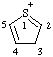 | 3H-1λ4-thiophen-1-ylium |
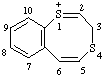 | 3H-1λ4-benzo[e][1,4]dithiocin-1-ylium 3H-1l4,4-benzodithiocin-1-ylium (ref 3h) |
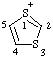 | 1λ4,3-dithiol-1-ylium |
Contents of this section
Note: Cationic heteroatoms of this type result usually from the involvement of a "free" electron pair of the heteroatom in the skeletal bonding of the heterocycle, either in the formation of a ring or in the double bond system of the ring. These cations cannot be treated systematically by either or both of the methods described in Rules RC-82.1.1 or RC-82.2.2 as such. They have been described by trivial names (ref 2ii), named by replacement methods (see RC-82.4), or simply indicated as being cationic by adding the suffix "-ium" to the name of the neutral heterocycle (ref 2jj), regardless of the formal derivation of the cation. None of these methods is satisfactory for a broad range of structural types.RC-82.3.1. The λ-convention with "-ylium". A cationic heterocycle having a cationic center on a heteroatom that has one more skeletal bond than it has in the corresponding neutral heterocycle is named by adding the operational suffix "-ylium" to the name of a neutral parent heterocycle for which the λ-convention (ref 16) has been used to describe a nonstandard bonding state of the heteroatom and that heteroatom has at least one hydrogen atom in the neutral heterocycle on which the "-ylium" suffix can operate.
Note: For certain cationic heterocycles of this type, especially those with cationic centers on heteroatoms from the second period elements, it might seem more acceptable to use replacement nomenclature (see RC-82.4) or to derive the name by the removal of two hydrogen atoms (didehydro) from a cation formed by addition of a hydron, for example, 4a-azonianaphthalene or 2,5-didehydro-2H-quinolizin-5-ium, respectively, for the cation known also as quinolizinium (ref 2jj). The latter method, however can become quite cumbersome in some cases, requiring both hydro and dehydro prefixes; for example, the structure in the first example below would have to be named 1,2-didehydro-2,3-dihydrothiophen-1-ium.Examples:
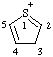 | 3H-1λ4-thiophen-1-ylium |
 | 3H-1λ4-benzo[e][1,4]dithiocin-1-ylium 3H-1l4,4-benzodithiocin-1-ylium (ref 3h) |
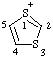 | 1λ4,3-dithiol-1-ylium |
Note This cation is commonly called 1,3-dithiolium, but it must be noted that this name also could refer to following structure:
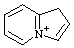 | 1H-4λ5-indolizin-4-ylium 3a-azonia-1H-indene (see RC-82.4) |
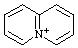 | 5λ5-quinolizin-5-ylium 4a-azonianaphthalene (see RC-82.4) quinolizinium [traditional name (ref 2jj)] |
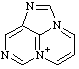 | 4H-7λ5-pyrimido[1,2,3-cd]purin-7-ylium 3H-1,2a,7-triaza-5a-azoniaacenaphthylene (see RC-82.4) |
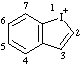 | 1λ3-benzo[b]iodol-1-ylium 1λ3-benzoiodol-1-ylium (ref 3h) |
Note: For recommendations to be published (ref 5) on elision in names of fused ring systems, see the footnote to example 4 under RC-81.3.3.2.
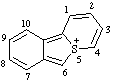 | 5H-5λ4-benzo[3,4]thieno[1,2-a]thiopyran-5-ylium 5H-5λ4-thiopyrano[2,1-a][2]benzothiophen-5-ylium (ref 3h) benzo[3,4]thieno[1,2-a]thiopyrylium (RC-82.3.2) |
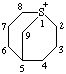 | 1λ4-thiabicyclo[3.3.1]nonan-1-ylium |
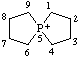 | 5λ5-phosphaspiro[4.4]nonan-5-ylium |
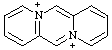 | 5λ5,11λ5-dipyrido[1,2-a:1',2'-d]pyrazine-5,1-bis(ylium) 4a,8a-diazoniaanthracene (see RC-82.4) |
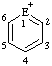 | 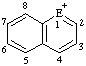 | 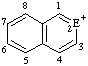 | |||
| E=O: pyrylium E=S: thiopyrylium | E=O: chromenylium E=S: thiochromenylium | E=O: isochromenylium E=S: isothiochromenylium | |||
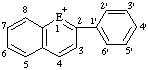 | 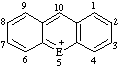 | ||||
| E=O: flavylium E=S: thioflavylium | E=O: xanthylium E=S: thioxanthylium | ||||
Note 1: This cationic replacement prefix for bismuth is formed by adding "-onia" to the element name (ref 4r).Examples:Note 2: Although the name "carbenia" has been proposed as a replacement prefix for the tricoordinate carbon cation (an "-ylia" cationic center) (ref 20), the name "carbanylia" would be consistent with the principles of these recommendations. The term "carbonia" cannot be used as a replacement prefix for a pentacoordinate carbon cationic center, because of previous usage of the term "carbonium" for a tricoordinate carbon cationic center (see also footnote to RC-82.1.1.1).
Note 3: The only replacement prefix of this type noted in the previous rules was borylia (ref 4s).
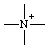 or or 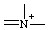 | azonia |
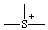 or or 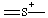 | thionia |
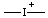 | iodonia |
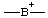 | boranylia |
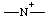 | azanylia |
Cationic replacement prefixes are cited and the skeletal positions they represent are numbered, where there is a choice, in the same order as the corresponding neutral replacement ("a") prefixes given in Table I of Rule B-1.1 (ref 3d) or in Table I in the Appendix to Section D of the 1979 IUPAC organic Rules (ref 4d). Cationic replacement prefixes are cited immediately after the corresponding neutral replacement prefix (ref 3i) occurring in the same name in the order "-onia" followed by "-ylia"; and, where there is a choice, are preferred to the corresponding neutral skeletal atom for assignment of low locants (ref 2mm) in the order "-ylia" followed by "-onia" (see also RC-82.5.2).
Note: In general, the Commission favors the use of the suffixes "-ium" and "-ylium" with nonreplacement names of parent hydrides or with neutral replacement names, to the use of cationic replacement prefixes. In addition, names that do not require designation of skeletal atoms in nonstandard valency states by the λ-convention (ref 16) are preferred (see also Note 5 to RC-82.2.2.2).Examples:
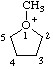 | 1-methyl-1-oxoniacyclopentane 1-methyltetrahydrofuran-1-ium (RC-82.1.1.2) |
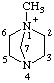 | 1-methyl-4-aza-1-azoniabicyclo[2.2.1]heptane 1-methyl-1,4-diazabicyclo[2.2.1]heptan-1-ium (RC-82.1.1.2) |
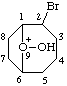 | 2-bromo-9-hydroxy-9-oxoniabicyclo[4.2.1]nonane 2-bromo-9-hydroxy-9-oxabicyclo[4.2.1]nonan-9-ium (RC-81.1.1.2) |
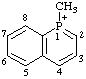 | 1-methyl-1-phosphonianaphthalene 1-methylphosphinolin-1-ium (RC-82.1.1.2) 1-methylbenzo[b]phosphinin-1-ium (ref 11) (RC-82.1.1.2) 1-methyl-1-benzophosphinin-1-ium (ref 3h, 11) (RC-82.1.1.2) |
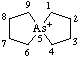 | 5-arsoniaspiro[4.4]nonane 5λ5-arsaspiro[4.4]nonan-5-ylium (RC-82.3.1) |
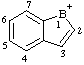 | 1-boranyliaindene 1H-1-borainden-1-ylium (RC-82.2.2.2) 1H-benzo[b]borol-1-ylium (RC-82.2.2.2) 1H-1-benzoborol-1-ylium (ref 3h) (RC-82.2.2.2) |
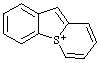 | 4a-thioniafluorene 4aH-4aλ4-thiafluoren-4a-ylium (RC-82.3.1) 5H-5λ4-benzo[4,5]thieno[1,2-a]thiopyrylium (RC-82.3.1 and RC-82.3.2) 5H-5λ4-thiopyrano[1,2-a][1]benzothiophen-5-ylium (ref 3h) (RC-82.3.1) |
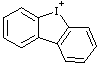 | 9-iodoniafluorene 5H-5λ3-dibenzoiodol-5-ylium (RC-82.3.1) 9H-9λ3-iodafluoren-9-ylium (RC-82.3.1) |
Note: For recommendations to be published (ref 5) on elision in names of fused ring systems, see the note to example 4 under RC-81.3.3.2.
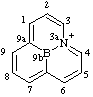 | 3a-azonia-9b-boraphenalene 3aλ5-aza-9b-boraphenalen-3a-ylium (RC-82.3.1) |
1. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 ed., Pergamon Press, Oxford, 1979, 559 p.
2. Reference 1, Section C, pp. 79-322: [f] Rule C-16.1, p. 108; [ii] Rule C-83.2, p. 139; [jj] Rule C-83.1, pp. 137-9, Note 2, p. 138; [kk] Subsection C-0.6, pp. 123-7; [mm] Rule C-62.1, pp. 124-5.
3. Reference 1, Section B, pp. 53-76: [d] Rule B-1.1, pp. 53-5, Table I, p. 53; [g] Rule B-4, p. 68-70; [h] Rule B3.5, pp. 67-8; [i] Rules B-6. pp. 71-2; B-10.1 (in part), p. 72; and B-11 (in part).
4. Reference 1, Section D, pp. 323-471: [d] Appendix, Table I, pp. 459-60; [q] Rule D-1.6, pp. 334-15; [r] Rule D-5.34, p. 393; [s] Rule D-7.63, pp. 449-50.
5. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford, 1993.
11. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Revision of the Extended Hantzsch-Widman System of Nomenclature for Heteromonocycles, Recommendations 1982, Pure Appl. Chem. 55, 409-16, 1983.
16. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Treatment of Variable Valence in Organic Nomenclature (Lambda Convention), Recommendations 1983, Pure Appl. Chem. 56, 769-78 (1984).
20. J. H. Fletcher, O. C. Dermer, and R. B. Fox, Nomenclature of Organic Compounds, Principles and Practice, American Chemical Society, Washington, D. C., 1974 (Advances in Chemistry Series, No. 126), pp. 219-20.