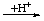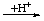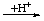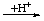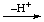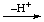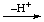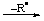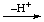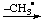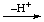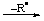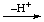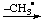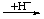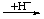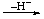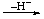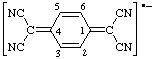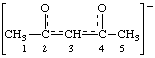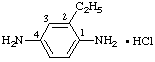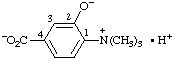Revised Nomenclature for Radicals, Ions, Radical Ions and Related Species
(IUPAC Recommendations 1993)
Preamble and Introduction
Continued from Contents
Contents of this section
Preamble
The 1979 edition of the IUPAC Nomenclature of Organic Chemistry (ref 1), hereinafter called the 1979 IUPAC Organic Rules, contains a subsection codifying nomenclature recommendations for radicals, ions, and radical ions (ref 2a), which are illustrated by various recommendations in Sections B (ref 3a), C (ref 2b), and D (ref 4a). Although those recommendations embody all the requirements for a general system of nomenclature for radicals, ions, and radical ions, they have often been interpreted rather narrowly and do not deal thoroughly with some types of compounds, for example, those containing different formal charges in the same parent structure.
Note. The adjective "free", used in earlier editions of the IUPAC Organic Rules (ref 2a), is not used in these recommendations. It was used previously to distinguish between radicals as distinct chemical species and the term radical used to describe a substituting group in substitutive nomenclature. In subsequent recommendations on organic nomenclature, the Commission intends to use the term "group" rather than "radical" to designate a substituting group. This revision retains the essential principles of the 1979 IUPAC Organic Rules (ref l) for naming organic radicals, ions, and radical ions (ref 2a, 2b, 3a, 4a) and extends them to include parent compounds of the nonmetallic and certain semimetallic elements and to some other characteristic groups. Established recommendations from other IUPAC Nomenclature Commissions have been incorporated and, where appropriate, alternative methods under study by the Commission on Nomenclature of Organic Chemistry are noted.
It is not intended for these revised recommendations to deal exhaustively with all aspects of nomenclature for radicals, ions, and radical ions. For example, delocalization is not included even though it was mentioned briefly in the 1979 IUPAC Organic Rules (ref 2c); this aspect of their nomenclature is currently under study by the Commission. The recommendations presented here are concerned only with classical valence structures and do not deal with concepts such as paired and unpaired electronic configurations.
These recommendations do not legislate for a particular name when more than one is given. Like Section C in the 1979 IUPAC Organic Rules (ref 2), they provide guidelines for the generation of unambiguous names corresponding to the structural information available. When more than one name appears, the first is the one that reflects the recommendation just preceding, followed by names generated by subsections elsewhere in these recommendations or in previously published IUPAC recommendations, or that reflect established usage elsewhere. Occasionally, a trivial name or a "traditional name", i.e., a name that is, or has been, in common use, is given even though not preferred by these recommendations. Finally, when the difference between a recommended name and a name that would be generated by applying the recommendations in the 1979 IUPAC Organic Rules (ref l) may be somewhat subtle, the name that would be generated according to the 1979 IUPAC Organic Rules (ref l) is given, but only for comparison, and identified by a parenthetical note beginning with the phrase "according to" or the word "previously".
These recommendations replace or modify most of Subsection C-0.8 "Free Radicals, Ions, and Radical Ions", in Section C: 'Characteristic Groups Containing Carbon, Hydrogen, Oxygen, Nitrogen, Halogen, Sulfur, Selenium, and/or Tellurium" (ref 2a), and other recommendations in Sections B (ref 3a), C (ref 2b), and D (ref 4a) of the 1979 edition of the IUPAC Nomenclature of Organic Chemistry (ref l).
These revised recommendations are also published in a brief form (ref 5).
Introduction
In organic nomenclature, most radicals, ions, and related species are considered formally as derived from parent hydrides or characteristic groups by the loss of hydrogen atoms, or by the addition or loss of hydrogen ions. [Acyl radicals and corresponding cations may be considered as exceptions derived formally from acids by loss of -OH groups as hydroxyl radicals or hydroxide ions, respectively (see RC-81.2.2 and RC-82.2.3.2)]. These operations change the number of substitutable hydrogen atoms at the radical or ionic centers and are described mainly by the operational suffixes or prefixes shown in Table 1. The suffixes may be added individually or in combination to the name of a parent hydride, or to some of the traditional suffixes used to express principal characteristic groups in substitutive nomenclature.
Note. Operational suffixes are affixes attached to the end of the name of a parent hydride or functional suffix to indicate that the number of substitutable hydrogen atoms is different from that implied by the parent name or suffix name, for example, "-ium", "-ylium", "-ide", and the traditional subtractive suffixes such as "-yl", "-ene", and "-yne".
Table 1: Suffixes and Substituent Prefix Endings for Describing Radical and Ionic Centers
| Operation | | Suffix | | Substituent
Prefix Ending* |
| 1. Addition of H+ | | -onium
-ium | | -oniumyl
-iumyl |
| EHx |  | [EHx+1]+ |  | -[EHx]+ |
| SH2
sulfane |  | [SH3]+
sulfonium |  | -[SH2]+
sulfoniumyl |
| CH4
methane | 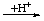 | [CH5]+
methanium |  | -[CH4]+
methaniumyl |
| C5H5N
pyridine | 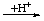 | [C5H6N]+
pyridinium |  | -[C5H5N]+
pyridiniumyl |
| 2. Loss of H+ | | | | |
| a | | -ide | | -idyl |
| EHx | 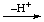 | [EHx-1]- |  | -[EHx-2]- |
| PH3
phosphane | 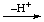 | [PH2]-
phosphanide |  | [PH]-
phosphanidyl |
| CH4
methane | 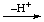 | [CH3]-
methanide |  | [CH2]-
methanidyl |
| b.** | | -ate | | -ato*** |
| R-E(X)xHy |  | [R-E(X)x]y- | 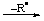 | -[E(X)x]y- |
| CH3-SO3H
methanesulfonic acid | 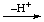 | [CH3-SO3]-
methanesulfonate | 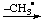 | -[SO3]-
sulfonato |
| c.** | | -ate | | -ido |
| R-XH | 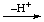 | R-X- | 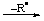 | -X- |
| CH3-SH
methanethiol | 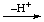 | CH3-S-
methanethiolate | 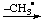 | -S-
sulfido |
| | | | | |
| 3. Addition of H- | | -uide | | -uidyl |
| EHx | 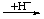 | [EHx+1]- |  | -[EHx]- |
| BH3
borane | 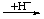 | [BH4]-
boranuide**** |  | -[BH3]-
boranuidyl |
| 4. Loss of H- | | -ylium | | -yliumyl |
| EHx | 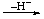 | [EHx-1]+ |  | -[EHx-2]+ |
| CH4
methane | 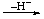 | [CH3]+
methylium |  | -[CH2]+
methyliumyl |
| 5. Loss of H• | | -yl | | ylo....yl |
| EHx |  | [EHx-1]• |  | -[EHx-2]• |
| CH4
methane |  | [CH3]•
methyl |  | -[CH2]•
ylomethyl |
* Although these prefixes are illustrated here for a mononuclear species, it is understood that the free valency need not reside at the ionic or radical center.
** X = O, S, Se, Te
*** Used for acid characteristic groups only, attachment being through the characteristic atom, for example, -CO2-, carboxylato.
**** In inorganic nomenclature, this anion is named tetrahydroborate(l-) (ref 6a).
These recommendations are concerned only with formal operations for naming radicals, ions, radical ions, and neutral compounds with compensating ionic centers, and related species. They do not include general principles of nomenclature systems, such as the expression of unsaturation or partial saturation in parent hydrides, indicated and "added" hydrogen, locant positions, or numbering priorities, except for those involving the operational suffixes noted in Table 1. However, for convenience, modifications to the 1979 IUPAC Organic Rules (ref l) to be published in the first part of a complete revision of the 1979 edition (ref 5) that are used in these recommendations are outlined briefly in RC-80.
Even though, traditionally, abbreviated or trivial names for acyclic radicals and ions may be commonly used and even preferred in these recommendations, systematic names for acyclic parent hydrides may be used to generate names of radicals and ions using the operational affixes given in Table 1. These systematic names for acyclic parent hydrides are given in RC-80.9.1 (mononuclear), RC-80.9.2 (polynuclear hydrocarbon), and RC-80.9.3 (polynuclear other than hydrocarbon).
Except for the descriptive method of naming radical ions (RC-85.1), these recommendations provide methods for describing specific structures of radicals, ions, radical ions, and related substances. Methods for naming radicals and ions whose structures are indefinite are not included in these recommendations. In the absence of specific IUPAC recommendations, various methods have been used, such as omission of locants and the use of descriptive phrases, sometimes preceding, but usually following the name of the neutral parent structure, as illustrated by the following examples.
Note. In these recommendations, a radical ion in an empirical formula is denoted by a superscript dot followed by the appropriate charge sign (ref 7). It is recognized that in mass spectroscopy the opposite sequence is used (ref 8), following well-established tradition in that field.
Examples:
1. Removal of electrons.
| [CH2=CH-CH3]•+ | propene radical cation |
2. Addition of electrons.
a. 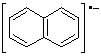 | naphthalene radical anion |
b. 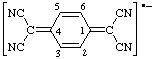 | 2,2'-(cyclohexa-2,5-diene-1,4-diylidene)dimalononitrile radical anion |
3. Removal of hydride ions.
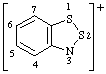 | 3H-benzo[d][1,2,3]dithiazole ion(1+)
3H-1,2,3-benzodithiazole ion(l+) |
4. Removal of hydrons.
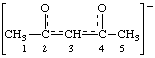 | pentane-2,4-dione ion(l-)
acetylacetonate ion |
Note. The name hydron is a generic name for the hydrogen cation, i.e., the naturally occurring mixture of protons, deuterons, and tritons (ref 9). The name proton is restricted to the hydrogen cation having the mass number 1, i.e., 1H+.
5. Addition of hydrons
a. 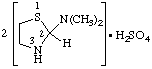 | bis(N,N-dimethylthiazolidin-2-amine) sulfate
bis[2-(dimethylamino)thiazolidine] sulfate |
b. 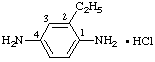 | 2-ethylbenzene-1,4-diamine monohydrochloride |
Note. When the counterion is unknown, terms like "conjugated monoacid" or "hydronated" have been used. When the hydrons have been "substituted", i.e., the unknown cationic center is an "onium" center, terms like "methiodide" and "ethochloride" have been used. However, descriptions like "addition compound of .... with methyl iodide" or "....with iodomethane" are preferred.
6. Zwitterions (internally compensating charges).
a. 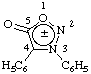 | 3,4-diphenyl-1,2,3-oxadiazolidin-5-one zwitterionic didehydro derivative |
b. 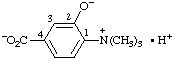 | (4-carboxy-2-hydroxyphenyl)trimethylammonium hydroxide inner salt
4-carboxy-2-hydroxy-N,N,N-trimethylanilinium hydroxide inner salt |
References for this section
1. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 ed., Pergamon Press, Oxford, 1979, 559 p.
2. Reference 1, Section C, pp. 79-322: [a] Subsection C-0.8, pp. 133-43; [b] Rules C-107, pp. 146-7; C-206 (in part), pp. 155-6; C-511.3, p. 212; C-511.4 (in part), p. 213; C-551, pp. 227-8; C-816, pp. 259-61; C-861, pp. 275-6; C-921.6, p. 287; C-931.1, pp. 289-90; C-931.2 (in part); p. 290; C-931.3 (in part), p. 290; C-973, p. 299; and C-974 (in part), pp. 299-300; [c] Rule C-83.1, Note 1, p. 138.
3. Reference 1, Section B, pp. 53-76: [a] Rules B-6, pp. 71-2; B-10.1 (in part), p. 72; and B-11 (in part), p. 73.
4. Reference 1, Section D, pp. 323-471: [a] Rules D-1.63, p. 336; D-3.73, p. 371; D-4.53, p. 379; D-5.3, pp. 392-3; D-5.42, p. 394; D-5.52 (in part), p. 396; D-5.8, p. 408; D-6.85, pp. 425-6; D-6.86, p. 426; D-6.87, p. 426; and D-7.6 (in part), pp. 447-54.
5. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford, 1993.
6. International Union of Pure and Applied Chemistry. Inorganic Chemistry Division. Commission on Nomenclature of Inorganic Chemistry, Nomenclature of Inorganic Chemistry, Recommendations 1990, G. J. Leigh, ed., Blackwell Scientific Publications, Oxford, England, 1990, 289 p. [a] Sections I-8.3.3.5, p. 111; and I-11.5.1, pp. 232-3.
7. International Union of Pure and Applied Chemistry, Compendium of Chemical Terminology (IUPAC Recommendations), V. Gold, K. L. Loening, A. D. McNaught, P. Sehmi, compilers, Blackwell Scientific Publications, Oxford, 1987, 456 p.
8. International Union of Pure and Applied Chemistry. Physical Chemistry Division. Commission on Molecular Structure and Spectroscopy, "Recommendations for Nomenclature and Symbolism for Mass Spectroscopy, Recommendations 1991", Pure Appl. Chem. 63, 1541-66 (1991).
9. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Physical Organic Chemistry, "Names for Hydrogen Atoms, Ions, or Groups and for Reactions Involving Them", Recommendations 1988, Pure Appl. Chem. 60, 1115-6 (1988).
Continue with RC-80 General Principles
Return to ions and radicals home page.
Return to chemical nomenclature home page.