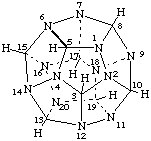
1,2,4,6,7,9,11,12,14,16,18,20-Dodecaaza(C20-Ih)[5]fullerane
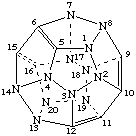
1,2,3,4,7,8,13,14,17,18,19,20-Dodecaaza(C20-Ih)[5]fullerene
Contents of this section
Fullerenes in which carbon atoms have been replaced by heteroatoms having standard bonding numbers or by bonding numbers indicated by the &lamda;-convention (ref. 7) are named by citing the 'a' prefixes of skeletal replacement nomenclature (ref. 8a) in front of the name of the fullerene.
The parent name is fullerene if double bonds are present or are possible in the fullerene; if double bonds are not possible the parent name is fullerane. This is illustrated by the dodecaaza(C20-Ih)[5]fullerane and dodecaaza(C20-Ih)[5]fullerene structures shown below.


1,2,3,4,7,8,13,14,17,18,19,20-Dodecaaza(C20-Ih)[5]fullerene
Note. The double bonds in this structure are for illustrative purposes only. Double bonds are not shown in fullerenes, fulleroids, or fullerene component structures elsewhere in this report.The heteroatoms include all elements capable of being tricoordinate, including metals and semimetals; no heteroatoms have attached hydrogen atoms. All carbons atoms of a fullerene may be replaced by the same or by different heteroatoms. Replacement of carbon atoms by trivalent heteroatoms may result in the need for indicated hydrogen.
Examples:
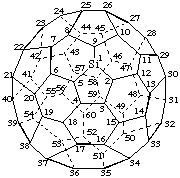
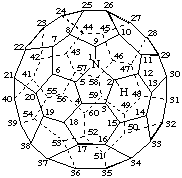
2H-1-Aza(C60-Ih)[5,6]fullerene
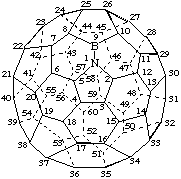
1-Aza-9-bora(C60-Ih)[5,6]fullerene
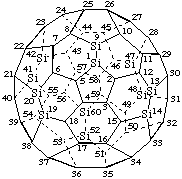
1,9,13,14,19,20,41,42,47,48,52,60-Dodecasila(C60-Ih)[5,6]fullerene
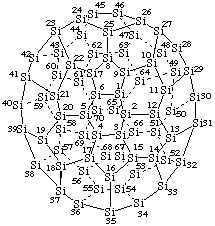
Heptacontasila(C70-D5h(6))[5,6]fullerene
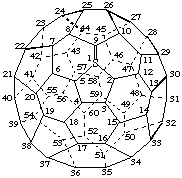
1&lamda;4-Thia(C60-Ih)[5,6]fullerene
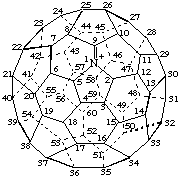
Azonia(C60-Ih)[5,6]fullerene
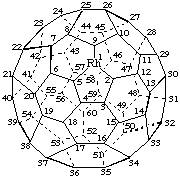
Rhoda(C60-Ih)[5,6]fullerene
Fu-5.2. Skeletal replacement ('a') nomenclature for structurally modified fullerenes.
When 'homo', 'nor', 'cyclo', or 'seco' co-occur with replacement terms, such as 'aza' and 'oxa', the replacement prefixes are cited in order of their senority in front of any structure modifying prefixes. Structure modifying prefixes have preference over replacement prefixes for low locants.
Examples:
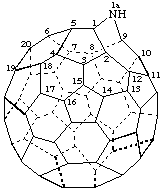
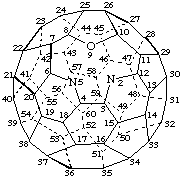
9-Oxa-2,5-diaza-1-nor(C60-Ih)[5,6]fullerene
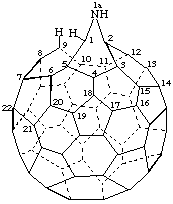
1aH-1a-Aza-1,9-seco-1(2)a-homo(C60-Ih)[5,6]fullerene
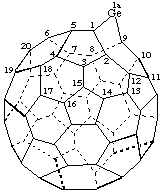
1a&lamda;2-Germa-1(9)a-homo(C60-Ih)[5,6]fullerene
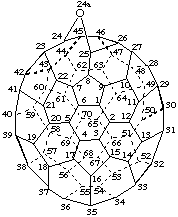
24a-Oxa-24(25)a-homo(C70-D5h(6))[5,6]fullerene
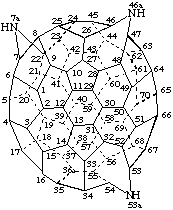
7aH,46aH,53aH-7a,46a,53a-Triaza-7(22)a,46(47)a,53(54)a-trihomo(C70-D5h(6))[5,6]fullerene
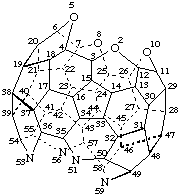
2,5,8,10-Tetraoxa-51,53,56,59-tetraaza-1,9,52,60-tetranor(C60-Ih)[5,6]fullerene
7. International Union of Pure and Applied Chemistry. Division of Organic Chemistry. Commission on Nomenclature of Organic Chemistry, "Treatment of Variable Valence in Organic Nomenclature (Lambda Convention) (Recommendations 1983)". Pure Appl. Chem. 1984, 56, 769-778.
8. International Union of Pure and Applied Chemistry. Division of Organic Chemistry. Commission on Nomenclature of Organic Chemistry, A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford, 1993: (a) Appendix, R-9.3, Table 33, p. 182; (b) R-2.4.4, Ring Assemblies, pp. 53-55; (c) R-0.2.4.2, Lowest set of locants, p. 17.