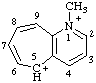
 | 1-methyl-5H-cyclohepta[b]pyridin-1-ium-5-ylium 1-methyl-5H-1-azoniabenzocyclohepten-5-ylium |
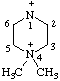 | 4,4-dimethylpiperazin-4-ium-1-ylium |
Contents of this section
RC-82.5.1. Polycations with cationic centers identically derived at two or more positions on the same parent hydride are named using appropriate numerical prefixes and operational suffixes as given in RC-82.1.1.2 and RC-82.2.2.2 or replacement prefixes as given in RC-82.4.
RC-82.5.2. "Ium" and "ylium" centers in the same parent hydride. Cationic compounds with two or more cationic centers in the same parent hydride structure, at least one being derived formally by the addition of a hydron to a skeletal position and at least one by the loss of a hydrogen atom as a hydride ion from another skeletal position, are named by replacing the final "e" of the parent hydride name by the operational suffix "-ium", followed by the operational suffix "-ylium", each preceded, where necessary, by appropriate numerical or multiplicative prefixes and locants (see RC-82.1.1.2 and RC-82.2.2.2).
Where there is a choice, low locants of the parent hydride are given first to the cationic centers regardless of their type, and then to "-ylium" cationic centers.
Examples:
 | 1-methyl-5H-cyclohepta[b]pyridin-1-ium-5-ylium 1-methyl-5H-1-azoniabenzocyclohepten-5-ylium |
 | 4,4-dimethylpiperazin-4-ium-1-ylium |
Dications and polycations so derived from diacids and polyacids are named by adding numerical prefixes, such as "di-" and "tri-", to the term "acidium".
Examples:
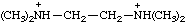 | N,N,N',N'-tetramethylethane-1,2-bis(aminium) N,N,N',N'-tetramethylethylenebis(aminium) |
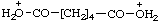 | hexanedioic diacidium |
Examples:
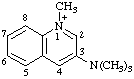 | N,N,N,1-tetramethylquinolin-1-ium-3-aminium |
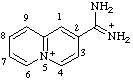 | 5λ5-quinolizin-5-ylium-2-carboximidamidium (see footnote to Table 4 in RC-82.1.2.1) |
Example:
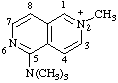 | N,N,N,2-tetramethyl-2,6-naphthyridin-2-ium-5-aminium (not N,N,N,6-tetramethyl-2,6-napthyridin-6-ium-1-aminium) |
Note: The multiplicative prefixes "bis-", "tris-", etc. are used to avoid the possibility of confusion that could arise from the use of the numerical prefixes "di-", "tri-", etc.Examples:
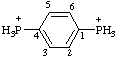 | 1,4-phenylenebis(phosphonium) |
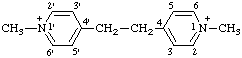 | 1,1'-dimethyl-4,4'-ethylenedipyridin-1-ium (IUPAC multiplicative method; see RC-80.11) 4,4'-ethylenebis[1-methylpyridin-1-ium] (CAS multiplicative method; see RC-80.11) |
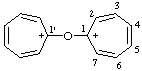 | 1,1'-oxydicyclohepta-2,4,6-trien-1-ylium |
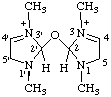 | 1,1',3,3'-tetramethyl-2,2'-oxybis(2,5-dihydro-1H-imidazol-3-ium) (IUPAC multiplicative method; see RC-80.11) 2,2'-oxybis[1,3-dimethyl-2,5-dihydro-1H-imidazole-3-ium] (CAS multiplicative method; see RC-80.11) |
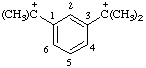 | 2,2'-(1,3-phenylene)dipropan-2-ylium (see RC-82.2.2.2 and RC-80.8) 1,1'-dimethyl-1,1'-(1,3-phenylene)diethylium (see RC-82.2.2.1 and RC-80.8) (IUPAC multiplicative method; see RC-80.11) 1,1'-(1,3-phenylene)bis[1-methylethylium] (see (RC-82.2.2.1 and RC-80.8) (CAS multiplicative method; see RC-80.11) |
Note: Accordingly, cationic centers derived from two or more of the same characteristic group are not described in terms of that characteristic group using appropriate multiplicative prefixes.Examples:
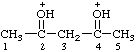 | pentane-2,4-diylidenebis(oxonium) (see RC-80.8) (1,3-dimethylpropan-1,3-diyl)bis(oxonium) (see RC-80.8) |
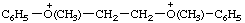 | O,O'-dimethyl-O,O'-diphenyl[ethylenebis(oxonium)] (IUPAC multiplicative method; see RC-80.11) ethylenebis[methylphenyloxonium] (CAS multiplicative method; see RC-80.11) |
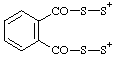 | phthaloylbis(disulfanylium) |
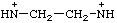 | ethylenebis(azanylium) ethylenebis(aminylium) |
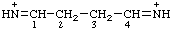 | butane-1,4-diylidenebis(azanylium) butane-1,4-diylidenebis(aminylium) |
| +O-CH2-CH2-O+ | ethylenebis(oxidanylium) |
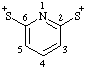 | pyridine-2,6-diylbis(sulfanylium) |
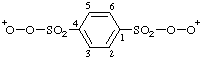 | benzene-1,4-disulfonylbis(dioxidanylium) |
| +O-S-SO2-CH2-CH2-SO2-S-O+ | [ethane-1,2-disulfonylbis(sulfanediyl)]bis(oxidanylium) |
Examples:
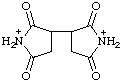 | 2,2',5,5'-tetraoxo[3,3'-bipyrrolidine)-1,1'-diium |
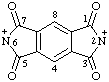 | 1,3,5,7-tetraoxo-1,2,3,5,6,7-hexahydrobenzo[1,2-c;4,5-c']dipyrrole-2,6-bis(ylium) (RC-82.2.2.4) 1,3,5,7-tetraoxo-5,7-dihydrobenzo[1,2-c:4,5-c']di-pyrrole-2,6(1H,3H)-bis(ylium) (RC-82.2.2.4) |
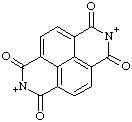 | 1,3,6,8-tetraoxo-1,2,3,6,7,8-hexahydrobenzo[lmn][3,8]phenanthrolin-7-ium-2-ylium (RC-82.2.2.4) 1,3,6,8-tetraoxo-3,6,7,8-tetrahydrobenzo[lmn][3,8]phenanthrolin-7-ium-2(1H)-ylium (RC-82.2.2.4) |
RC-82.5.8.1. Prefixes for expressing the mononuclear parent cations described by RC-82.1.1.1 as monovalent substituents are formed by changing the "-onium" ending of the parent cation to "-onio", (ref 2nn).
Examples:
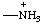 | ammonio |
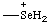 | selenonio |
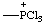 | trichlorophosphonio |
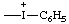 | phenyliodonio |
Examples:
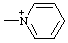 | pyridinio |
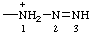 | triaz-2-en-1-io |
Note 1: The operational suffix "-ylidene" is to be used only to describe the presence of a double bond between the substituting group and a parent hydride or parent substituent (see RC-80.5).Examples:Note 2: This method is also applicable to the cationic prefix groups considered in RC-82.5.8.1 and RC-82.5.8.2.
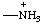 | ammoniumyl ammonio (RC-82.5.8.1) |
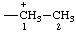 | ethan-1-ium-1-yl |
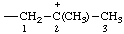 | 2-methylpropan-2-ylium-1-yl |
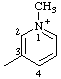 | 1-methylpyridin-1-ium-3-yl |
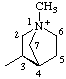 | 1-methyl-1-azoniabicyclo[2.2.1]heptan-3-yl (RC-82.4) 1-methyl-1-azabicyclo[2.2.1]heptan-1-ium-3-yl (RC-82.1.1.2) |
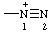 | diazyn-1-ium-1-yl (traditional name: diazonio) |
Note: The traditional name "diazonio" describes both structures,and -N=N+, whereas the systematic name given here describes only the first of these structures; the name diazen-2-ylium-1-yl describes the second structure.
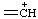 | methanyliumylidene |
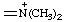 | dimethylazaniumylidene |
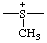 | methylsulfaniumdiyl |
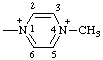 | 4-methylpyrazine-1,4-diium-1-yl |
(a) the maximum number of cationic centers of any kind, including cationic suffix groups [This is in agreement with the principles for choosing a preferred component for naming fused heterocyclic ring systems (ref 3j)];Further choice, if necessary, is made by applying the general criteria for chains and rings given in the 1979 IUPAC Organic Rules (ref 2cc).(b) the maximum number of "ylium" cationic centers;
(c) the maximum number of senior cationic centers, according to the nature of the cationic atom, in the same order as for the corresponding replacement prefixes (ref 3d, 11).
Examples:
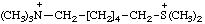 | dimethyl[6-(trimethylammonio)hexyl]sulfonium (RC-82.1.1.1, RC-82.5.8.1, and RC-80.8) 6-(dimethylsulfonio)-N,N,N-trimethylhexan-1-aminium (RC-82.1.2.1 and RC-82.5.8.1) 2,9,9-trimethyl-2-thionia-9-azoniadecane (RC-82.4) |
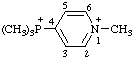 | 1-methyl-4-(trimethylphosphoniumyl)pyridin-1-ium (RC-82.5.8.3) 1-methyl-4-(trimethylphosphonio)pyridin-1-ium (RC-82.5.8.1) |
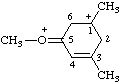 | 1,3-dimethyl-5-(methyloxoniumylidene)cyclohex-3-en-1-ylium |
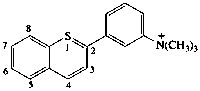 | 2-[3-(trimethylammonio)phenyl]-1λ4-benzo[b]thiopyrylium (RC-82.5.8.1 and RC-82.3.2) 2-[3-(trimethylammoniumyl)phenyl]-1-benzothiopyrylium (ref 3h) (RC-82.5.8.3 and RC-82.3.2) |
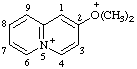 | 2-(dimethyloxoniumyl)-5λ5-quinolizin-5-ylium (RC-82.3.1 and RC-82.5.8.3) 2-(dimethyloxonio)-5λ5-quinolizin-5-ylium (RC-82.3.1 and RC-82.5.8.1) |
1. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 ed., Pergamon Press, Oxford, 1979, 559 p.
2. Reference 1, Section C, pp. 79-322: [q] Rules C-72, p. 130-1; and C-73, p. 132; [cc] Subsections C-0.13, pp. 97-101; and C-0.14, pp. 101-5; [nn] Rules C-82.1, p. 134-5, Table V, p. 135; and C-816.3, p. 260; [pp] Rule C-82.1, p. 134-5, Table V, p. 135, footnote.
3. Reference 1, Section B, pp. 53-76: [d] Rule B-1.1, pp. 53-5, Table I, p. 53; [h] Rule B-3.5, pp. 67-8; [j] Rule B-3.1, pp. 64-5.
11. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Revision of the Extended Hantzsch-Widman System of Nomenclature for Heteromonocycles, Recommendations 1982, Pure Appl. Chem. 55, 409-16, 1983.