Nomenclature of Tetrapyrroles
Continued from TP-4 Reduced Porphyrins Including Chlorins
TP-5 Ring-Expanded and Ring-Contracted Systems
Contents
TP-5 Ring-expanded and ring-contracted systems
TP-5.1 Ring expansion
TP-5.2 Skeletal replacement of ring-expanded systems
TP-5.3 Ring-contracted systems
TP-5.4 Sapphyrin
References for this section
Rule Tetrapyrroles 5. Ring-Expanded and Ring-Contracted Systems
TP-5.1. Ring Expansion. Inclusion of a methylene group (-CH2-) into a porphyrin ring sector is indicated by the prefix "homo" in accord with the guidelines established for natural products. The homo prefix is preceded by a locant indicating the position of the inserted group. A locant for the inserted group is generated by adding the letter "a" to the locant of the highest possible numbered atom of the ring-sector that is not a bridgehead or junction to which the group is added.
Note 1. IUPAC, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 edition. Pergamon, 1979, Rule F-4.5  . [See also RF-4.2 in 1999 revised rules]
. [See also RF-4.2 in 1999 revised rules]
Note 2. Bridgehead and junction atoms are excluded to avoid ambiguity.
To denote ring expansion of the fundamental porphyrin nucleus, "homo" immediately precedes "porphyrin". To denote ring expansion of a trivially named porphyrin or reduced porphyrin, "homo" immediately precedes the appropriate trivial name.
Examples:
| 1. | 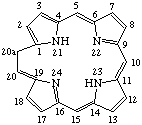 | 20a-Homoporphyrin |
| 2. | 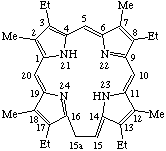 | 15a-Homoetioporphyrin III |
TP-5.2. Skeletal Replacement of Ring-expanded Systems. Replacement prefixes with appropriate locants are added in front of the name of the ring-expanded porphyrin.
Note General practice varies in related areas such as steroids and natural products as to the relative placement of ring modifying and skeletal replacement prefixes. Further study is needed. See IUPAC provisional Rule F-4.11  . [See also RF-4.7.4 for order of prefixes and RF-5 for replacement in 1999 revised rules]
. [See also RF-4.7.4 for order of prefixes and RF-5 for replacement in 1999 revised rules]
Examples:
| 1. | 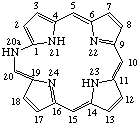 | 20a-Aza-20a-homoporphyrin |
| 2. | 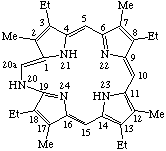 | 20H-20-Aza-20a-homoetioporphyrin 1 |
Note "Indicated hydrogen" distinguishes this structure from the closely related one:
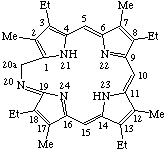 | 20-Aza-20a-homoetioporphyrin I |
Because atom 19 is a bridgehead, the locant 19a may be ambiguous.
TP-5.3. Ring-contracted systems. The trivial names "corrin" and "corrole" [ref 7] are retained for the ring-contracted structures shown in Fig. 10 and Fig. 11 respectively. Skeletal replacement analogs are named by prefixing the appropriate locants with multiplicative and replacement prefixes to the parent name "corrole". Other ring-contracted porphyrins are named systematically according to the rules for naming heterocycles.
Note 1. For other trivial names such as cobyrinic acid, cobinic acid and vitamin B-12 that are retained for substituted derivatives of these ring systems, see IUPAC-IUB Commission on Biochemical Nomenclature, "Nomenclature of Corrinoids, Rules Approved 1975." Pure Appl. Chem. 48, 495-502 (1976).
Note 2. As for skeletal replacement in porphyrins, replacement of saturated nitrogen atoms of corrin and corrole precede replacement of the unsaturated nitrogen atoms. See TP-1.5.
Note 3. See IUPAC Rules B-1 to B-15.2.
Note 4. The ring contraction prefix "nor", recommended for natural products in IUPAC provisional Rule F-4.4 [See RF-4.1 in 1999 revised rules], at present denotes only the loss of a methylene (-CH2-) group from a ring system. Generally, ring contraction by use of "nor" has been restricted to saturated systems such as steroids. Extension of the meaning of "nor" to indicate loss of either a methylene (-CH2-) or methyne (-CH=) group needs further study with regard to indication of hydrogen present in the ring, priority of citation and numbering of heteroatoms and other ring modifying prefixes.
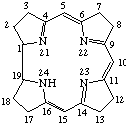
Fig. 10. Corrin.
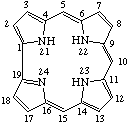
Fig. 11. Corrole.
Examples:
| 1. | 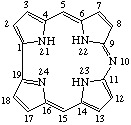 | 10-Azacorrole |
| 2. | 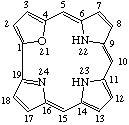 | 21-Oxacorrole |
TP-5.4. Sapphyrin. The pentapyrrolic macrocycle Fig. 12 is called sapphyrin [ref 8], and is numbered as shown.
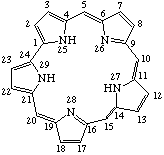
Fig. 12. Sapphyrin.
References for this section
7. A. W. Johnson and I. T. Kay, J. Chem. Soc., 1620 (1965).
8. R. B. Woodward, Chemical Society Meeting on Aromaticity, Sheffield, 1966. M. J. Broadhurst, R. Grigg, and A. W. Johnson, J. Chem. Soc. Perkin Trans. 1, 2111 (1972).
Continued with TP-6 and TP-7 Linear Tetrapyrroles
Return to Tetrapyrrole nomenclature homepage
Return to IUBMB Biochemical Nomenclature homepage.
Return to IUPAC Chemical Nomenclature homepage.
. [See also RF-4.2 in 1999 revised rules]









