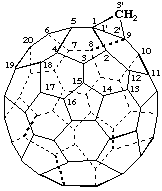
3'H-Cyclopropa[1,9](C60-Ih)[5,6]fullerene
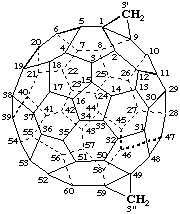
3'H,3"H-Dicyclopropa[1,9:49,59](C60-Ih)[5,6]fullerene
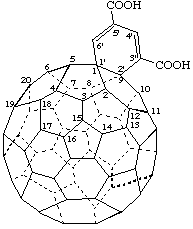
Benzo[1,9](C60-Ih)[5,6]fullerene-3',5'-dicarboxylic acid
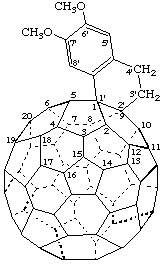
6',7'-Dimethoxy-3',4'-dihydronaphtho[1',2':1,9](C60-Ih)[5,6]fullerene
Note: Although when the nonfullerene component is a simple ring, such as cyclopropane or oxirene, the additional atoms could be cited by means of bridge prefixes, such as methano and epoxy, for consistency it is recommended that all be named as fused systems. Both methods were included in the preliminary survey (ref. 1). It is clear that as the attached organic system becomes more complex, the formation of bridge names becomes more difficult. For example, the CAS Ring Systems Handbook (ref. 10) contains many examples of a fullerene fused to two organic rings which themselves are connected by a bridge. For bridged fullerenes, see Fu-8.Organic rings and ring systems, including monocyclic rings and all polycyclic ring systems except spiro ring systems, are always cited as prefixes to the name of the fullerene or modified fullerene. Spiro systems that might be considered as fused to a fullerene are treated as spiro systems between modified fullerenes or fused fullerene systems and other components (see Fu-9). Each system retains its own name and numbering both for indicating fusion sites and for indicating positions of substitution. The fullerene locants are always unprimed and primes are added to the locants of the fused organic rings in the order described below. The fusion is described by citing the primed locants of the organic ring component and the unprimed locants of the fullerene in that order, enclosed in brackets and separated by a colon. Locants for monocyclic hydrocarbon components are usually omitted.
Note: CAS prefixes the fullerene name to that of a heterocyclic ring system when the latter has a heteroatomic component preferred to the fullerene component (ref. 3). For example, a fullerene component with no heteroatoms would be prefixed to any heterocyclic component, but a nitrogenous heterocyclic component would be prefixed to an nitrogenous fullerene component. This method was chosen for consistency with normal organic fusion nomenclature where heterocycles are preferred as base components. When the heterocycle is a monocycle, a fused ring system used as a base component in ordinary fusion nomenclature, or a heterocyclic ring system that is bridged, the fusion site is described by citing the unprimed locants of the fullerene component followed by the letter locant for the fusion side of the heterocycle. However, when the heterocycle is itself a fused ring system, a bridged fused system that is not a base component, or a bridged nonfused heterocycle, the fusion locants of the fullerene component are primed and those of the heterocycle are unprimed. As a result in the latter case, the fusion locants are not the same as the locants used for substitution, which are unprimed for the fullerene component and primed for the nonfullerene component, a situation that could be confusing. This situation is defended by the notion that locants within brackets in fusion names are those of the components, and are not the locants of the complete fused system. When the fullerene is always the base component, this confusion does not occur; in normal fusion nomenclature, the final fused ring system is renumbered, so the possibility of confusion does not exist.Examples:


3'H,3"H-Dicyclopropa[1,9:49,59](C60-Ih)[5,6]fullerene

Benzo[1,9](C60-Ih)[5,6]fullerene-3',5'-dicarboxylic acid

6',7'-Dimethoxy-3',4'-dihydronaphtho[1',2':1,9](C60-Ih)[5,6]fullerene
Note: Hydro prefixes are treated as nondetachable in these recommendations.
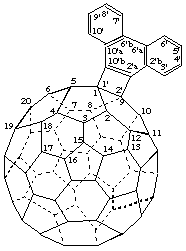
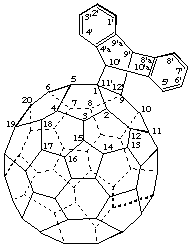
9',10'-Dihydro([9,10]ethanoanthra)[11',12':1,9](C60-Ih)[5,6]fullerene
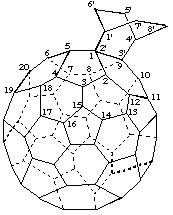
Bicyclo[2.2.2]octano[2',3':1,9](C60-Ih)[5,6]fullerene
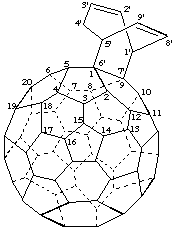
Bicyclo[3.2.2]nona[2,8]dieno[6',7':1,9](C60-Ih)[5,6]fullerene
(not bicyclo[3.2.2]nona[2,6]dieno[8',9':1,9](C60-Ih)[5,6]fullerene;
there is a choice in numbering and the fusion site is preferred for lower locants)
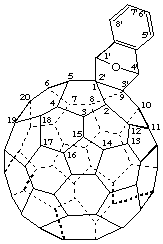
1',4'-Dihydro([1,4]epoxynaphtho)[2',3':1,9](C60-Ih)[5,6]fullerene
Note: The CA index name is [5,6]fullereno-C60-Ih-[1,9]-d][1,3]dioxole.
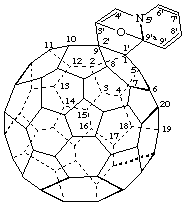
Note: The CA index name is [5,6]fullereno-C60-Ih-[1,9-a][3,9a]epoxy[9aH]quinolizine.
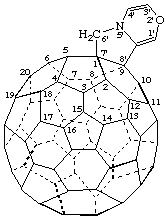
Note: The CA index name is [5,6]fullereno-C60-Ih-[1',9':7,8]pyrrolo[2,1-c][1,4]oxazine.
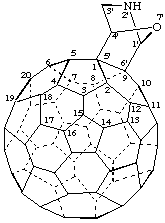
Note: The CA index name is [5,6]fullereno-C60-Ih-[7]oxa[2]azabicyclo[2.2.1]heptane.
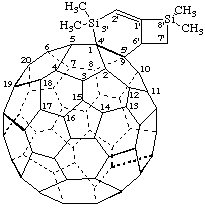
Note: The CA index name is 3',3',8',8'-tetramethyl[5,6]fullereno-C60-Ih-[1'.9':4,5][3,8]disilabicyclo[4.2.0]oct-1-ene.When two or more of the same nonfullerene component are fused to a fullerene, primes are assigned to the locants of the components according to the increasing value of the lower fullerene fusion locant. When different nonfullerene components are attached to a fullerene, primes are assigned in the alphabetical order of citation of the fusion prefix in the name reading from left to right, respecting the criterion for multiples of the same nonfullerene component given above.
Note 1: As shown by examples five and six below, the fullerene fusion locants are assigned based on the lowest set of locants for all fusion sites at the first point of difference when compared term by term in ascending numerical order.Examples:Note 2: Fullerene fusion locants are not assigned on the basis of the lowest locants for nonfullerene fusion components first cited in the name.
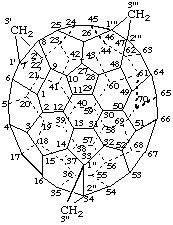
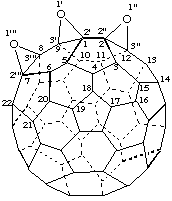
Trisoxireno[2',3':1,9; 2",3":2,12; 2''',3''':7,8](C60-Ih)[5,6]fullerene
Note: The CA index name is [5,6]fullereno-C60-Ih-[1,9-b:2,12-b':3,15-b"]trisoxirene.
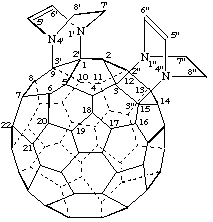
Note: The CA index name is [5,6]fullereno-C60-Ih-[1",9":2,3;3",15":2',3']bis[1,4]diazabicyclo[2.2.2]oct[5]ene.
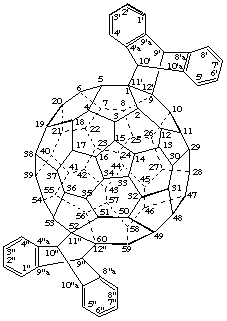
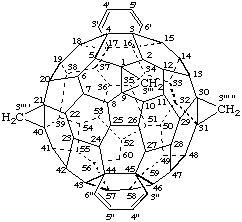
3'''H,3''' 'H,3''' "H-Dibenzo[16,17:44,45]tricyclopropa[1,9:21,40:30,31](C60-Ih)-[5,6]fullerene
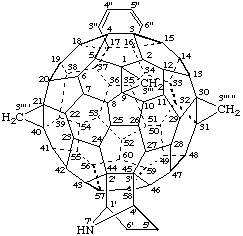
3'''H,3''' 'H,3''' "H-([7]Azabicyclo[2.2.1]heptano)[2',3':44,45]benzo[16,17]tricyclopropa- [1,9:21,40:30,31](C60-Ih)[5,6]fullerene
Note: The CA index name would be 3''H,3'''H,3''''H-benzo[16',17']tricyclopropa[1',9':21',40':30',31'][5,6]fullereno-C60-Ih- [44',45':2,3][7]azabicyclo[2.2.1]heptane.The fusion principles described above for fullerenes are fully applicable to modified fullerenes.
Examples:
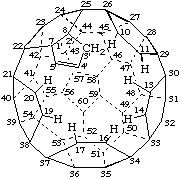
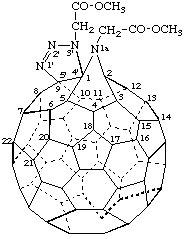
Dimethyl 2,2'-[1aH,3'H-[1,2,3]triazolo[4',5':1,9]-1a-aza-1(2)a-homo(C60-Ih)-[5,6]fullerene-1a,3'-diyl]diacetate
Dimethyl 1aH,3'H-[1,2,3]triazolo[4',5':1,9]-1a-aza-1(2)a-homo(C60-Ih)[5,6]-fullerene-1a,3'-diacetate
(a conjunctive name)
Note: The uninverted CA index name is dimethyl 3'H-[1,2,3]triazolo[4',5':1,9]-2a-aza-1,2(2a)-homo[5,6]fullerene-C60-Ih-2a,3'-diacetate.
1. International Union of Pure and Applied Chemistry. "Nomenclature and Terminology of Fullerenes: A Preliminary Survey". Pure Appl. Chem. 1997, 69, 1411-1434.
3. A. L. Goodson, C. L. Gladys, and D. E. Worst, "Numbering and Naming of Fullerenes by Chemical Abstracts Service". J. Chem. Inf. Comp. Sci. 1995, 35, 969-978.
9. International Union of Pure and Applied Chemistry. Division of Organic Chemistry. Commission on Nomenclature of Organic Chemistry, "Nomenclature of Fused and Bridged Fused Ring Systems (IUPAC Recommendations 1998)". Pure Appl. Chem. 1998, 70, 143-216.
10. Chemical Abstracts Service, Ring Systems Handbook, 1998 Edition, American Chemical Society, Washington, D. C., 1998.