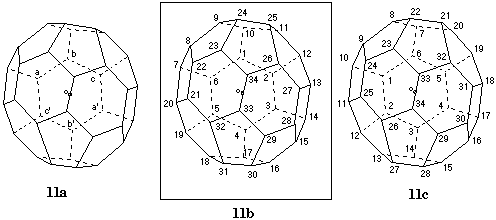
Fig. 11. Systematic numbering of (f,sA)(C34-C2)[5,6]fullerene
The only C2 axis in this fullerene is the reference axis. It passes through the center of the six-membered ring at the far end and bisects a bond at the near end of the structure in Fig. 11a. According to Fu-3.1.2 the numbering should begin in the ring which contains three pairs of symmetry-equivalent atoms indicated as a/a', b/b', and c/c'. There are six possible pathways to be evaluated: a to b to c; a to c' to b'; b to c to a'; b to a to c'; c to a' to b'; and c to b to a. Of these, a to b to c and b to a to c' do not yield a contiguous helical numbering pathway, whereas the other four pathways do. Since they terminate at an atom belonging to the bond bisected by the C2 axis, a selection among them cannot be made using Fu-3.1.3. However, application of Fu-3.1.4 solves the problem. First, pathways b to c to a' (Fig. 11b) and a to c' to b' (Fig. 11c), that begin with 6,6,5 atoms, are preferred over the other two pathways that begin with 6,5,5 atoms. Then, inspection of the atom-ranking shows that the numbering in Fig. 11b is preferred to that in Fig. 11c since it contains a 6,6,5 rather than a 6,5,5 atom at position 21. The stereodescriptor for the shown enantiomer of this inherently chiral fullerene is (f,sA) [ref 4].

Fig. 11. Systematic numbering of (f,sA)(C34-C2)[5,6]fullerene
References for this section
3. A.L. Goodson, C.L. Gladys and D.E. Worst, J. Chem. Inf. Comp. Sci. 1995, 35, 969-978.
4. International Union of Pure and Applied Chemistry, Division of Organic Chemistry, Commission on Nomenclature of Organic Chemistry. "Nomenclature for the (C60-Ih) and (C70-D5h(6))[5,6]fullerenes (IUPAC recommendations 2002)", Pure Appl. Chem. 2002, 74, 629-695.
5. P.W. Fowler and D.E. Manolopoulos, An Atlas of Fullerenes, Clarendon Press, Oxford, 1995