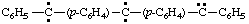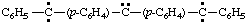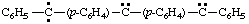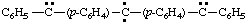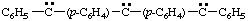Revised Nomenclature for Radicals, Ions, Radical Ions and Related Species
(IUPAC Recommendations 1993)
RC-81.3. Multiple radical centers
Continued from RC-81.2. Radical centers on characteristic groups
Contents of this section
- RC-81.3. Multiple radical centers
- RC-81.3.1. Polyradicals with radical centers on different skeletal atoms of parent hydrides or hydro derivatives
- RC-81.3.2. Assemblies of parent radicals
- RC-81.3.3. Other polyradicals
RC-81.3. Multiple radical centers
RC-81.3.1. Polyradicals with radical centers on different skeletal atoms of parent hydrides or hydro derivatives formally derived by removal of one or more hydrogen atoms from each of two or more skeletal atoms are named by using the appropriate operational suffixes, "-yl", "-ylidene", and "-ylidyne", as prescribed by RC-81.1, each preceded by numerical prefixes, as needed, and the locant of the skeletal position for each of the hydrogen atoms removed.
Examples:
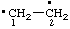 | ethane-1,2-diyl
[traditional name: ethylene (ref 10p)] |
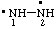 | diazane-1,2-diyl
[traditional name: hydrazo (ref 2bb, 13b)] |
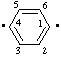 | benzene-1,4-diyl
[traditional names: p-phenylene or 1,4-phenylene (ref 10q)] |
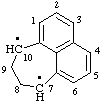 | 7,8,9,10-tetrahydrocyclohepta[de]naphthalene-7,10-diyl |
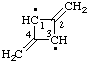 | 2,4-dimethylidenecyclobutane-1,3-diy1
[2,4-dimethylene-1,3-cyclobutylene,
according to the 1979 IUPAC Organic Rules (ref 10q)] |
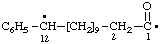 | 1-oxo-12-phenyldodecane-1,12-diyl |
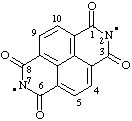 | 1,3,6,8-tetraoxo-1,3,6,8-tetrahydrobenzo[lmn][3,8]phenanthroline-2,7-diyl |
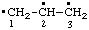 | propane-1,2,3-triyl |
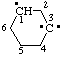 and/or and/or 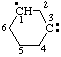 | cyclohexan-1-yl-3-ylidene |
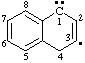 and/or and/or 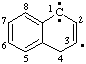 | 1,4-dihydronaphthalen-3-yl-1-ylidene
(See RC-81.1.4) naphthalen-3-yl-1(4H)-ylidene
(see RC-81.1.4) |
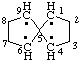 | spiro[4.4]nonane-1,4,6,9-tetrayl |
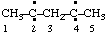
and/or
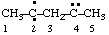
and/or
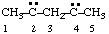 | pentane-2,4-diylidene |
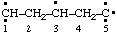
and/or
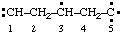
and/or
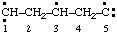
and/or
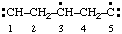 | pentan-3-yl-1-yliden-5-ylidyne  |
RC-81.3.2. Assemblies of parent radicals. Polyradicals with radical centers identically derived from the same parent hydride or the same characteristic group (except for polyacyl or polyimide radicals), but located in different parts of the structure, are named if possible, according to the principles of nomenclature for assemblies of identical units (ref 2q) on the basis of a parent radical (see RC-81.1) using the multiplicative prefixes "-bis", "-tris", etc., where necessary to avoid possible confusion that may arise from the use of the numerical prefixes "di-", "tri-", etc.
Note. Accordingly, radical centers identically derived from two or more of the same characteristic group are not described in terms of that characteristic group using appropriate multiplicative prefixes.
Polyacyl radicals are included in RC-81.2.2.
Polyimide radicals are named as polyradicals derived from heterocyclic ring systems (see RC-81.3.1).
Examples:
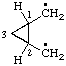 | cyclopropane-1,2-diyldimethyl |
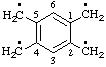 | benzene-1,2,4,5-tetrayltetramethyl |
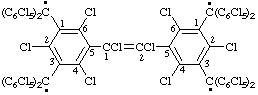
(a) IUPAC Multiplicative method (see RC-80.11)
octakis(pentachlorophenyl)[(1,2-dichloroethene-1,2-diyl)bis(2,4, 6-trichlorobenzene-5,1,3-triyl)]tetramethyl
(b) CAS multiplicative method (see RC-80.11)
[(1,2-dichloroethene-1,2-diyl)bis(2,4,6-trichlorobenzene-5,1,3-triyl)]tetrakis[bis(pentachlorophenyl)methyl]
Note. Since the benzenetriyl component of the multiplying prefix is not the central unit, it is numbered so that the pair of locants describing its attachment to the parent radical, methyl, are the lowest pair and, to reduce the possibility of ambiguity, are cited last, i.e., nearer to the name of the parent radical.
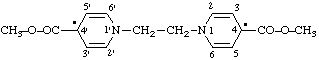
(a) IUPAC Multiplicative method (see RC-80.11)
4,4'-bis(methoxycarbonyl)-1,1'-ethylenebis(1,4-dihydropyridin-4-y l) (see RC-81.1.4)
4,4'-bis(methoxycarbonyl)-1,1'-ethylenedipyridin-4(1H)-yl (see RC-81.1.4)
(b) CAS multiplicative method (see RC-80.11)
1,1'-ethylenebis[4-(methoxycarbonyl)-1,4-dihydropyridin-4-yl] (see RC-81.1.4)
1,1'-ethylenebis[4-(methoxycarbonyl)pyridin-4(1H)-yl] (see RC-81.1.4)
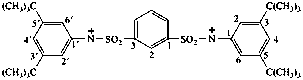
(a) IUPAC multiplicative method (see RC-80.11)
3,3',5,5'-tetra-tert-butyl-N,N'-(1,3-phenylenedisulfonyl)dianilinyl (see RC-80.8)
3,3',5,5'-tetrakis(2-methylpropan-2-yl)-N,N'-(1,3-phenylenedisulfonyl)bis(benzenaminyl) (see RC-80.8)
(b) CAS multiplicative method (see RC-80.11)
N,N'-[1,3-phenylenebis(sulfonyl)]bis[3,5-di-tert-butylanilinyl] (see RC-80.8)
N,N'-[1,3-phenylenebis(sulfonyl)]bis[3,5-bis(2-methylpropan-2-yl )benzenaminy1] (see RC-80.8)
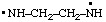 | ethylenebis(azanyl)
ethylenebis(aminyl) |
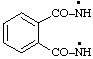 | phthaloylbis(azanyl)
phthaloylbis(aminyl) |
| •N=C=N• | methanediylidenebis(azanyl)
methanediylidenebis(aminyl) |
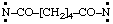
and/or
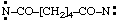
and/or
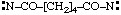 | hexanedioylbis(nitrene) (RC-81.1.3.1)
hexanedioylbis(aminylene) (RC-81.1.3.1)
hexanedioylbis(λ1-azane) (RC-81.1.3.3) |
| •O-C(CH3)2-CH2-C(CH3)2-O• | 2,4-dimethylpentane-2,4-diylbis(oxidanyl) (see RC-80.8)
1,1,3,3-tetramethylpropane-1,3-diylbis(oxidanyl) (see RC-80.8) |
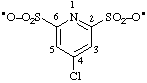 | (4-chloropyridine-2,6-disulfonyl)bis(oxidanyl) |
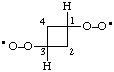 | cyclobutane-1,3-diylbis(dioxidanyl) |
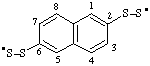 | naphthalene-2,6-diylbis(disulfanyl) |
RC-81.3.3. Other polyradicals. Polyradicals in which all of the radical centers are in the same structure, but cannot be included in a parent radical derived from a parent hydride according to RC-81.1 (see RC81.3.1) or by using multiplicative nomenclature (see RC-81.3.2), or when it may not be desirable to do so, are named by selecting as the parent radical that part of the structure containing the largest number of radical centers [see RC-81.3.3.2(a)]; the parts of the structure that contain the other radical centers are then expressed as prefixes.
RC-81.3.3.1. Radical centers in substituent groups. The presence of a radical center in a substituent that is to be cited as a prefix is expressed by the prefix "ylo-", indicating the removal of a hydrogen atom. This prefix is attached directly, i.e., nondetachably, to the parent substituent prefix, which is derived by usual methods (ref 1, 5). The removal of two or more hydrogen atoms from a substituent cited as a prefix is indicated by the appropriate numerical prefix, for example, "diylo-". The prefix "ylo-" is also used with prefixes normally used additively, such as oxy and carbonyl.
Examples:
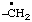 | ylomethyl |
| -O• | ylooxy (not ylohydroxy) |
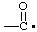 | ylocarbonyl |
| -CO-O• | (ylooxy)carbonyl |
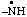 | yloamino |
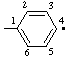 | 4-ylophenyl |
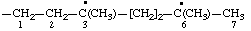 | 3,6-dimethyl-3,6-diyloheptyl (see RC-80.8) |
RC-81.3.3.2. Choice of parent structure. Although any portion of a polyradical structure that can be named by the principles of these recommendations can be the parent structure, it is often useful to have some guidelines for making this choice. Thus, when necessary or desirable, a parent radical may be chosen by applying the following criteria in order until a definitive choice is attained:
(a) the maximum number of radical centers of any kind in a single parent structure (see also RC-81.3.1);
(b) the maximum number of radical centers that can be expressed by means of a multiplicative name (see also RC-81.3.2);
(c) the maximum number of "senior" radical centers, according to the nature of the skeletal atom at which the radical center is considered to reside, in the same order as for the corresponding replacement prefixes (ref 3d, 11). 
Further choice, if necessary, may be made by applying the general criteria for chains and rings given in the 1979 edition of the organic nomenclature rules (ref 2cc).
Examples:
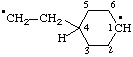 | 4-(2-yloethyl)cyclohexyl
(cyclohexyl is preferred to ethyl) |
| •CH2-C(CH3)2-O• | 1,1-dimethyl-2-yloethoxyl (see RC-80.8)
2-methyl(1-ylopropan-2-yloxyl) (see RC-80.8)
( ... oxyl is preferred to propyl) |
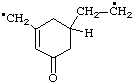 | 2-[5-oxo-3-(ylomethyl)cyclohex-3-en-1-yl]ethyl
(ethyl is preferred to methyl) |
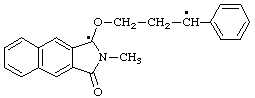
2-methyl-3-oxo-1-(3-phenyl-3-ylopropoxy)-2,3-dihydro-1H-benzo[f]isindol-1-yl
(benzoisoindolyl is preferred to propyl)
Note The practice of eliding a final "o" or "a" before a following vowel from certain fusion prefix names, as described by the footnote to Rule 21.4 in previous editions of the IUPAC Organic Rules (ref l), will no longer be recommended in the forthcoming revision of the 1979 edition (ref 5).
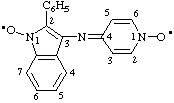
2-phenyl-3-[(1-ylooxy-1,4-dihydropyridin-4-ylidene)amino](1H-indol-1-yloxyl)
(see RC-81.1.4)
2-phenyl-3-[(1-ylooxypyridin-4(1H)-ylidene)amino(1H-indol-1-yloxyl
(see RC-81.1.4)
(indolyloxyl is preferred to pyridinyloxyl)
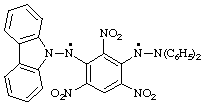
N-[3-(2,2-diphenyl-1-ylohydrazino)-2,4,6-trinitrophenyl]-9H-carbazol-9-aminyl
(radical centers on characteristic groups are preferred to parent hydride radicals;
and carbazolaminyl is preferred to anilino or benzenaminyl)
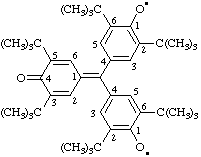
4,4'-[(3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methylene]bis(,6-di-tert-butyl-4,1-phenylene)bis(oxidanyl)
(IUPAC and CAS multiplicative methods; see RC-80.11)
(tert-butyl = 2-methylpropan-2-yl; see RC-80.8)
Note In order to eliminate possible ambiguity, the locants of the divalent radical phenylene, a component of the multipart multiplying prefix but not the central component, are cited so that the locant "1", the position of attachment to the parent radical, oxidanyl, is nearer to its name.
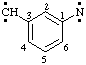 and/or
and/or 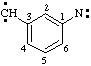 and/or
and/or 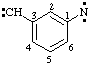
3-(diylomethyl)phenyl]nitrene (RC-81.1.3.1)
[3-(diylomethyl)phenyl]aminylene (RC-81.1.3.1)
[3-(diylo-λ1-methanyl)phenyl]-λ1-azane (RC-81.1.3.3)
(nitrene or aminylene or λ1-azane is preferred to carbene or methylene)
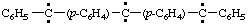
and/or
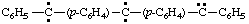
and/or
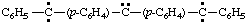
and/or
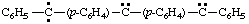
and/or
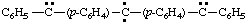
and/or
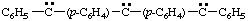
(a) IUPAC multiplicative method (see RC-80.11)
diphenyl(diylomethylenedi-4,1-phenylene)dicarbene (RC-81.1.3.1)
diphenyl(diylomethylenedi-4,1-phenylene)bis(methylene) (RC-81.1.3.1)
diphenyl(λ2-methanediyldi-4,1-phenylene)di-λ2-methane (RC-81.1.3.3)
(b) CAS multiplicative method (see RC-80.11)
(diylomethylenedi-4,1-phenylene)bis[phenylcarbene] (RC-81.1.3.1)
(diylomethylenedi-4,1-phenylene)bis[phenylmethylene] (RC-81.1.3.1)
(λ2-methanediyldi-4,1-phenylene)bis[phenyl-λ2-methane] (RC-81.1.3.3)
(c) Substitutive method
bis[4-[phenyl(diylomethyl)]phenyl]carbene (RC-81.1.3.1)
bis[4-[phenyl(diylomethyl)]phenyl]methylene (RC-81.1.3.1)
bis[4-(phenyl-λ2-methanyl)phenyl]-λ2-methane (RC-81.1.3.3)
Note For locant order of phenylene see previous footnote
References for this section
1. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 ed., Pergamon Press, Oxford, 1979, 559 p.
2. Reference 1, Section C, pp. 79-322: [q] Rules C-72, p. 130-1; and C-73, p. 132; [bb] Rule C-921.2, pp. 285-6; [cc] Subsections C-0.13, pp. 97-101; and C-0.14, pp. 101-5.
3. Reference 1, Section B, pp. 53-76: [d] Rule B-1.1, pp. 53-5, Table I, p. 53.
5. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford, 1993.
10. Reference 1, Section A, pp. 5-52: [p] Rule A-4.2, p. 14; [q] Rule A-11.6, pp. 17-8.
11. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Revision of the Extended Hantzsch-Widman System of Nomenclature for Heteromonocycles, Recommendations 1982", Pure Appl. Chem. 55, 409-16, 1983.
Continue with RC-82. Cations
Return to ions and radicals home page.
Return to chemical nomenclature home page.
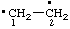
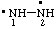
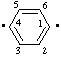


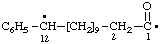

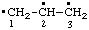
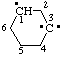 and/or
and/or 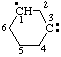
 and/or
and/or 

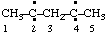
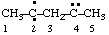
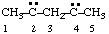
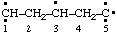
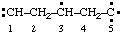
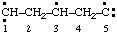
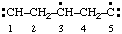


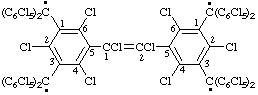
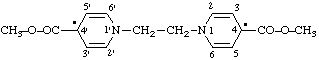

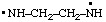

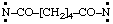
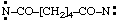
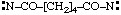
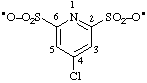


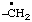
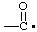
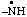

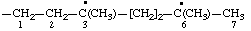
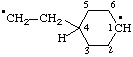

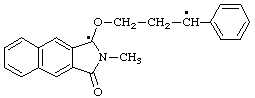



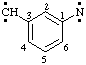 and/or
and/or 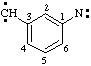 and/or
and/or