,6]fullerene
References for this section
1. International Union of Pure and Applied Chemistry. "Nomenclature and Terminology of Fullerenes: A Preliminary Survey". Pure Appl. Chem. 1997, 69, 1411-1434.
3. A. L. Goodson, C. L. Gladys, and D. E. Worst, "Numbering and Naming of Fullerenes by Chemical Abstracts Service". J. Chem. Inf. Comp. Sci. 1995, 35, 969-978.
6. International Union of Pure and Applied Chemistry. Division of Organic Chemistry. Commission on Nomenclature of Organic Chemistry, "Revised Section F: Natural Products and Related Compounds (IUPAC Recommendations 1999)". Pure Appl. Chem. 1999, 71, 587-643.
Continue with Fu-5 Heterofullerenes
Return to Fullerenes home page.
Return to IUPAC chemical nomenclature home page.














