| H3C• | methyl |
| H3Ge• | germyl |
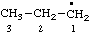 | propyl |
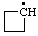 | cyclobutyl |
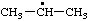 | 1-methylethyl propan-2-yl (see RC-81.1.2 and RC-80.8) isopropyl (traditional name; see item 1, Appendix, List A) |
Contents of this section
RC-81.0. Introduction. For the purposes of organic nomenclature, a parent radical is a molecular compound formally derived by the removal of one or more hydrogen atoms from one or more atoms of a parent hydride, a parent compound, or a hydro derivative of either. An atom at which the resulting (nonbonding) electron(s) is(are) considered to reside is called a radical center. An atom with one free (nonbonding) electron is a monovalent radical center; an atom with two (or three) free (nonbonding) electrons, which may be paired, i.e., having antiparallel spins (singlet), or unpaired, i.e., having parallel spins (triplet), but which cannot be nonbonding electron pairs ("lone pairs") of that atom in the parent structure is called a divalent (or trivalent) radical center. A parent radical with two or more monovalent radical centers is called a diradical, triradical, etc.
RC-81.1. Radical centers in parent hydrides
RC-81.1.1. Monovalent radical centers in saturated acyclic and monocyclic hydrocarbons, and the mononuclear EH4 parent hydrides of the carbon family. A monovalent radical center derived formally by removal of one hydrogen atom from a terminal atom of a saturated, unbranched acyclic hydrocarbon; from an atom of a saturated monocyclic hydrocarbon; or from any of the mononuclear parent hydrides methane (CH4), silane (SiH4), germane (GeH4), stannane (SnH4), or plumbane (PbH4) is named by replacing the "-ane" ending of the name of the parent hydride by "-yl" (ref 4f, 10i).
Examples:
| H3C• | methyl |
| H3Ge• | germyl |
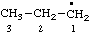 | propyl |
 | cyclobutyl |
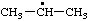 | 1-methylethyl propan-2-yl (see RC-81.1.2 and RC-80.8) isopropyl (traditional name; see item 1, Appendix, List A) |
Note 1. This recommendation is consistent with the generalized principles for naming and numbering acyclic substituent prefixes as given in Section D of the 1979 IUPAC Organic Rules (ref 4c, 10f) rather than the principles of Section A (ref 10g) and the ACS silicon rules (ref 12a), where the principal chain must terminate at the free valence position (see also RC-80.8).Examples:Note 2. The systematic names phosphane and arsane (ref 4g, 6b) must be used for naming the radicals derived from the mononuclear parent hydrides PH3 and AsH3 according to this recommendation, because use of the traditional names phosphine and arsine (ref 4g, 6b) will generate the names phosphinyl (ref 12b) and arsinyl (ref 13b), which are traditional prefix names for the oxo phosphoric and arsenic acid groups H2P(O)- and H2As(O)-, respectively, as substituents. Even though the names phosphinoyl and arsinoyl are recommended in the 1979 IUPAC Organic Rules for these groups (ref 4h), ambiguity would still exist.
Note 3. The traditional names phosphino, arsino, stibino, and bismuthino (ref 4i) are less acceptable alternative names for the radicals H2P•, H2As•, H2Sb•, and H2Bi•.
Note 4. The name "aminyl" for the parent radical H2N• used in the 1979 IUPAC Organic Rules (ref 2r) is continued in these recommendations as an alternative to "azanyl", derived from the parent hydride name azane (ref 6b, l4), even though "amine" is not used as a parent hydride name for NH3 herein (see also footnote to Table 2 in RC-80.9.1).
Note 5. Although the name "amine" would be quite consistent with parent hydride names, such as phosphine and arsine, and is used as such by Beilstein, it is not used as a parent hydride name in these recommendations because it has also been added to the name of a radical to form the name of a parent compound (ref 15), for example butylamine. In these recommendations, the term "amine" is used only as a suffix to denote the attachment of the -NH2 group to a parent hydride, for example, pyridin-2-amine.
Note 6. The names of the radicals HO•, and HO-O• are hydroxyl (ref 6c) and hydroperoxyl (ref 2s), respectively, but, in keeping with tradition, are not used as parent radical names in these recommendations. For names of such "substituted" radicals, see RC-81.2.4.
| HS• | sulfanyl [trivial name: mercapto (ref 2k)] |
Note. The name mercapto is not used as a parent radical name in these recommendations. For names of such "substituted" radicals, see RC-81.2.5.
| H2N• | azanyl aminyl (traditional name: amino) |
Note. The trivial name amidogen is used for H2N• in CAS index nomenclature (ref 13c)
| H2B• | boranyl (previously (ref 4j) and in inorganic nomenclature recommendations (ref 6d), boryl) |
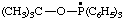 | [(2-methylpropan-2-yl)oxyltriphenyl-λ5-phosphanyl (ref 16) (see RC-80.8) tert-butoxytriphenylphosphoranyl (ref 4k) (see RC-80.8) |
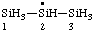 | trisilan-2-yl |
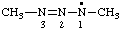 | 1,3-dimethyltriaz-2-en-1-yl |
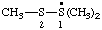 | 1,1,2-trimethyl-1λ4-disulfan-1-yl (ref 16) |
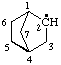 | bicyclo[2.2.1]heptan-2-yl [previously (ref 10m), bicyclo[2.2.1]hept-2-yl] |
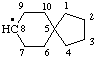 | spiro[4.5]decan-8-yl [previously (ref 10n), spiro[4.5]dec-8-yl] |
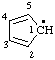 | cyclopenta-2,4-dien-1-yl |
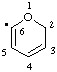 | 2H-pyran-6-yl |
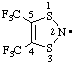 | 4,5-bis(trifluoromethyl)-1,3,2-dithiazol-2-yl |
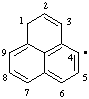 | 1H-phenalen-4-yl |
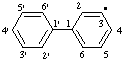 | 1,1'-biphenyl-3-yl |
Nomenclature methods for describing divalent and trivalent radical centers are complicated by current definitions of the term "radical" (ref 7, 17). According to these definitions and many experts in the field, the free (nonbonding) electrons of a radical must be unpaired and therefore a divalent radical center in which the free (nonbonding) electrons are paired (singlet state) is not considered to be a radical. Hence, there is objection to the use of "radical suffixes", such as "-diyl" and "-ylidene", to describe such divalent radical centers. However, in these recommendations, the radical suffixes "-ylidene" and "-ylidyne" are used to describe divalent and trivalent radical centers regardless of the electronic state of the free (nonbonding) electrons. Alternative suffixes, such as "-ylidene" and "-diyl", should not be used to differentiate between a singlet and a triplet.state. If necessary, such a distinction should be made by adding the separate word "singlet" or "triplet" to the name of the divalent radical.
RC-81.1.3.1. Carbenes, carbynes, nitrenes, and silylenes. Divalent radical centers derived formally by the removal of two hydrogen atoms from the mononuclear parent hydrides CH4, NH3, and SiH4, i.e., 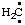 and/or H2C:,
and/or H2C:, 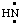 and/or HN:, and
and/or HN:, and 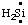 and/or H2Si:, respectively, may be named carbene (or methylene) (ref 2t), nitrene [or aminylene (ref 2r)], and silylene, respectively. The mononuclear parent hydride derived formally by removal of three hydrogen atoms from CH4, i.e.,
and/or H2Si:, respectively, may be named carbene (or methylene) (ref 2t), nitrene [or aminylene (ref 2r)], and silylene, respectively. The mononuclear parent hydride derived formally by removal of three hydrogen atoms from CH4, i.e., 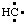 and/or
and/or 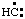 , may be named carbyne. Derivatives of these parent hydrides are named according to the usual principles of substitutive nomenclature.
, may be named carbyne. Derivatives of these parent hydrides are named according to the usual principles of substitutive nomenclature.
Note 1. The name nitrene is widely used in the literature; the name imino also has been used. The trivial name imidogen is used in CAS index nomenclature (ref 13c).Examples:Note 2. Because the compound CH2=SiH2 is quite well known in the literature as silene (ref 18), this name cannot be used for the mononuclear parent radical
and/or H2Si:. The name "silylene" (ref 12a) is widely used in the literature and in CAS index nomenclature (ref 13c).
Note 3. A generally accepted principle of substitutive nomenclature does not allow a structure containing a carbene carbon atom in a carbon chain or ring to be named as a derivative of carbene or methylene; therefore, such structures should be named according to RC-81.1.3.2 or RC-81.1.3.3, below. However, such radicals are included in the general class known as carbenes.
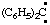 and/or (C6H5)2C: and/or (C6H5)2C: | diphenylcarbene diphenylmethylene |
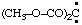 and/or (CH3-O-CO)2C: and/or (CH3-O-CO)2C: | bis(methoxycarbonyl)carbene bis(methoxycarbonyl)methylene |
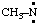 and/or CH3-N: and/or CH3-N: | methylnitrene methylaminylene |
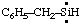 and/or and/or 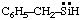 | benzylsilylene |
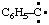 and/or and/or 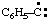 | phenylcarbyne |
Note. Although the traditional names methylene (ref 2t), aminylene (ref 2r), and silylene (ref 12a), are used in RC-81.1.3.1 above, the suffix "-ylene" for describing other divalent radical centers is no longer recommended (see RC-80.5).Examples:
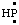 and/or HP: and/or HP: | phosphanylidene [the traditional name is phosphinidene (ref 12b), but phosphinediyl according to the 1979 IUPAC Organic Rules (ref 4m)] |
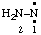 and/or and/or 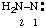 | diazanylidene hydrazinylidene (traditional parent hydride name (ref 14)) (trivial name: hydrazono (ref 2u)) (not aminonitrene) |
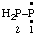 and/or and/or 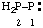 | diphosphanylidene |
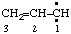 and/or and/or 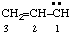 | prop-2-en-1-ylidene |
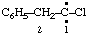 and/or and/or 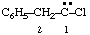 | 1-chloro-2-phenylethylidene (not benzylchlorocarbene) |
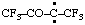 and/or and/or 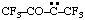 | 1,1,1,4,4,4-hexafluoro-3-oxobutan-2-ylidene (see RC-80.8) [3,3,3-trifluoro-2-oxo-1-(trifluoromethyl)propylidene (see RC-80.8) |
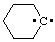 and/or and/or 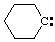 | cyclohexylidene |
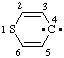 and/or and/or 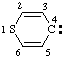 | 4H-thiopyran-4-ylidene |
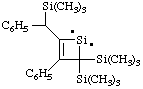 and/or
and/or 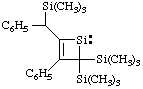
3-phenyl-2-[phenyl(trimethylsilyl)methyl]-4,4-bis(trimethylsilyl)-1-silacyclobut-2-en-1-ylidene
[replacement name (ref 3g)]
3-phenyl-4-[phenyl(trimethylsilyl)methyl]-2,2-bis(trimethylsilyl)-1,2-dihydrosilet-1-ylidene
(Hantzsch-Widman name (ref 3e, 11))
Note. The trivial name "benzyl" is not used as a substituent prefix when a-substituted (see footnote to item 17 in the Appendix, List A).
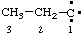 and/or and/or 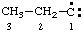 | propylidyne |
Note. Use of the λ-convention (ref 16) to describe a radical center derived by the loss of two hydrogen atoms from a skeletal atom that is in a valence state higher than the standard one is ambiguous because it would not be clear whether the name was describing a standard valence state or a divalent radical center derived from the higher valence state. However, the use of the λ-convention to describe a radical center that is in a lower valence state than the standard one may be useful for naming radical ions (see RC-85.2).Examples:
 and/or Cl2C: and/or Cl2C: | dichloro-λ2-methane |
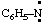 and/or C6H5-N: and/or C6H5-N: | phenyl-λ1-azane |
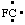 and/or and/or 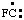 | fluoro-λ1-methane |
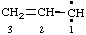 and/or and/or 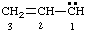 | 1λ2-prop-2-ene |
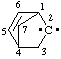 and/or and/or 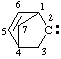 | 2λ2-bicyclo[2.2.1]hept-5-ene |
Note. This procedure is exactly analogous to the use of "indicated hydrogen" to describe saturated ring positions that remain after the introduction of suffixes for principal characteristic groups, such as "-one", into the name of a parent ring or ring system that otherwise expresses the maximum number of noncumulative double bonds (ref 2g).Example:
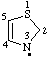 | 2,3-dihydrothiazol-3-yl thiazol-3(2H)-yl |
1. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 ed., Pergamon Press, Oxford, 1979, 559 p.
2. Reference 1, Section C, pp. 79-322: [a] Subsection C-0.8, pp. 133-43; [f] Rule C-16.1, p. 108; [g] Rule C-315.1, p. 172; [k] Rule C-502, pp. 210-1; [r] Rule C-81.2, p. 134; [s] Rules C-218.1, p. 161; and C-81.1, pp. 133-4; [t] Rule C-81.1, pp. 133-4; [u] Rule C-922.1, p. 288.
3. Reference 1, Section B, pp. 53-76: [e] Rule B1.1, pp. 53-5; [f] Rule B-5.11, p. 70; [g] Rule B-4, p. 68-70.
4. Reference 1, Section D, pp. 323-471: [c] Rule D-4.14, p. 374-5, esp. footnote, p. 374; and Rule D-6.12, pp. 409-10, Note. p. 410; [g] Rule D-5.11, p. 384, footnote; [h] Rule D-5.66, pp. 403-4; [i] Rule D-5.12, pp. 385-6; [j] Rule D-7.3, pp. 433-4; [k] Rule D-5.74, P. 407; [m] Rule D-5.14, p. 387.
6. International Union of Pure and Applied Chemistry. Inorganic Chemistry Division. Commission on Nomenclature of Inorganic Chemistry, Nomenclature of Inorganic Chemistry, Recommendations 1990, G. J. Leigh, ed., Blackwell Scientific Publications, Oxford, England, 1990, 289 p. [b] Section I-7.2.2.1, pp. 83-5, Table 17.2; [c] Section I-8.4.2.2, p. 113; [d] Section I-8.4.2.3, pp. 114-5.
7. International Union of Pure and Applied Chemistry, Compendium of Chemical Terminology (IUPAC Recommendations), V. Gold, K. L. Loening, A. D. McNaught, P. Sehmi, compilers, Blackwell Scientific Publications, Oxford, 1987, 456 p.
10. Reference 1, Section A, pp. 5-52: [f] Rule A-4, p. 14, ** footnote; [g] Rules A-1.2, p. 5; A-2.25, pp. 6-7; A-3.5, p. 13; A-4.1, p. 14; and A-4.4, p. 15; [i] Rules A-1.2, p. 5; and A-11.2, p. 16; [j] Rule A-11.4, p. 17; [k] Rule A-24.2, p. 29-30, Exceptions, p. 30; [m] Rule A-31.4, p. 32; [n] Rule A-43, pp. 41-2.
11. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Revision of the Extended Hantzsch-Widman System of Nomenclature for Heteromonocycles, Recommendations 1982, Pure Appl. Chem. 55, 409-16, 1983; [a] ibid. Recommendation RB-1.5; [b] ibid. Recommendation RB-1.2.
12. American Chemical Society, "Report of the ACS Nomenclature, Spelling and Pronunciation Committee for the First Half of 1952": [a] F. Organosilicon Compounds, Chem. Eng. News 30, 4517-22 (1952); [b] E. Organic Compounds Containing Phosphorus, ibid., 4515-22.
13. American Chemical Society, "Chemical Substance Index Names", Appendix IV in the 1987-1991 Chemical Abstracts Index Guide, Chemical Abstracts Service, Columbus, Ohio, 1992: [b] §294, Illustrative List of Substituent Prefixes, pp. 236I-242I; [c] §187, p. 176I.
14. International Union of Pure and Applied Chemistry. Inorganic Chemistry Division. Commission on Nomenclature of Inorganic Chemistry, "Nomenclature of Inorganic Chemistry: II.2. The Nomenclature of Hydrides of Nitrogen and Derived Cations, Anions, and Ligands (Recommmendations 1981)" Pure Appl. Chem. 54, 2545-52 (1982), Rule II.2.1, p. 2546.
15. American Chemical Society, "The Naming and Indexing of Chemical Substances from Chemical Abstracts" [a reprint of the Introduction to the Subject Index of Volume 56 (January-June 1962) of Chemical Abstracts, Chemical Abstracts Service, Columbus, Ohio, 1962, §255-7, pp. 41N-42N].
16. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Treatment of Variable Valence in Organic Nomenclature (Lambda Convention), Recommendations 1983, Pure Appl. Chem. 56, 769-78 (1984).
17. International Union of Pure and Applied Chemistry. Organic Chemistry Division, (a) Commission on Physical Organic Chemistry, Glossary of Terms Used in Physical Organic Chemistry, Recommendations 1982, Pure Appl. Chem. 55, 1281-371 (1983); (b) Commission on Photochemistry, "Glossary of Terms Used in Photochemistry, Recommendations 1988", Pure Appl. Chem. 60, 1055-106 (1988).
18. See, for example, G. Raabe and J. Michl, Chem. Rev. 85, 419-509 (1985), Part III, pp. 442-84.