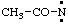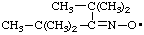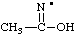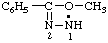Revised Nomenclature for Radicals, Ions, Radical Ions and Related Species
(IUPAC Recommendations 1993)
RC-81.2. Radical centers on characteristic groups
Continued from RC-81.1. Radical Centers in Parent Hydrides
Contents of this section
- RC-81.2. Radical centers on characteristic groups
- RC-81.2.1. Established or traditional names
- RC-81.2.2. Acyl radicals
- RC-81.2.3. Radical centers on nitrogen atoms of amine, imine, amide, or imide characteristic groups
- RC-81.2.4. Radical centers on oxygen atoms of acid, peroxy acid, hydroxy, or hydroperoxy characteristic groups
- RC-81.2.5. Radical centers on sulfur atoms of thio acid, thiol, or dithiohydroperoxy characteristic groups
- RC-81.2.6. Radical centers on other characteristic groups
RC-81.2. Radical centers on characteristic groups. Although radical centers located on characteristic groups can be described on the basis of parent radicals derived from parent hydrides as given above in RC-81.1, it is often much more convenient and/or desirable to describe a radical center on the basis of a larger parent structure, such as a parent hydride together with a principal characteristic group, or to use names that have become established in the literature. The following subsections provide recommendations for describing radical centers on some. but not all, of the characteristic groups of substitutive nomenclature.
RC-81.2.1. Established or traditional names. Names for describing radical centers on certain characteristic groups have become established in the literature. Some of these "traditional" names follow the recommendation given in the 1979 IUPAC Organic Rules (ref 2t), whereby an "l" is added to the name of the substituent prefix for the characteristic group, if a terminal "l" is not already present. Thus, HO2C• is "carboxyl" (ref 19); HO• and HOO• are "hydroxyl" (ref 6c) and "hydroperoxyl" (ref 2s), respectively, (see also Note 6 to RC-81.1.2); 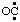 (and/or OC:) is carbonyl (ref 6c);
(and/or OC:) is carbonyl (ref 6c); 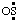 (and/or OS:) and
(and/or OS:) and 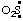 (and/or O2S:) are "sulfinyl" (ref 2v, 6c) and "sulfonyl" (ref 2v, 6c), respectively, and their selenium and tellurium analogs are quite analogous, for example,
(and/or O2S:) are "sulfinyl" (ref 2v, 6c) and "sulfonyl" (ref 2v, 6c), respectively, and their selenium and tellurium analogs are quite analogous, for example, 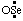 (and/or OSe:) is "seleninyl" (ref 2j, 6c); and •ClO2 and •ClO3 are chloryl (ref 6c) and perchloryl (ref 6c), respectively. Contracted names, such as "acetoxyl" and "methoxyl", and even the term "oxyl" (see RC-81.2.4), may also be considered to have been derived in this manner.
(and/or OSe:) is "seleninyl" (ref 2j, 6c); and •ClO2 and •ClO3 are chloryl (ref 6c) and perchloryl (ref 6c), respectively. Contracted names, such as "acetoxyl" and "methoxyl", and even the term "oxyl" (see RC-81.2.4), may also be considered to have been derived in this manner.
A few other established names may be considered to have been derived from a substituent prefix name ending in "o", by replacing the "o" with "yl". Thus, NC• is "cyanyl" (ref 19); CN• is "isocyanyl" (ref 19); ON• and O2N• are "nitrosyl" (ref 6c) and "nitryl" (ref 6c), respectively; •N3 is "azidyl" (ref 19); and •ClO is "chlorosyl" (ref 6c). The name "aminyl" (ref 2r) and the term "thiyl" (see RC-81.2.5) can also be considered as having been derived in this manner. However, this method cannot be classified as a general one, because it can easily lead to the same name for other groups. For example, replacing the "o" of "sulfino", the substituent prefix name for HO2S-, by "yl" leads directly to the name for 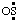 (and/or OS:), given above; and the method cannot be applied to monovalent halo radicals, because the resulting names are the names for O2E- groups, for example, "chloryl" (ref 2w, 6c).
(and/or OS:), given above; and the method cannot be applied to monovalent halo radicals, because the resulting names are the names for O2E- groups, for example, "chloryl" (ref 2w, 6c).
Note. To avoid any possibility of ambiguity, it is only necessary to keep clearly in mind that this name is derived from "azido", the name for the N3 group as a substituent prefix, and not from "azide", the name for the N3- anion. No confusion should occur if it is remembered that the azide anion lacks hydrogen atoms, which are necessary to generate the "-idyl" suffix for describing systematically an anionic substituent as a prefix (see RC-83.4.7.3).
RC-81.2.2. Acyl radicals, i.e., radicals with at least one chalcogen or nitrogen atom attached to the radical center by a (formal) double bond and which may be considered to have been derived formally by the loss of the hydroxy group from all acid characteristic groups expressed by the name of the acid are named by replacing the "-ic acid" or "-carboxylic acid" ending of the name of the acid with "-yl", "-oyl", or "-carbonyl", according to the method for forming the name of the corresponding acid halide (ref 2x, 4n).
Note. Compound prefixes for naming acyl substituents (ref 2h) are not used in these recommendations for acyl radicals or ions (see RC-80.6).
Examples:
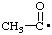 | acetyl |
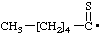 | hexanethioyl |
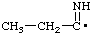 | propionimidoyl |
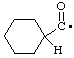 | cyclohexanecarbonyl |
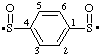 | benzene-1,4-disulfinyl |
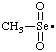 | methaneselenonyl |
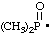 | dimethylphosphinoyl |
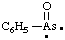 and/or and/or 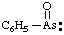 | phenylarsonoyl |
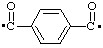 | terephthaloyl |
RC-81.2.3. Radical centers on nitrogen atoms of amine, imine, amide, or imide characteristic groups.
RC-81.2.3.1. Monovalent radical centers. A monovalent radical center formally derived by the removal of one (or the) hydrogen atom from the nitrogen atom of an amine, imine, amide, or imide characteristic group is named by adding a suffix "-aminyl", "-iminyl", or "-amidyl", "carboxamidyl", "-sulfonamidyl", "-dicarboximidyl", etc., to the name of the parent hydride attached to the characteristic group with the radical center, eliding the final "e", if any, from the name of the parent hydride; or by replacing the final "e" of a trivial name for an amine, imine, amide, or imide by "-yl".
Note 1. Amides of carboximidic and sulfinimidic acids were named as amidines, carboxamidines, or sulfinamidines in the 1979 IUPAC Organic Rules (ref 2y); the corresponding suffixes for monovalent radical centers on these suffixes would be "-amidinyl", "-carboxamidinyl", and "-sulfinamidinyl".
Note 2. Although not used in these recommendations for radical centers derived from amine, imine, or amide characteristic groups, the names "azanyl" and "aminyl" (ref 2r) can be used as parent radical names in the same way that "amine" has been used as a parent hydride name for derivatives of ammonia (see also footnote to Table 2 in RC-80.9.1 and Note 4 to RC-81.1.2)
Note 3. Trivial and semisystematic names preferred for naming organic compounds according to IUPAC recommendations are used in the first cited names for the examples below.
Examples:
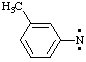
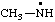 | methanaminyl |
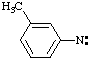
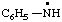 | anilinyl
benzenaminyl |
| CH3-CH2-CH=N• | propan-1-iminyl (ref 2z)
propylideneaminyl (ref 2aa) (see Note 2, above) |
| (CH3)3P=N• | trimethyl-λ5-phosphaniminyl (ref 2z, 16)
(trimethylphosphoranylidene)aminyl (ref 2aa, 4p, 12b)
(see Note 2, above)
trimethylphosphine imidyl (ref 12b) |
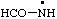 | formamidyl |
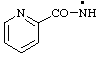 | pyridine-2-carboxamidyl |
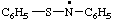 | N-(phenylsulfanyl)anilinyl
N-(phenylsulfanyl)benzenaminyl
(not N-phenylbenzenesulfenamidyl; see RC-80.7) |
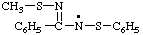 | N-(methylsulfanyl)-N-(phenylsulfanyl)benzenecarboximidamidyl
(See Note 1 above) |
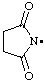 | succinimidyl
2,5-dioxopyrrolidin-1-yl (RC-81.1.2) |
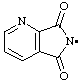 | pyridine-2,3-dicarboximidyl
5,7-dioxo-5,7-dihydro-6H-pyrrolo[3,4-b]pyridin-6-yl (RC-81.1.2) |
RC-81.2.3.2. Divalent radical centers. A divalent radical center formally derived by removal of both hydrogen atoms from an amine or amide characteristic group is named as a derivative of nitrene, aminylene (see RC-81.1.3.1), or λ1-azane (see RC-81.1.3.3).
Examples:
RC-81.2.4. Radical centers on oxygen atoms of acid, peroxy acid, hydroxy, or hydroperoxy characteristic groups. A radical center derived formally by the removal of the hydrogen atom from the oxygen atom of an acid, peroxy acid, hydroxy, or hydroperoxy characteristic group is named on the basis of a compound parent radical name formed by combining the name for the parent hydride or acid residue attached to the oxygen atom with the term "oxyl" or "peroxyl", for example, pentyloxyl, pentan-2-ylperoxyl. The contracted forms methoxyl, ethoxyl, propoxyl, butoxyl, phenoxyl, acetoxyl, and aminoxyl are retained. Substituents to such compound parent radicals are expressed by prefixes in the usual manner. Except for the contracted forms noted above, the compound parent radical name is enclosed in parentheses or brackets (see third example below).
Note 1. The term peroxyl is more consistent with terms like peroxo, peroxy, and peroxide, even though "dioxyl" is used in the 1979 IUPAC organic Rules (ref 2t).
Note 2. The names hydroxyl (ref 6c) and hydroperoxyl (ref 2s) for the radicals HO• and HO-O•, respectively (see also Note 6 to RC-81.1.2 and RC-81.2.1), are not used when the hydrogen atom has been substituted by another atom or group.
Examples:
| CH3-O• | methoxyl |
| Cl-CH2-CO-O• | chloroacetoxyl |
| CH3-O-CH2-CS-O• | methoxy[(thioacetyl)oxyl] |
Note. The contracted form thioacetoxyl is not used because it could be ambiguous. Brackets enclose the compound parent radical name in the presence of the substituent since curves are used to unambiguously describe the structure in the name for the compound parent radical itself.
| CH3-[CH2]4-CO-O-O• | hexanoylperoxyl |
| (Cl-CH2)2N-O• | bis(chloromethyl)aminoxyl |
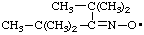 | (2,2,4,4-tetramethylpentan-3-ylidene)aminoxyl
(see RC-80.8)
(1-tert-butyl-2,2-dimethylpropylidene)aminoxyl
(see RC-80.8) |
Note. Aminoxyl radicals are named by radicofunctional nomenclature in CAS index nomenclature by using the functional parent compound name "nitroxide" as a class name (ref 13c), for example, bis(chloromethyl) nitroxide.
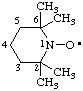 | 2,2,6,6-tetramethyl(piperidin-1-yloxyl) |
RC-81.2.5. Radical centers on sulfur atoms of thio acid, thiol, or dithiohydroperoxy characteristic groups. Although a radical center formally derived by the removal of the hydrogen atom from the sulfur atom of a thio acid, thiol, or thio hydroperoxy characteristic group can be described in the same manner as a radical center on the corresponding oxygen characteristic group (see RC-81.2.4) using the terms "-thiyl" and "perthiyl", usage in the literature is not consistent and therefore it is recommended that these radical centers be named on the basis of the parent radical names sulfanyl and disulfanyl (see RC-81.1.2).
Note. For instance both the names benzenethiyl, presumably derived from benzenethiol, and phenylthiyl, by analogy with phenyloxyl, the uncontracted form of phenoxyl (see RC-81.2.4), are used.
Examples:
| CH3-CO-S• | acetylsulfanyl
acetylthiyl (by analogy with RC-81.2.4) |
| C6H5-S• | phenyIsulfanyl
phenylthiyl (by analogy with RC-81.2.4)
(not benzenesulfenyl; see RC-80.7) |
| CH3-C(CH3)2-S-S• | (2-methylpropan-2-yl)disulfanyl (see RC-80.8)
tert-butyldisulfanyl (see RC-80.8)
tert-butylperthiyl (by analogy with RC-81.2.4) |
| Cl-CH2-CS-S• | [chloro(thioacetyl)lsulfanyl
chloro[(thioacetyl)thiyl] (by analogy with RC-81.2.4) |
RC-81.2.6. Radical centers on other characteristic groups. A radical center formally derived by the removal of hydrogen atoms from characteristic groups other than those described above in RC-81.2.1 through RC-81.2.5 is named preferably on the basis of a parent radical named according to RC-81.1 above.
Examples:
| CH3-CH2-O-S• | ethoxysulfanyl |
| C6H5-CO-S-O-O• | (benzoylsulfanyl)dioxidanyl
benzoyl(sulfanylperoxyl) (see RC-81.2.4) |
| C6H5-O-S-S-O• | (phenoxydisulfanyl)oxidanyl
phenoxy(disulfanyloxyl) (see RC-81.2.4) |
| C6H5-CO-Se• | benzoylselanyl |
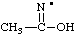 | (1-hydroxyethylidene)azanyl
(1-hydroxyethylidene)aminyl
1-hydroxyethan-1-iminyl (see RC-81.2.3.1) |
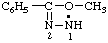 | 2-[methoxy(phenyl)methylidene]hydrazyl |
Note. The trivial name benzyl is not used as a substituent prefix when a-substituted; see footnote to item 17 in the Appendix, List A.
References for this section
1. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 ed., Pergamon Press, Oxford, 1979, 559 p.
2. Reference 1, Section C, pp. 79-322: [h] Rule C-403.2, pp. 185, 188, Note, p. 188; and Rule C-631.1, pp. 230-1; [j] Rule C-701.1, p. 247-8, Table XIII; p. 248; [r] Rule C-81.2, p. 134; [s] Rules C-218.1, p. 161; and C-81.1, pp. 133-4; [t] Rule C-81.1, pp. 133-4; [u] Rule C-922.1, p. 288; [v] Rule C-631.2, p. 231; [w] Rule C-106.2, p. 146; [x] Rules C-403, pp. 185, 188; C-481, pp. 206-7; C-641.7, pp. 236-7; and C-642.4, pp. 240-1; [y] Rules C-641.9, pp. 238-9; C-951.1, p. 292; C-951.2, p. 292; and C-951.3, p. 293; [z] Rule C-815.3(a), pp. 258-9; [aa] Rule C-815.3(b), pp. 258-9.
4. Reference 1, Section D, pp. 323-471: [n] Rules D-5.66, pp. 403-4; and D-5.67, p. 404; [p] Rule D-5.71, p. 406.
6. International Union of Pure and Applied Chemistry. Inorganic Chemistry Division. Commission on Nomenclature of Inorganic Chemistry, Nomenclature of Inorganic Chemistry, Recommendations 1990, G. J. Leigh, ed., Blackwell Scientific Publications, Oxford, England, 1990, 289 p. [c] Section I-8.4.2.2, p. 113.
12. American Chemical Society, "Report of the ACS Nomenclature, Spelling and Pronunciation Committee for the First Half of 1952": [b] E. Organic Compounds Containing Phosphorus, ibid., 4515-22.
13. American Chemical Society, "Chemical Substance Index Names", Appendix IV in the 1987-1991 Chemical Abstracts Index Guide, Chemical Abstracts Service, Columbus, Ohio, 1992: [c] §187, p. 176I.
16. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Treatment of Variable Valence in Organic Nomenclature (Lambda Convention), Recommendations 1983, Pure Appl. Chem. 56, 769-78 (1984).
19. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Physical Organic Chemistry, "Nomenclature for Organic Chemical Transformations, Recommendations 1988", Pure Appl. Chem. 61, 725-68 (1989), Table I, p. 730.
Continue with RC-81.3. Multiple radical centers
Return to ions and radicals home page.
Return to chemical nomenclature home page.
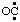 (and/or OC:) is carbonyl (ref 6c);
(and/or OC:) is carbonyl (ref 6c); 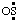 (and/or OS:) and
(and/or OS:) and 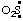 (and/or O2S:) are "sulfinyl" (ref 2v, 6c) and "sulfonyl" (ref 2v, 6c), respectively, and their selenium and tellurium analogs are quite analogous, for example,
(and/or O2S:) are "sulfinyl" (ref 2v, 6c) and "sulfonyl" (ref 2v, 6c), respectively, and their selenium and tellurium analogs are quite analogous, for example, 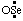 (and/or OSe:) is "seleninyl" (ref 2j, 6c); and •ClO2 and •ClO3 are chloryl (ref 6c) and perchloryl (ref 6c), respectively. Contracted names, such as "acetoxyl" and "methoxyl", and even the term "oxyl" (see RC-81.2.4), may also be considered to have been derived in this manner.
(and/or OSe:) is "seleninyl" (ref 2j, 6c); and •ClO2 and •ClO3 are chloryl (ref 6c) and perchloryl (ref 6c), respectively. Contracted names, such as "acetoxyl" and "methoxyl", and even the term "oxyl" (see RC-81.2.4), may also be considered to have been derived in this manner.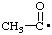
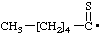
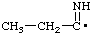

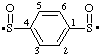
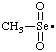
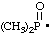
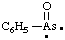 and/or
and/or 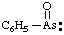

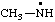
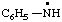
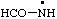
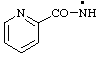
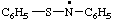
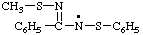
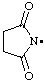

 and/or
and/or