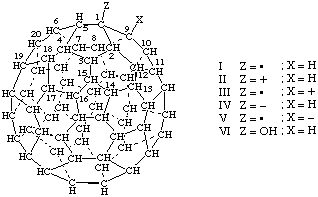
| I | (C60-Ih)[5,6]Fulleran-1-yl | IV | (C60-Ih)[5,6]Fulleran-1-ide |
| II | (C60-Ih)[5,6]Fulleran-1-ylium | V | (C60-Ih)[5,6]Fulleran-9-id-1-yl |
| III | (C60-Ih)[5,6]Fulleran-9-ylium-1-yl | VI | (C60-Ih)[5,6]Fulleran-1-ol |
Contents of this section
Fulleranes are fully saturated fullerenes, i.e, CnHn, and their derivatives, radicals, and ions, can be named by application of the principles of substitutive nomenclature normal to saturated hydrocarbons.
Examples:

| I | (C60-Ih)[5,6]Fulleran-1-yl | IV | (C60-Ih)[5,6]Fulleran-1-ide |
| II | (C60-Ih)[5,6]Fulleran-1-ylium | V | (C60-Ih)[5,6]Fulleran-9-id-1-yl |
| III | (C60-Ih)[5,6]Fulleran-9-ylium-1-yl | VI | (C60-Ih)[5,6]Fulleran-1-ol |
Fullerenes however, do not have hydrogen atoms for direct substitution and thus their derivatives, radicals, and ions, are named by application of the principles of substitutive nomenclature applicable to fusion atoms of fused ring systems, for example, the 4a and 8a positions of naphthalene. The characteristic group names and substituent prefix names normal to substitutive nomenclature are used.
Note: The lack of hydrogen atoms for substitution in fullerenes led to the conclusion in the preliminary survey (ref. 1) that atoms and groups attached to fullerenes should be designated as addends and not substituents. This would imply that principles of additive nomenclature should be used which might lead to names such as dibromo[60]fullerene. However, this leads to difficulties since such names are, in fact, formed by coordination nomenclature (bromo is the current ligand name for Br), a nomenclature method not approved for use with carbon compounds; the additive operation in the nomenclature of organic compounds would produce names such as [60]fullerene dibromide, for C60Br2. Since such additive names have been discarded in the nomenclature of organic compounds, where substitutive names are preferred, the only acceptable method is to use substitutive nomenclature, in which atoms and groups cited as prefixes substitute hydrogen atoms previously added by means of the additive prefix 'hydro'; or groups cited as suffixes require the concomitant addition of hydrogen atoms cited as 'added hydrogens'. Thus, names such as 1,9-dibromo-1,9-dihydro(C60-Ih)[5,6]fullerene and (C60-Ih)[5,6]fulleren-1(9H)-ol result, names that are fully in accordance with the principles, rules, and conventions of substitutive nomenclature. This approach provides a completely consistent method for naming fullerenes with atoms or groups attached to skeletal carbon atoms and all compounds derived from them.
Examples (radicals, ions, and radical ions):
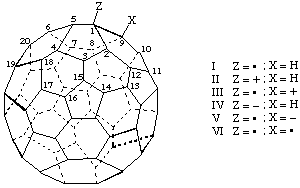
| I | (C60-Ih)[5,6]Fulleren-1(9H)-yl | IV | (C60-Ih)[5,6]Fulleren-1(9H)-ide |
| II | (C60-Ih)[5,6]Fulleren-1(9H)-ylium | V | (C60-Ih)[5,6]Fulleren-9-id-1(9H)-yl |
| III | (C60-Ih)[5,6]Fulleren-9-ylium-1(9H)-yl | VI | (C60-Ih)[5,6]Fullerene-1,9-diyl |
Note 1: The hydrogen needed for the -ide or -ylium suffix of examples III and V is provided by the added hydrogen reqiuired by the -yl suffix.Note 2: Added hydrogen is not used for example VI since the multiple suffixes simply remove a double bond, as in naphthalene-4a,8a-diol.
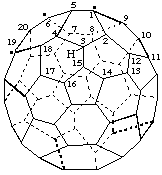
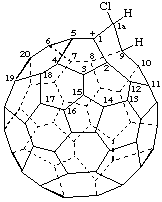
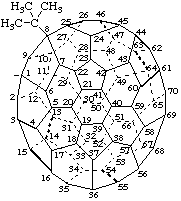
8-tert-Butyl(C70-D5h(6))[5,6]fulleren-1(8H)-ide
Note: The uninverted CA index name is 8-(1,1-dimethylethyl)-1,8-dihydro[5,6]fullerene-C70-D5h(6) ion(1-).
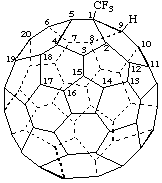
Note: Hydro prefixes are treated as nondetachable in these recommendations.
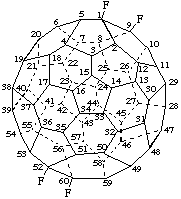
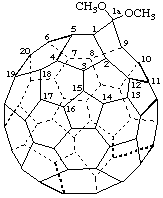
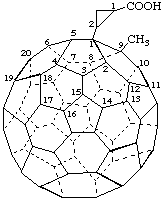
1a,1a-Dimethoxy-1aH-1(9)a-homo(C60-Ih)[5,6]fullerene
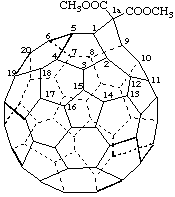
Dimethyl 1aH-1(9)a-homo(C60-Ih)[5,6]fullerene-1a,1a-dicarboxylate
For the C60-Ih and C70-D5h(6) fullerenes and fulleranes the high symmetry can result in difficulty in determining the lowest set of locants for derivatives. Numbering in (C60-Ih)[5,6]fullerene can begin at any atom of any one of the twelve five-membered rings and proceed around the chosen five-membered ring in either direction.
Example:
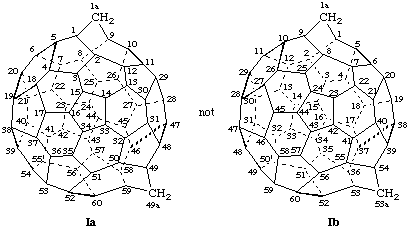
In (C70-D5h(6))[5,6]fullerene numbering can begin at four positions of each of the five six-membered rings in the middle of the fullerene structure perpendicular to the C2 axis, as shown by the enlarged dots in Ia, below, but may proceed in only one direction from each position. The correct numbering is shown in Ib and incorrect numberings in Ic and Id.
Example:
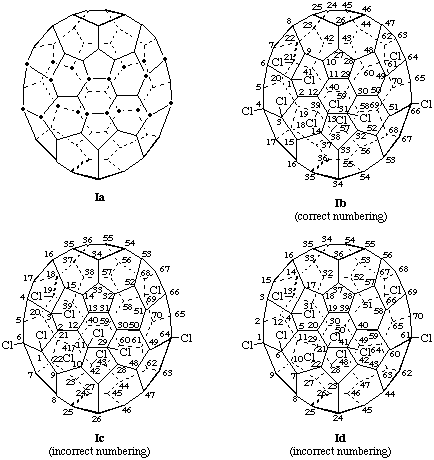
Explanation: All but one of the chlorine substituents fall at one of the possible starting points for numbering the (C70-D5h(6))[5,6]fullerene (see Ia). However, one of the candidate benzene rings has three chlorine atoms and thus would obviously provide the lowest initial locants of the locant set. Of the three possible numberings starting in this benzene ring, 1,3,4… is lower than 1,3,6…., or 1,4,6…]
(For an on-line program to assign lowest locants click here.)
Fu-6.4. Fullerenes and fulleranes as substituents
When a fullerene is a substituent to a more preferred parent compound, the name of the appropriate fullerene free radical (see above) is used as its substituent prefix name.
Examples:
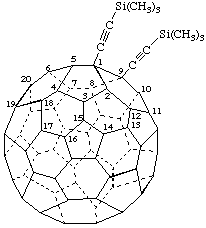
[(C60-Ih)[5,6]Fullerene-1,9-diyldiethyne-2,1-diyl]bis[trimethylsilane]
or
Hexamethyl[(C60-Ih)[5,6]fullerene-1,9-diyldiethyne-2,1-diyl]bis[silane]
Note: The first name is a multiplicative name following the method used by CA index nomenclature. The second name is a multiplicative name following the method currently recommended by IUPAC.
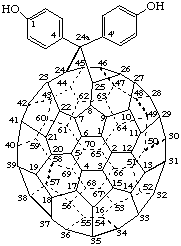
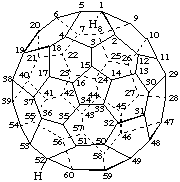
All substituents are assumed to project away from the fullerene cage. However, some fullerene substituents are small enough so that they can project into the fullerene cage. To describe such substituents, the term endo, enclosed in parentheses, is cited after the appropriate locant.
Examples:
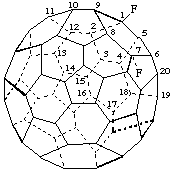
1,7(endo)-Difluoro-1,7-dihydro(C60-Ih)[5,6]fullerene
1. International Union of Pure and Applied Chemistry. "Nomenclature and Terminology of Fullerenes: A Preliminary Survey". Pure Appl. Chem. 1997, 69, 1411-1434.