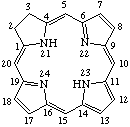
Fig. 7.Chlorin
2,3-Dihydroporphyrin
Continued from TP-3 Semisystematic Porphyrin Names
TP-4 Reduced porphyrins including chlorins
TP-4.1 Unsubstituted reduced porphyrins
TP-4.2 Substituted reduced porphyrins. Systematic chlorin names
TP-4.3 Substituted reduced porphyrins, and close relatives of the chlorophylls. Trivial names and numbering
TP-4.4 Substituted chlorins. Semisystematic chlorin names
Reference for this section
TP-4.1. Unsubstituted Reduced Porphyrins. The most common reduced porphyrins are dihydroporphyrins in which saturated carbon atoms are located at the nonfused carbon atoms of one of the pyrrole rings. The parent compound of this series is called chlorin, which, defined in terms of the unsubstituted porphyrin ring, is 2,3-dihydroporphyrin (see Fig. 7). Tetrahydroporphyrins in which the saturated carbon atoms are located at nonfused carbon atoms of two diagonally opposite pyrrole rings are bacteriochlorins: tetrahydroporphyrins with adjacent pyrrole rings reduced in this way are called isobacteriochlorins. Hexahydroporphyrins in which the nitrogen atoms and four meso positions are saturated are porphyrinogens. The unsubstituted bacteriochlorin, isobacteriochlorin and porphyrinogen ring system are shown together with their systematic names and numbering in Fig. 8, Fig. 8a and Fig. 9 respectively. Further hydrogenated derivatives of these specially named structures are to be named systematically as hydroporphyrins in preference to hydrochlorins, hydrobacteriochlorins, hydroisobacteriochlorins or hydroporphyrinogens. Other unsubstituted reduced porphyrin ring systems are also to be named systematically according to TP-1.7.

Fig. 7.Chlorin
2,3-Dihydroporphyrin
Note The presence of hydrogen atoms at positions 21 and 23 is implied by the name "porphyrin".
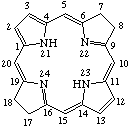
Fig. 8.Bacteriochlorin
7,8,17,18-Tetrahydroporphyrin
Note The 2,3,12,13-tetrahydroporphyrin with implied hydrogen atoms at 21 and 23 is not structurally possible.
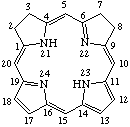
Fig. 8a. Isobacteriochlorin
2,3,7,8-tetrahydroporphyrin
Note The presence of hydrogen atoms at positions 21 and 23 is implied by the name "porphyrin".
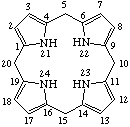
Fig. 9. Porphyrinogen
5,10,15,20,22,24-Hexahydroporphyrin
Note The presence of hydrogen atoms at positions 21 and 23 is implied by the name "porphyrin".
Examples:
| 1. | 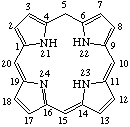 | 5,22-Dihydroporphyrin (formerly named phlorin) |
| 2. | 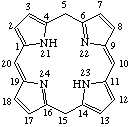 | 5,15-Dihydroporphyrin (formerly porphodimethene) |
| 3. | 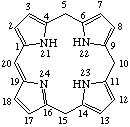 | 5,10,15,22-Tetrahydroporphyrin (formerly porphomethene) |
Note 1. See also Appendix 1.Note 2. See also TP-1.7.
Example:
Note For more examples see TP-4.4.
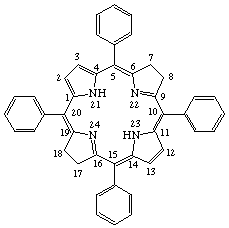 | 5,10,15,20-Tetraphenylbacteriochlorin |
TP-4.3.1. The four trivial names listed in Table 4 may be used to name 17,18-dihydro derivatives of the four corresponding trivially named porphyrins in Table 2. These names may also be used to form semisystematic names as directed in TP-4.4. 17,18-Dihydro derivatives of other trivially named porphyrins are named systematically as directed in TP4.2.
Table 4. Trivially named substituted chlorins corresponding to 17,18-dihydro derivatives of trivially named porphyrins
| Trivial name | Rank No. | Substituents and locants | ||||||||
| 2 | 3 | 7 | 8 | 12 | 13 | 15 | 17 | 18 | ||
| Phyllochlorin | 2 | Me | Et | Me | Et | Me | H | Me | Cet,H | Me,H |
| Phytochlorin | 4 | Me | Et | Me | Et | Me | -C(O)-CH2 | Cet,H | Me,H | |
| Pyrrochlorin | 1 | Me | Et | Me | Et | Me | H | H | Cet,H | Me,H |
| Rhodochlorin | 3 | Me | Et | Me | Et | Me | -CO2H | H | Cet,H | Me,H |
TP-4.3.2. Additional trivial names used to name chlorins, bacteriochlorins and porphyrins may be derived from the common chlorophyll names listed in TP-8.4 and Appendix 1. Compounds that may be formally derived by demetallation of the corresponding substituted naturally occurring chlorophyll shown in TP-8.4 are called pheophytins (if the ester of the 17-propionic acid group is phytyl) or pheofarnesins (if the ester of the 17-propionic acid group is farnesyl). In naming such compounds, the word "pheophytin" (or "pheofarnesin") replaces "chlorophyll" in the original name. Thus demetallation of bacteriochlorophyll a gives bacteriopheophytin a.
Note At present this method is limited to the phytyl and farnesyl ester groups. Even though a geranylgeranyl ester has been reported for bacteriochlorophyll, no trivial name has been accepted for the demetallation analog.
Compounds that are demetallated and also possess a free propionic acid or acrylic acid residue at position 17 are called pheophorbides, and the names are related to those of the chlorophyll in the same way. Thus, on demetallation and hydrolysis of the phytyl ester, chlorophyll a gives pheophorbide a.
Note The ending "-ide" should not be confused with its meaning by IUPAC Rule C-84.3 in which it would designate an anion formed by removal of a proton from a carbon atom.
Examples:
| 1. | 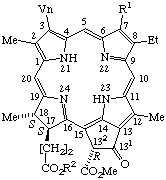 | Pheophytin a R1 =-CH3; R2 = phytyl Pheophytin b R1 = -CHO; R2 = phytyl Pheophorbide a R1 = -CH3; R2 = -H Pheophorbide b R1 = -CHO; R2 = -H Pheophorbide a methyl ester R1 -CH3; R2 = -CH3 |
Note Phytyl =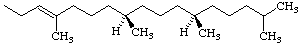
(2E)-(7R,11R)-3,7,11,15-tetramethyl-2-hexadecenyl.
| 2. | 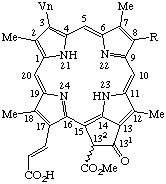 | Pheophorbide c1 R = -CH2CH3 Pheophorbide c2 R = -CH=CH2 |
Note 1. Chlorophylls c1 and c2 (which have a free acrylic acid residue at C-17) on demetallation yield the pheophorbides c1 and c2, and are porphyrins rather than chlorins.Note 2. The configuration of the double bond is E, and is to be implied by these names. If it is necessary to specify the absolute configuration at C-132, it should be indicated by R or S according to TP-0.3.
| 3. | 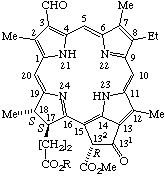 | Pheophytin d R = phytyl Pheophorbide d R = -H |
| 4. | 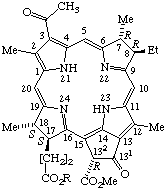 | Bacteriopheophytin a R = phytyl Bacteriopheofarnesin a R = farnesyl Bacteriopheophorbide a R = -H |
Note trans,trans-Farnesyl is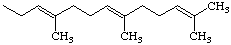
(2E,6E)-3,7,11-trimethyl-2,6,10-dodecatrienyl
| 5. | 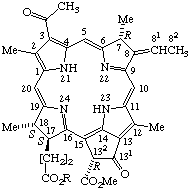 | Bacteriopheophytin b R = phytyl |
Note If it is necessary to specify the sterochemistry of the 8(81) double bond, it should be indicated by E or Z according to TP-0.3.
| 6. | 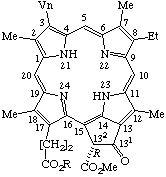 | 17,18-Didehydropheophytin a R = phytyl 17,18-Didehydropheophorbide a R = -H |
Note The names protopheophytin a and protopheophorbide a would be derived according to this rule from the Fischer name, protochlorophyll a. However "protochlorophyll a" is not included in the list of chlorophyll names in TP-8.4 because its substitution pattern differs from that of protoporphyrin (an ethyl group at position 8 is a vinyl group in protoporphyrin). Hence "protopheophytin a" and "protopheophorbide a" are not acceptable names in this document. These structures are easily named using the subtractive prefix "didehydro" combined with derivative terms, pheophytin a or pheophorbide a based on the parent structure, chlorophyll a.
| 7. | 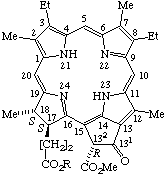 | Mesopheophytin a R = Phytyl Mesopheophorbide a R = -H |
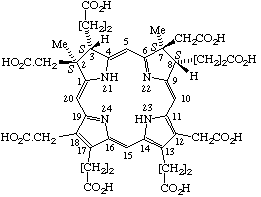
Sirohydrochlorin
TP-4.4. Substituted Chlorins. Semisystematic Chlorin Names. As an alternative to systematic chlorin names formed according to TP-4.2, chlorin derivatives closely related to the four trivially named chlorins in Table 4 of TP-4.3.1 may be named semisystematically. Subtractive and/or substitutive prefixes (or suffix) are combined with the appropriate trivial name. The procedures for forming semisystematic porphyrin names in TP-3 are followed.
Examples:
| 1. | 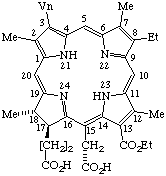 | Semisystematic: 31,32-Didehydrorhodochlorin- -15-acetic acid 13-ethyl ester Systematic: (2S,3S)-20-(Carboxymethyl)-18-(ethoxycarbonyl)- Fischer: Chlorin e6 (as a monoethyl ester) |
| 2. | 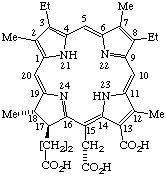 | Semisystematic: Rhodochlorin-15-acetic acid Systematic: (2S,3S)-18-Carboxy-20-(carboxymethyl)-8,13- Fischer: Mesochlorin e6 |
| 3. | 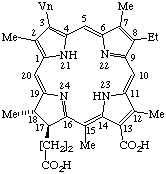 | Semisystematic: 31,32-Didehydro-15-methylrhodochlorin Systematic: (2S,3S)-18-Carboxy-13-ethyl-3,7,12,17,20- Fischer: Chlorin e4 |
| 4. | 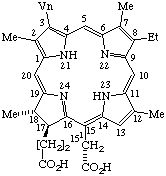 | Semisystematic: 31,32-Didehydrophyllochlorin-151-carboxylic acid Systematic: (2S,3S)-20-(Carboxymethyl)-13-ethyl-3,7,12,17- Fischer: Isochlorin e4 |
| 5. | 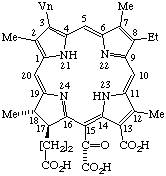 | Semisystematic: 31,32-Didehydrorhodochlorin-15-glyoxylic acid Systematic: (2S,3S)-18-Carboxy-13-ethyl-3,7,12,17-tetramethyl- Fischer: Purpurin 7 |
| 6. | 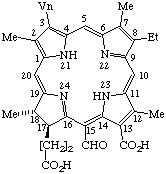 | Semisystematic: 31,32-Didehydro-15-formylrhodochlorin Systematic: (2S,3S)-18-Carboxy-13-ethyl-20-formyl-3,7,12,17- Fischer: Purpurin 5 |
13. A. R. Battersby and E. McDonald, B12 (ed. D. Dolphin) Volume 1, p.107, Wiley, New York, 1982.