| H3C- | methyl anion |
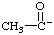 | acetyl anion |
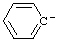 | phenyl anion |
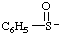 | benzenesulfinyl anion |
| CH3-NH- | methanaminyl anion methylazanyl anion |
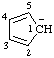 | cyclopenta-2,4-dien-1-yl anion |
| (C6H5)2C2- | diphenylmethylene dianion |
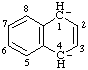 | 1,4-dihydronaphthalene-1,4-diyl dianion |
Contents of this section
RC-83.0. Introduction. For the purposes of organic nomenclature, an anion is a molecular entity carrying at least one unit of negative charge formally derived from a parent hydride, a parent compound, or a hydro derivative of either, by the removal of one or more hydrogen atoms as hydrons, by the addition of one or more hydride ions, or by a combination of these operations. An atom at which the negative charge is considered to reside is called an anionic center. Anions with two or more anionic centers are called dianions, trianions, etc.
Note: Hydron is a generic name for the hydrogen cation, i.e., the naturally occurring mixture of protons, deuterons, and tritons (ref 9). The name proton is restricted to the hydrogen cation having the mass number 1, i.e., 1H+.RC-83.1. Anionic compounds with anionic centers derived formally by removal of hydrons.
RC-83.1.1. Radicofunctional names. Anionic compounds that can be considered as being derived formally by addition of electrons to the corresponding radical (see RC-81) may be named by adding the class name "anion" as a separate word after the name of the radical. Polyanions are indicated by adding the numerical prefixes "di-", "tri-", etc., as appropriate, to the class name.
Note: Although such names are useful for anions where all anionic centers are located in a single parent structure, the system cannot be extended to polyanions where one or more of the anionic centers must be expressed in a substituent to a parent structure.Examples:
| H3C- | methyl anion |
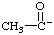 | acetyl anion |
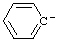 | phenyl anion |
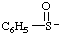 | benzenesulfinyl anion |
| CH3-NH- | methanaminyl anion methylazanyl anion |
 | cyclopenta-2,4-dien-1-yl anion |
| (C6H5)2C2- | diphenylmethylene dianion |
 | 1,4-dihydronaphthalene-1,4-diyl dianion |
RC-83.1.2.1. The operational suffix "-ide". An anionic center derived formally by the removal of one or more hydrons from any position of a neutral parent hydride is named by replacing the final "e" of the name of the parent hydride, if present, by the operational suffix "-ide", or by adding the suffixes "-ide", "-diide", etc., to the name of the parent hydride.
The trivial names "amide" and "imide" for the anions H2N- and HN2-, respectively (ref 6f, 14), are retained, and may be used as parent anions in substitutive nomenclature, but see also RC-83.1.5. The trivial name "acetylide" for the anion [C2]2- is also retained (ref 6g).
Note 1: Contracted names for hydrocarbon anions, such as methide, are not recommended, since other contracted names, such as boride, sulfide, and phosphide, cannot be used because they are names for the monoatomic anions B3-, S2-, and P3-, respectively (ref 6h).Examples:Note 2: The names for the anions HO-, HO-O-, and HS-, HS-S-, etc., are hydroxide (ref 6f), hydroperoxide (ref 21a), and hydrosulfide (ref 21b), hydrodisulfide, etc., respectively, but, in keeping with tradition, are not used as parent anion names in substitutive nomenclature. For the substituted anions, see RC-83.1.4 and RC-83.1.6.
| CH3-NH- | methylamide methylazanide (see also RC-83-1.5) |
| (CH3)2P- | dimethylphosphanide (ref 4g, 6b) dimethylphosphinide |
Note: Although the traditional names phosphine, arsine, etc. (ref 4g, 6b), can be used for deriving parent anion names, the systematic names are preferred.
| (CH3-CH2-CH2-CH2)3Sn- | tributylstannanide |
| HC | methylidynesilanide |
| (C6H5)2B-CH2- | (diphenylboryl)methanide |
| (NC)3C- | tricyanomethanide |
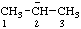 | propan-2-ide |
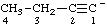 | but-1-yn-1-ide |
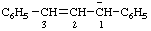 | 1,3-diphenylprop-2-en-1-ide |
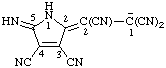
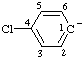 | 4-chlorobenzen-1-ide |
 | cyclopenta-2,4-dien-1-ide |
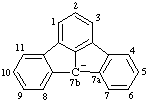 | 7bH-indeno[1,2,3-jk]fluoren-7b-ide |
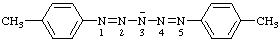 | 1,5-di-p-tolylpentaaza-1,4-dien-3-ide |
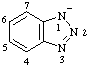 | 1H-benzotriazol-1-ide |
| C6H5-N2- | phenylimide |
| (C6H5)2C2- | diphenylmethanediide |
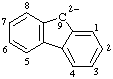 | 9H-fluorene-9,9-diide |
 | 1,4-dihydronaphthalene-1,4-diide |
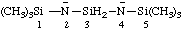 | 1,1,1,5,5,5-hexamethyltrisilazane-2,4-diide |
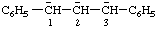 | 1,3-diphenylpropane-1,2,3-triide |
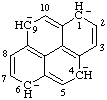 | 1,4,6,9-tetrahydropyrene-1,4,6,9-tetraide |
In the first method (ref 2g), the "hydro" derivative is described by specifying the hydrogen atom of the dihydro pair that remains after the anionic center has been created by citing an italic capital H(H) and the locant for the skeletal atom at which that hydrogen atom resides, both enclosed in a set of parentheses and inserted into the name immediately after the locant for the anionic center.
Note: This procedure is exactly analogous to the use of "indicated hydrogen" to describe saturated ring positions that remain after the introduction of suffixes for principal characteristic groups, such as "-one", into the name of a ring or ring system that otherwise expresses the maximum number of noncumulative double bonds (ref 2g).Examples:
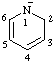 | 1,2-dihydropyridin-1-ide pyridin-1(2H)-ide |
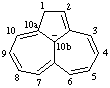 | 1,10b-dihydrocyclopenta[ef]heptalen-10b-ide cyclopenta[ef]heptalen-10b(1H)-ide |
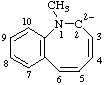 | 1-methyl-1,2-dihydrobenzo[b]azocine-2,2-diide 1-methyl-1-benzoazocine-2,2(1H)-diide (ref 3h) |
Note: For recommendations to be published (ref 5) on elision in names of fused ring systems, see the footnote to example 4 under RC-81.3.3.2.RC-83.1.3. Anionic centers on chalcogen atoms of acid characteristic groups.
RC-83.1.3.1. Acid groups expressed as suffixes. An anion formed by the loss of the hydrogen atom as a hydron from the chalcogen atom of each acid characteristic group expressed by an "ic acid" suffix (except for mixed chalcogen peroxy acids, such as R-CS-O-OH or R-CO-S-SH), or implied by a semisystematic or trivial "-ic acid" name is named by replacing the "-ic acid" ending of the acid name by the suffix "-ate".
Note: Application of this method to mixed chalcogen peroxy acids does not necessarily produce an unambiguous name.Examples:
Note: Even though it is common practice to represent the structure of such acid anions in a condensed form, such as CO2- and -SO3-, anionic acid groups are shown here in an extended form, as -CO-O- and -S(O)2-O- in order to explicitly show nonbonding electron pairs at anionic centers, as is done throughout these recommendations for radical and ionic centers.
| CH3-CO-O- | acetate |
| C6H5-CO-O-O- | peroxybenzoate perbenzoate [from perbenzoic acid (ref 2qq)] |
| C6H5-SO2-O- | benzenesulfonate |
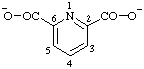 | pyridine-2,6-dicarboxylate |
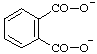 | phthalate |
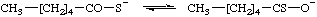 hexanethioate
hexanethioate
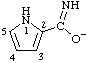 | 1H-pyrrole-2-carboximidate |
| OHC-CH2-CH2-CO-O- | succinaldehydate |
| -O-CO-[CH(OH)]2-CO-O- | tartrate (a contraction of "tartarate", a name derived according to this recommendation) |
Examples:
| (C6H5)2PO- | diphenylphosphinate |
| CH3-AsO(-O-)2 | methylarsonate |
Note 1: According to IUPAC inorganic nomenclature recommendations, the word hydrogen should be attached directly to the name of the anion (ref 6i); in this guide to IUPAC organic nomenclature practices, the word hydrogen is separated from the name of the anion, a continuation of the practice used in previous organic rules and recommendations (ref 2rr).If specificity is required, the anionic center should be named on the basis of a fully ionized structure according to RC-83.1.3.1 or RC-83.1.3.2, and the free acid or ester groups expressed as substituents. Names of this type are included for the examples below.Note 2: Although locants prefixed to the hydrogen term can provide specificity in some cases, this is not always possible. The lack of specificity in this method can be an advantage when positions of remaining hydrogen atoms or charge cannot be determined, or when such specificity is neither necessary nor desirable. This method retains a correlation with the functionality of the corresponding neutral compound.
Examples:
| HO2C-[CH2]5-CO-O- | hydrogen heptanedioate 6-carboxyhexanoate |
| CH3-CH2-O-OC-CH(C6H5)-CO-O- | ethyl phenylmalonate (ethoxycarbonyl)phenylacetate |
| C6H5-P(O)(OH)-O- | hydrogen phenylphosphonate hydroxy(phenyl)phosphinate |
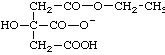 | O3-ethyl O1-hydrogen citrate O4-ethyl 2-(carboxymethyl)-2-hydroxybutanedioate O4-hydrogen 2-[(ethoxycarbonyl)methyl]-2-hydroxybutanedioate 2-(carboxymethyl)-3-(ethoxycarbonyl)-2-hydroxypropanoate |
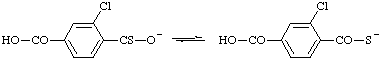
RC-83.1.4.1. Anions from C1 - C4 acyclic alcohols, phenols, or hydroxylamine. The traditional contracted radicofunctional names (ref 2ss) such as methoxide (from methyl oxide) and aminoxide (presumably from aminyl oxide) are the names for anions formally derived by the loss of the hydrogen atom as a hydron from the oxygen atom of these hydroxy compounds. The unbranched acyclic structures are parent anions for substitutive nomenclature; the branched structures, such as isopropoxide (from isopropyl oxide), are not.
Examples:
| Cl-CH2-CH2-O- | 2-chloroethoxide |
| (CH3)2C(Cl)-O- | 1-chloro-1-methylethoxide 2-chloropropan-2-olate (see RC-83.1.4.2) |
| (CH3)N-O- | dimethylaminoxide |
Note 1: The limited practice of naming anions derived in this way from alcohols in radicofunctional nomenclature by adding the suffix "ate" to the class name "alcohol", or by changing the ending "yl alcohol" to "ylate" (in some languages other than English), permitted by the previous edition of the IUPAC Organic Rules (ref 2tt), is not included in these revised recommendations.Examples:Note 2: The trivial names picric acid (ref 2uu) (2,4,6-trinitrophenol) and styphnic acid (ref 2uu) (2,4,6-trinitrobenzene-1,3-diol) are used to derive the names picrate and styphnate for the corresponding anions with anionic centers on the (both) oxygen atom(s), according to RC-83.1.3.1, just as if an actual acid characteristic group were present.
Note 3: The names hydroxide, hydroperoxide, and hydrosulfide, hydrodisulfide, etc., for the anions HO-, HOO-, and HS-, HS-S-, etc., respectively, are not used when the hydrogen atom has been substituted by another atom or group (see Note 2 to RC-83.1.2.1).
| CH3-[CH2]4-O- | pentan-1-olate |
| C6H11-Se- | cyclohexaneselenolate |
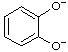 | pyrocatecholate benzene-1,2-bis(olate) |
Note: The multiplicative prefixes "bis-", "tris-", etc. are used to avoid the possibility of ambiguity by interpreting a suffix such as "-diolate" to mean a monoanion derived from a diol.RC-83.1.5. Monoanionic centers on the nitrogen atom of an amino characteristic group formally derived by the removal of one hydrogen atom from the nitrogen atom as a hydron may be named by replacing the final "e" of the amine suffix or parent compound name by the operational suffix "-ide", or by substitutive nomenclature based on the parent anion names amide or azanide (see RC-83.1.2.1).
Examples:
| CH3-CH2-NH- | ethanaminide ethylamide ethylazanide |
| C6H5-NH- | anilinide benzenaminide phenylamide phenylazanide |
Note: Suffixes such as "-imidide" and "-amidide" are not recommended and "ylideneaminide" names are not included herein.Examples:
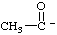 | 1-oxoethanide |
| CH3-O-O- | methyldioxidanide |
Note: Although the radicofunctional name methyl peroxide is in fact an accurate description for this anion, such names are not recommended herein because of the widespread use of such names to describe symmetrical peroxides, such as dimethyl peroxide.
| C6H5-S-O- | (phenylsulfanyl)oxidanide (not benzenesulfenate; see RC-80.7) |
| CH3-CS-O-O- | (thioacetyl)dioxidanide |
| (CH3)3C-O-O-O- | tert-butyltrioxidanide (see RC-80.8) (2-methylpropan-2-yl)trioxidanide (see RC-80.8) |
| CH3-CO-O-S- | acetoxysulfanide |
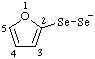 | (2-furyl)diselanide |
| CH3-O-S- | methoxysulfanide |
| CH3-C(OH)=N- | (1-hydroxyethylidene)amide (1-hydroxyethylidene)azanide |
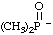 | dimethyloxo-λ5-phosphanide (ref 16) dimethyloxophosphoranide (ref 4p) |
| (CH3)3P=N- | (trimethyl-λ5-phosphanylidene)amide (ref 16) (trimethylphosphoranylidene)amide (ref 4p, 12b) (trimethyl-λ5-phosphanylidene)azanide (ref 16) (trimethylphosphoranylidene)azanide (ref 4p, 12b) |
| C6H5-CO-NH-N2- | 2-benzoyldiazane-1,1-diide 2-benzoylhydrazine-1,1-diide [traditional parent hydride name (ref 14)] |
| CH3-CO-NH- | acetylamide acetylazanide |
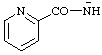 | pyridine-2-carbonylamide pyridine-2-carbonylazanide |
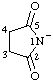 | 2,5-dioxopyrrolidin-1-ide |
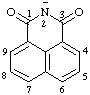 | 1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-2-ide (see RC-83.1.2.2) 1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-ide (see RC-83.1.2.2) |
Note: For recommendations to be published (ref 5) on elision in names of fused ring systems, see the note to example 4 under RC-81.3.3.2.
1. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 ed., Pergamon Press, Oxford, 1979, 559 p.
2. Reference 1, Section C, pp. 79-322: [f] Rule C-16.1, p. 108; [g] Rule C-315.1, p. 172; [qq] Rule C-441, pp. 196-7, Exceptions, p. 197; [rr] Rule C-462.1, p. 200; [ss] Rule C-206.2, p. 156, Exceptions; [tt] Rule C-206.2, p. 156, Note (1); [uu] Rule C-202.2, pp. 151-2.
3. Reference 1, Section B, pp. 53-76: [h] Rule B3.5, pp. 67-8.
4. Reference 1, Section D, pp. 323-471: [g] Rule D-5.11, p. 384, footnote; [p] Rule D-5.71, p. 406.
5. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford, 1993.
6. International Union of Pure and Applied Chemistry. Inorganic Chemistry Division. Commission on Nomenclature of Inorganic Chemistry, Nomenclature of Inorganic Chemistry, Recommendations 1990, G. J. Leigh, ed., Blackwell Scientific Publications, Oxford, England, 1990, 289 p. [b] Section I-7.2.2.1, pp. 83-5, Table 17.2; [f] Section I-8.3.3.3, p. 109; [g] Section I-8.3.3.2, p. 108-9; [h] Section I-8.3.2, p. 108; [i] Section I-9.8.1, p. 135-6.
9. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Physical Organic Chemistry, "Names for Hydrogen Atoms, Ions, or Groups and for Reactions Involving Them", Recommendations 1988, Pure Appl. Chem. 60, 1115-6 (1988).
14. International Union of Pure and Applied Chemistry. Inorganic Chemistry Division. Commission on Nomenclature of Inorganic Chemistry, "Nomenclature of Inorganic Chemistry: II.2. The Nomenclature of Hydrides of Nitrogen and Derived Cations, Anions, and Ligands (Recommmendations 1981)" Pure Appl. Chem. 54, 2545-52 (1982), Rule II.2.1, p. 2546.
16. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Treatment of Variable Valence in Organic Nomenclature (Lambda Convention), Recommendations 1983, Pure Appl. Chem. 56, 769-78 (1984).
21. B. P. Block, W. H. Powell, and W. C. Fernelius, Inorganic Chemical Nomenclature: Principles and Practice, American Chemical Society, Washington, D. C., 1990, 210 pp., [a] Subsection 9.5, pp. 82-3; [b] Subsection 9.4, pp. 81-2.