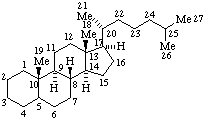
 | 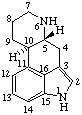 |
| Cholestane | Ergoline |
RF-4.2.1. The addition of a methylene (-CH2-) group between two skeletal atoms of a fundamental parent structure is described by the prefix "homo-"; the addition of two or more methylene groups is indicated by combining an appropriate numerical prefix with "homo-", e.g., "dihomo-", "trihomo-", etc. Positions of inserted methylene groups in the modified fundamental parent structure are indicated by the locants of the added methylene group which are cited in front of the prefixes, "homo-", "dihomo-", etc.
Note: Capital letters, associated with locants of added methylene groups, where needed, have been used with prefixes such as "homo-" and "dihomo-" to indicate insertion of methylene groups into particular rings. This system is used in Chemical Abstracts index nomenclature, but is not included in these Section F recommendations because it is not as general as the locant system recommended here.RF-4.2.2. Numbering of additional skeletal atoms.
The assignment of the locant to an added methylene group depends on whether it is considered to be inserted into an atomic connector or terminal acyclic segment (see also RF-4.1.1) or into a bond connector. A bond connector is a connection between any combination of bridgehead or ring junction atoms, rings or ring systems (i.e., ring assemblies), substituted skeletal atoms, or heteroatoms. The structures below illustrate atomic connectors, bond connectors, and terminal segments.
 |  |
| Cholestane | Ergoline |
Atomic connectors:
In cholestane: 1-4, 6-7, 11-12, 15-16, and 22-24.Terminal segments:
In ergoline: 2, 4, 7-9, and 12-14.
In cholestane: 18, 19, 21, 26, and 27.Bond connectors:
In ergoline: None.
In cholestane: 5-10, 8-9, 8-14, 9-10, 13-14, 13-17, and 17-20.RF-4.2.2.1. Methylene groups inserted into an atomic connector or into a terminal segment are identified by adding a letter "a", "b", etc., to the locant of the highest numbered skeletal atom of the atomic connector or terminal segment consistent with the location of double bonds remaining in the structure (compare example 2 below and example 3 under RF-4.2.2.2). If there are equivalent atomic connectors, the highest numbered atomic connector is chosen, and the methylene group is inserted after the highest numbered skeletal atom in that connector.
In ergoline: 1-15, 3-16, 5-6, 5-10, 10-11, 11-16, and 15-16.
Examples:
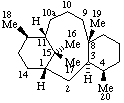 | 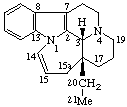 |
| 10a-Homotaxane | 15a-Homoeburnamenine |
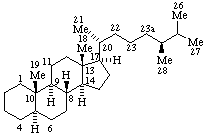 | 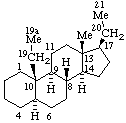 |
| 23a-Homo-5α-ergostane | 19a-Homo-5α-pregnane |
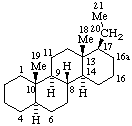 | 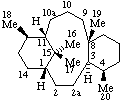 |
| 16a-Homo-5α-pregnane | 2a,10a-Dihomotaxane |
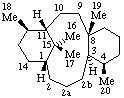 | 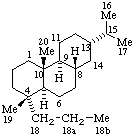 |
| 2a,2b-Dihomotaxane | 18a,18b-Dihomoabietane |
Note: Addition of acyclic side chains or extension of terminal segments of a side chain already attached to the basic skeleton of a fundamental parent structure may also be done by principles of substitutive nomenclature.RF-4.2.2.2. Methylene groups inserted into a bond connector are identified by citing both locants of the skeletal atoms terminating the bond connector enclosing the second (higher) number in parentheses, followed by a letter "a", "b", etc. according to the number of methylene groups.
Note: The insertion of a methylene group into a bond connector has been described by combining the capital letter(s) of the expanded ring(s) with the locant of the inserted atom derived by adding a letter "a", "b", etc., to one of the locants for the skeletal atoms terminating the ring bond connector [Rule 2S-7.4 (ref 9)].Examples:
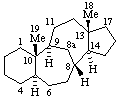
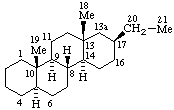 | 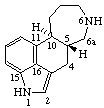 |
| 13(17)a-Homo-5α-pregnane | 5(6)a-Homoergoline |
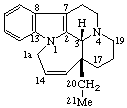 | 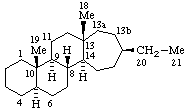 |
| 1(14)a-Homoeburnamenine | 13(17)a,13(17)b-Dihomo-5α-pregnane (has been called D(17a,17b)-Dihomo-5α-pregnane) |
RF-4.2.3. The insertion of a methylene group into a ring or a ring system of a fundamental parent structure that contains the maximum number of noncumulative double bonds or into a cyclic system of conjugated double bonds may create a saturated ring position that is indicated by "indicated hydrogen". The position of the inserted methylene group is prescribed by Rule RF-4.2.2, even though the saturated ring position may be elsewhere in the unsaturated ring system as denoted by the appropriate locant for the indicated hydrogen.
Examples:
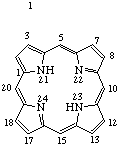 | 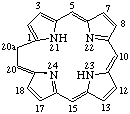 |
| Porphyrin (fundamental parent structure) | 20aH-20a-Homoporphyrin |
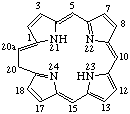
Note 1: Although it would be quite easy to justify the omission of indicated hydrogen when the saturated position is the same as the inserted methylene group, this should not be encouraged.Note 2: The name 20H-20a-Homoporphyrin describes an alternative tautomeric form shown below.
4. International Union of Pure and Applied Chemistry and International Union of Biochemistry, Joint Commission on Biochemical Nomenclature, "Nomenclature of Steroids", Pure Appl. Chem., 61, 1783-1822 (1989). [also in: Eur. J. Biochem., 186, 429-458 (1989) and pages xxx-lix in Dictionary of Steroids (Hill, R.A., Kirk, D.N., Makin, H.L.J. & Murphy, G.M., eds) Chapman & Hall, London 1991].
9. International Union of Pure and Applied Chemistry and International Union of Biochemistry, Commission on Biochemical Nomenclature, "The Nomenclature of Steroids Revised tentative rules, 1967.", Pure Appl. Chem., 31, 285-322 (1972). This 2nd edition has been superceded by the 1989 edition (ref 4).