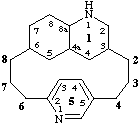
11,12,13,14,14a,15,16,17,18,18a-decahydro-1(3,6)quinolina-5(5,2)-pyridinacyclooctaphane
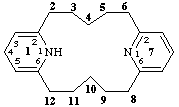
11,14-dihydro-1,7(2,6)-dipyridinacyclododecaphane
Contents of this section
Phane parent hydrides are composed of saturated and mancude components, i.e., amplificants, and alternating chains. A mancude component has the maximum number of noncumulative double bonds. The degree of hydrogenation of each component can be modified by applying the general rules recommended in the 1993 Guide to IUPAC Nomenclature of Organic Compounds by using the prefix 'hydro-' (ref. 3k) to indicate the addition of hydrogen atoms ; the prefix 'dehydro-' to indicate the removal of hydrogen atoms (ref. 3m) ; and the endings '-ene' and '-yne' (ref. 3n) to denote the subtraction of hydrogen atoms. The rule of lowest locants is always applied, as indicated below for specific cases.
The following rules are applied, in the order given, until a decision is reached.
When the name of an amplificant implies the presence of the maximum number of noncumulative double bonds, other states of hydrogenation can be indicated by use of the prefix 'hydro-' together with an appropriate numerical prefix, signifying the addition of hydrogen atoms. This operation is regarded as the reduction of double bonds. Thus, hydrogen atoms can only be added in pairs which are indicated by use of the numerical prefixes 'di-', 'tetra-', 'hexa-' cited before the prefix 'hydro-'(ref. 3k). Indicated hydrogen, if required by the parent hydride, is cited in front of the name of the phane parent hydride (see PhII-2). This method is applied as follows:
'Hydro-' prefixes are used to modify mancude heteromonocycles having retained names or named in accordance with the extended Hantzsch-Widman system (see ref. 3k). However, names for the corresponding fully saturated heteromonocycles that are retained names have Hantzsch-Widman names are preferred to those expressed by 'hydro-' prefixes, for example, oxolane and piperidine are preferred to tetrahydrofuran and hexahydropyridine, respectively (ref. 1a);
'Hydro-' prefixes are used to indicate all modifications of the degree of unsaturation of polycyclic carbocyclic or heterocyclic mancude parent hydrides, except for benzene. Retained names of partially hydrogenated parent hydrides, such as indane, chromane, isochromane, pyrroline, indoline and isoindoline, are not recommended as amplificants in phane nomenclature (ref. 1a).
Examples:

11,12,13,14,14a,15,16,17,18,18a-decahydro-1(3,6)quinolina-5(5,2)-pyridinacyclooctaphane

11,14-dihydro-1,7(2,6)-dipyridinacyclododecaphane
'Dehydro-' prefixes are used to indicate the removal of two contiguous hydrogen atoms from a mancude amplificant of a phane parent hydride system (ref. 3m).
Examples:
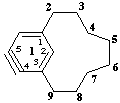
14,15-didehydro-1(1,3)-benzenacyclononaphane
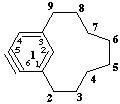
not 15,16-didehydro-1(1,3)-benzenacyclononaphane
PhII-5.3.1. Double and triple bonds.
The presence of one or more double or triple bonds in an otherwise saturated phane parent hydride, except in amplificants with Hantzsch-Widman names, is denoted by changing the final 'e' of the phane parent hydride name to '-ene' or '-yne', with appropriate multiplying prefixes to indicate the multiplicity of each type of unsaturated sites ( ref. 3n).
In phane nomenclature, the endings '-ene' and '-yne' are used to denote the presence of double and triple bonds in amplificants named as saturated rings or ring systems and in simplified parent skeletons.
The general method must, however, be adapted to phane nomenclature names, in which the term 'phane' is considered invariant to preserve the specificity of the class. As a consequence, the endings '-ene' and '-yne' are added to a phane name, with the appropriate multiplying prefixes, with elision of the letter 'e' of the 'phane' ending before the vowels 'e' and 'y'.
PhII-5.3.2. Double bonds in amplificants and in simplified phane skeletons.
Low locants are allocated for double bonds in accordance with the fixed numbering of phane parent hydrides and phane parent hydrides that are modified by skeletal replacement ('a') nomenclature. Traditionally, the degree of unsaturation in a benzene ring is never modified by using 'hydro-' prefixes; rather, cyclohexane is used as the amplificant name and later modified by changing the ending '-ane' to '-ene' and '-yne', as required.
Three kinds of locants are required to fully describe compounds derived from phane parent hydrides:
Examples:
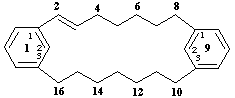
1,9(1,3)-dibenzenacyclohexadecaphan-2-ene
(the numeral '2' is a primary locant)
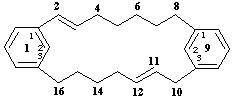
1,9(1,3)-dibenzenacyclohexadecaphane-2,11-diene
(the numerals '2' and '11' are primary locants)
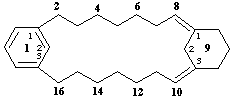
1(1,3)-benzena-9(1,3)-cyclohexanacyclohexadecaphane-8(91),93(10)-diene
[the superscripted numerals '91 and 93' are composite locants; '8(91) and 93(10)' are compound locants; compound locants are necessary to locate precisely the position of the double bonds]
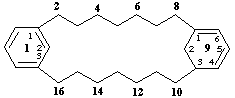
1(1,3)-benzena-9(1,3)-cyclohexanacyclohexadecaphane-91(96),94-diene
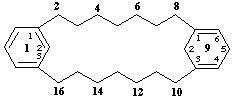
1(1,3)-benzena-9(1,3)-cyclohexanacyclohexadecaphane-91(96),93(94)-diene
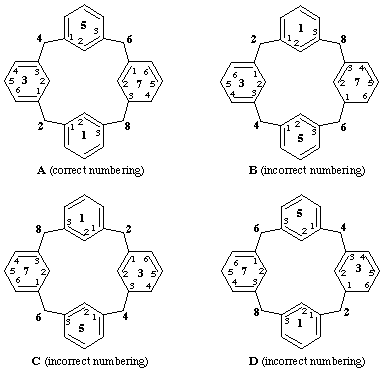
1,5(1,3)-dibenzena-3,7(1,3)-dicyclohexanacyclooctaphane-31(36),33(34),71(72),75-tetraene (A).
[not 1,5(1,3)-dibenzena-3,7(1,3)-dicyclohexanacyclooctaphane-31(36),33(34),72(73),74- tetraene (B);
nor 1,5(1,3)-dibenzena-3,7(1,3)-dicyclohexanacyclooctaphane-31(32),35,71(76),73(74)-tetraene (C);
nor 1,5(1,3)-dibenzena-3,7(1,3)-dicyclo-hexanacyclooctaphane-32(33),34,71(76),73(74)-tetraene (D)]
[The primary locant set '3,3,7,7' for all names are identical, but the set of composite locants '31,33,71,75' in name (A), ignoring the locant in parentheses, is lower than the locant sets '31,33,72,74' in (B), '31,35,71,73' in (C), or '32,34,71,73' in (D).]
Triple bonds in phane nomenclature are expressed by the ending '-yne'. Triple bonds located in interconnecting chains are denoted by primary locants (ref. 3n). If an amplificant would include one or more triple bonds, such as might be found in a large ring, composite locants, and compound locants, if required, would be necessary. Low locants are assigned to triple bonds, in the same way that as for double bonds.
Example:
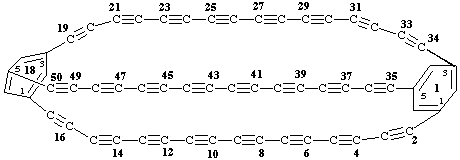
1,18(1,3,5)-dibenzenabicyclo[16.16.16]pentacontaphane-2,4,6,8,10,12,14,16,19,21,-
23,25,27,29,31,33,35,37,39,41,43,45,47,49-tetracosayne
PhII-5.3.4. Double and triple bonds in a phane structure.
Low locants are allocated to double and triple bonds first when considered together as a set in ascending order and, if a choice is still needed, to double bonds (ref. 3n).
Examples:
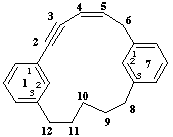
1,7(1,3)-dibenzenacyclododecaphan-4-en-2-yne
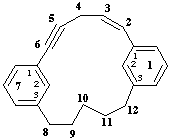
1,7(1,3)-dibenzenacyclododecaphan-5-en-2-yne
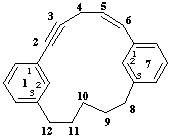
not 1,7(1,3)-dibenzenacyclododecaphan-5-en-2-yne
PhII-5.4. Double bonds between mancude amplificants and alternating atoms or chains.
Double bonds between mancude amplificants having indicated hydrogen atoms and alternating atoms or chains are denoted by '-ene' endings. Any remaining hydrogen atoms of the mancude amplificant are cited as 'indicated hydrogen' in front of the name and take precedence for low locants over '-ene' endings. This procedure also applies to double bonds between contiguous amplificants.
Examples:
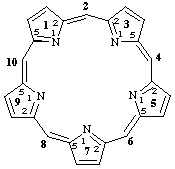
1,3,5,7,9(2,5)-pentapyrrolacyclodecaphane-12(2),35(4),55(6),75(8),95(10)-pentaene
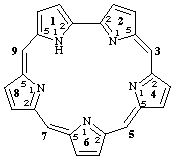
11H-1,2,4,6,8(2,5)-pentapyrrolacyclononaphane-25(3),45(5),65(7),85(9)-tetraene
31H-1,3,11,13(2,5)-tetrapyrrolacyclohenicosaphane-12(2),4,6,8,10(115),12(135),14,16,18,20-decaene
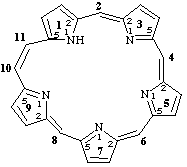
11H-1,3,5,7,9(2,5)-pentapyrrolacycloundecaphane-2(32),4(52),6(72),8(92),10-pentaene
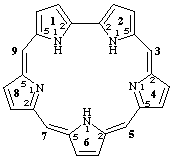
11H,21H,61H-1,2,4,6,8(2,5)-pentapyrrolacyclononaphane-3(42),5(62),65(7),85(9)-tetraene
[The trivial name for this pentapyrrolic macrocycle is sapphyrin (see ref. 5 for the numbering of this structure following the system for cyclic tetrapyrroles)]
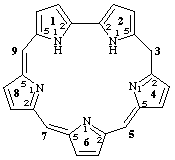
11H,21H-1,2,4,6,8(2,5)-pentapyrrolacyclononaphane-45(5),65(7),85(9)-triene
[not 11H,21H,3H-1,2,4,6,8(2,5)-pentapyrrolacyclononaphane-45(5),65(7),85(9)-triene; position 3 is naturally saturated in a phane structure and indicated hydrogen, i.e. 3H, is thus not allowed]
PhII-5.5. Double bonds between hydrogenated mancude amplificants and atoms or chains.
When double bonds are located between partially or fully hydrogenated mancude amplificants and acyclic components of a phane structure, double bonds are denoted by the ending '-ene' and the hydrogenation of the mancude amplificant is indicated by the usual prefix 'hydro-'. Names can be formed by the following two methods, method (a) being preferred.
For method (a) the order of operations is as follows:
1. insert the double bonds indicated by '-ene' endings;
2. add the maximum number of double bonds in the amplificant rings;
3. cite indicated hydrogen atoms, in accordance with the numbering of the amplificant;
4. saturate the appropriate double bonds using 'hydro-' prefixes.
This method, i.e., the introduction of double bonds denoted by the '-ene' endings followed by the insertion of the maximum number of noncumulative double bonds, is analogous to the method described in FR-8.1.2 (ref. 6) for naming bridged ring systems, in which the bridge is introduced followed by the addition of the maximum number of noncumulative double bonds.
For method (b), nondetachable 'hydro-' prefixes are used. Lowest locants are assigned first to indicated hydrogen, if present, then to 'hydro-' prefixes, and finally to double bonds denoted by '-ene' endings (see PhII-1.1).
Examples:
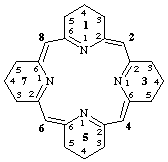
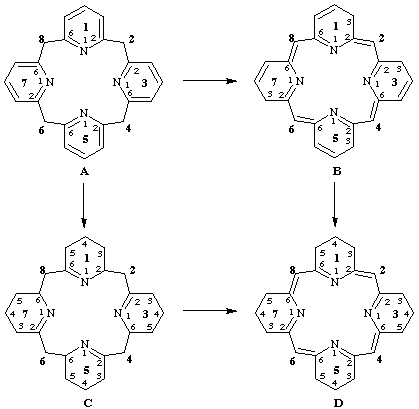
Method (a): 14,15,34,35,54,55,74,75-octahydro-13H,33H,53H,73H-1,3,5,7(2,6)-tetrapyridinacyclooctaphane-12(2),36(4),56(6),76(8)-tetraene
The formation of this name may be understood by referring to structures A, B, and D, below. The names for these structures are as follows:
A 1,3,5,7(2,6)-tetrapyridinacyclooctaphane
B 13H,33H,53H,73H-1,3,5,7(2,6)-tetrapyridinacyclooctaphane-12(2),36(4),56(6),76(8)-tetraene
D 14,15,34,35,54,55,74,75-octahydro-13H,33H,53H,73H-1,3,5,7(2,6)-tetrapyridinacyclooctaphane-12(2),36(4),56(6),76(8)-tetraene
Method (b): 12,13,14,15,33,34,35,36,53,54,55,56,73,74,75,76-hexadecahydro-1,3,5,7(2,6)-tetrapyridinacyclooctaphane-12(2),36(4),56(6),76(8)-tetraene
The formation of this name may be understood by referring to structures A, C, and D, below. The names for these structures are as follows:
A 1,3,5,7(2,6)-tetrapyridinacyclooctaphane
C 12,13,14,15,33,34,35,36,53,54,55,56,73,74,75,76-hexadecahydro-1,3,5,7(2,6)-tetrapyridinacyclooctaphane
D 12,13,14,15,33,34,35,36,53,54,55,56,73,74,75,76-hexadecahydro-1,3,5,7(2,6)-tetrapyridinacyclooctaphane-12(2),36(4),56(6),76(8)-tetraene
1. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry."Phane Nomenclature, Part I: Phane Parent Names (IUPAC Recommendations 1998)" Pure Appl. Chem. 70, 1513-1545 (1998). (a) PhI-2.2, pp. 1522-1523; (b) PhI-3.3, pp. 1534-1536. (See also IUPAC chemical nomenclature database https://iupac.qmul.ac.uk/phane/).
3. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry. A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, R. Panico, W. H. Powell and J.-C. Richer, eds. Blackwell Scientific Publications, Oxford, 1993. (k) R-3.1.2, pp. 60-61; (m) R-3.1.3, p. 61; (n) R-3.1.1, pp. 59-60. (See also http://www.acdlabs.com/iupac/nomenclature/).
5. International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). "Nomenclature of Tetrapyrroles, Recommendations 1986". Eur. J. Biochem. 178, 277-328 (1988), TP-5.4, p. 302; Biochemical Nomenclature and Related Documents, A Compendium, Second Edition, 1992, Portland Press Ltd, London, p. 303. (See also IUPAC chemical nomenclature database https://iupac.qmul.ac.uk/tetrapyrrole/).
6. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry. "Nomenclature of Fused and Bridged Fused Ring Systems (IUPAC Recommendations 1998" Pure Appl. Chem. 70, 143-216, 1998. (See also IUPAC chemical nomenclature database https://iupac.qmul.ac.uk/fusedring/).
Return to Phane nomenclature part II home page
Return to IUPAC nomenclature home page