TP-0.3. Unless implied by a trivial name, the absolute configuration of a tetrahedral chiral center is designated by an R or S symbol assigned by the sequence-rule procedure. Similarly, the stereochemistry about a double bond is denoted by the prefixes E or Z. The symbols are assigned in accord with procedures given in the international recommendations for stereochemistry and are normally cited with their corresponding locants, when necessary, in front of the whole name. Inversion of configuration or change in the configuration of a double bond from that implied by a name is expressed in front of the name in accord with IUPAC Rule F-6.3. [see RF-10.2 in the 1999 revision.]
Note IUPAC, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 edition. Pergamon, 1979, Rules E-2.2 and E-4.9.
TP-0.4. The use of the diphthong (ae) or the single letter (e) in such words as haem, aetioporphyrin etc., is left to editorial discretion. (This vowel becomes (ä) in German). Here the single letter (e) is used throughout, heme additionally taking a terminal (e).
Rule Tetrapyrroles 1. Fundamental Porphyrin Systems
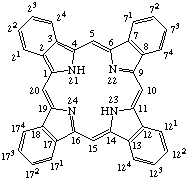
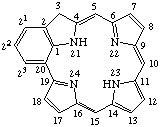
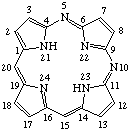
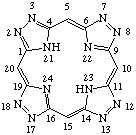
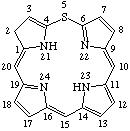
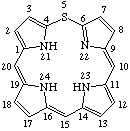
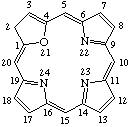
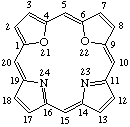
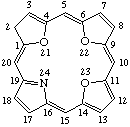
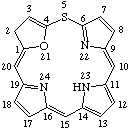
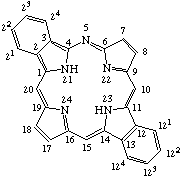
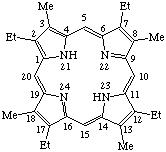
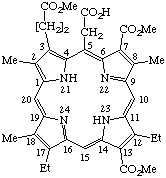
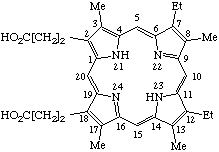
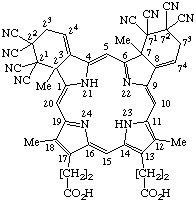
TP-1.1. Porphyrin ring system. The fundamental macrocyclic tetrapyrrolic ring system shown in Fig. 2 is named porphyrin. It is tautomeric with respect to the location of the two hydrogen atoms not involved in the peripheral conjugated system. The two hydrogen atoms may be associated with any two of the four nitrogen atoms. Unless stated to the contrary the representation of one tautomeric form does not imply the absence of another tautomer. However, for nomenclature purposes, the name "porhyrin" implies that the saturated nitrogen atoms are at positions 21 and 23 unless specifically indicated otherwise.
Fig. 2. Porphyrin 1-24 Numbering Scheme.
TP-1.2. Numbering. The 1-24 numbering system upon which the numbering system for corrinoids is based is adopted for the porphyrin nucleus and is shown in Fig. 2. The 2,3,7,8,12,13,17 and 18 positions have commonly been referred to generically as "beta-positions" (i.e. of the pyrrole rings). Similarly, positions at 1,4,6,9,11,14,16 and 19 have been referred to generically as "alpha-positions," while those at 5,10,15 and 20 are referred to generically as "meso-positions." However, in order to avoid possible ambiguity with stereochemical designations the use of these generic terms is discouraged.
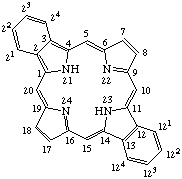
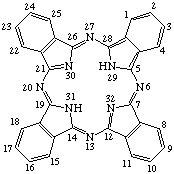
TP-1.3. Fused Systems. Compounds with extra rings ortho-fused or ortho-perifused to the porphyrin nucleus are of some importance. Tetrabenzoporphyrin is defined and the extra positions numbered as shown in Fig. 3. The name may be used without fusion letters to refer to tetrabenzo[b,g,l,q]porphyrin. Systems in which the porphyrin nucleus is fused with other cyclic hydrocarbons may be named by analogy to the IUPAC Rules B-3 and A-21 for naming fused ring systems, but adopting the porphyrin nucleus with the implied hydrogen atoms at positions 21 and 23 as the basic component to which other rings are fused. Fusion locants are derived according to IUPAC Rule A-21.5. Indicated hydrogen is determined on the basis of the presence of implied hydrogen atoms at positions 21 and 23 of the porphyrin ring in contrast to the form containing the maximum number of noncumulative double bonds as in IUPAC Rule A-21.6.
Note 2 See TP-1.6 for fused systems involving skeletally replaced porphyrins.
Note 3 Fusion of the porphyrin ring system with heterocyclic rings, particularly one containing a bivalent atom may affect the tautomerism of the nitrogen atoms of the porphyrin ring system and needs further consideration.
The component rings fused to the porphyrin base are numbered as substituent groups of the lowest possible position of the porphyrin ring. However, where the "extra" rings are formally derived, such as for lactones, from the reaction of noncyclic substituents implied by the trivial name the numbering of the noncyclized substituent is retained as illustrated by example 4 of Rule TP-3.1 and the examples of Rule TP-3.4.
Fig. 3. Tetrabenzoporphyrin.
Examples:
Note The name "porphine" has also been used widely for the structure in Fig. 2. For further discussion see A. H. Corwin in Organic Chemistry. (H. Gilman, ed.) Vol. 2. Wiley N. Y., 1943, p. 1272 and reference [ref 1].
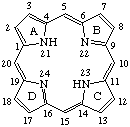
See IUPAC-IUB Commission on Biochemical Nomenclature, "Nomenclature of Corrinoids, Rules Approved 1975." Pure Appl. Chem. 48, 495-502 (1976).
Note 1 See also TP-3.4 for use of "cyclo".
Note See TP-2.1 Fig. 6. This practice is in contrast to established procedures of systematic organic nomenclature for orienting and numbering fused rings according to IUPAC Rules A-22 and B-3.4.