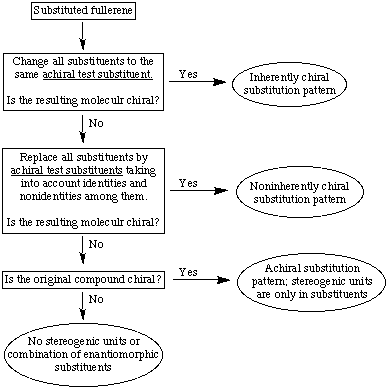
Contents of this section
Fu-17.1. Types of chiral fullerenes.
Four types of chiral fullerenes and/or fullerene derivatives can be distinguished depending on the origin of their chirality. Although the discussion that follows concerns substituted fullerenes, as described in Fu-6.2, it applies equally well to heterofullerenes and isotopically substituted fullerenes and their derivatives. Configurational specification for structurally modified fullerenes (fulleroids) is not discussed here; it will be considered in a later publication.
Type 1. Inherently chiral parent fullerenes. Since the C60-Ih and C70-D5h(6) parent fullerenes are not inherently chiral, they do not belong to this category. This type will only be illustrated here and more fully discussed in a later publication.
Type 2. Derivatives of achiral (and chiral) parent fullerenes in which the presence of substituents (see Fu-6.2), chiral or achiral, identical or different, on the fullerene skeleton, creates a chiral substitution pattern are said to have an inherently chiral substitution pattern.
Type 3. Derivatives of achiral parent fullerenes in which a chiral substitution (see Fu-6.2) pattern on the fullerene is due only to nonidentities among the substituents are said to have a noninherently chiral substitution pattern.
Type 4. Derivatives of achiral parent fullerenes in which the presence of chiral substituents (see Fu-6.2) does not create a chiral substitution pattern have the stereogenic elements located exclusively in the substituents.
Fu-17.2. The substitution test.
It must be recognized that a fullerene compound can belong to more than one of the above types when different stereogenic elements are superimposed in the same molecule. The above types of chirality can be classified by the simple substitution test shown in Scheme 1. To be precise, the scheme can be applied only to a single fullerene unit. In cases where there are more than one unit and more than one are chiral, each unit gets its own descriptor.
The substitution test consists of (a) replacing all substituents (see Fu-6.2) by the same achiral test substituent; (b) replacing nonidentical substituents by nonidentical achiral test substituents; and (c) consideration of the original molecule. After each step, the resulting structure of increasing complexity is checked for chirality; as soon as it is found, the type of chirality can be identified. (For an on-line program to assign chirality click here.)

Scheme 1. Classification of Fullerene Chirality by a Stepwise Substitution Pattern
Fu-17.3. Principles of the descriptor system.
The descriptor system presented herein is based on the fact that the numbering schemes proposed for fullerenes in this document are chiral (helical) and thus constitute an ideal reference for differentiating between enantiomers of chiral carbon cages and of fullerene derivatives having a chiral substitution pattern. A single descriptor is sufficient to specify the configuration of a chiral fullerene or of a chiral fullerene substitution pattern, regardless of the degree of its substitution or skeletal replacement.
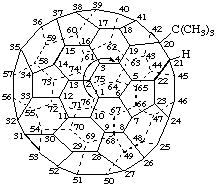
Note: This approach also applies to other numbering schemes in which consecutively numbered atoms form a helix.In a three dimensional model, the pathway following the sequence of numbered carbon atoms consists of a helix, or of helical segments, and is therefore chiral. As a consequence two isometric, mirror symmetric, numbering schemes can be applied to an achiral parent fullerene, as shown for (C60-Ih)[5,6]fullerene in Fig. 6. The arrows indicate the direction of the numbering (see below); clockwise on the left and anticlockwise on the right looking from outside the cage.
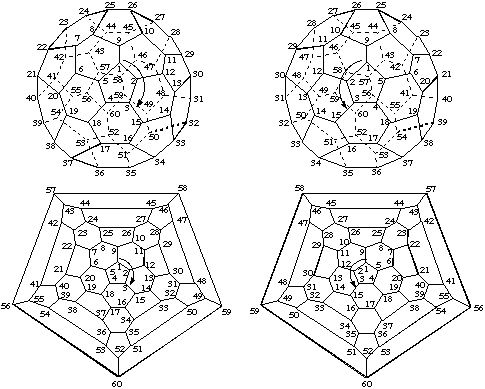
Fig. 6. Three Dimensional and Schlegel Diagrams of (C60-Ih)[5,6]Fullerene with Enantiomeric Numberings
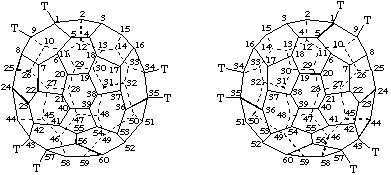
Note: In this document, the convention for Schlegel diagrams is that the central polygon is considered to be closer to the viewer.On the other hand, the numbering for a specific enantiomer of an inherently chiral fullerene is unique, and its mirror image must be applied to the other optical antipode. This is shown in Fig. 7 for the chiral (C76-D2)[5,6]fullerene. Again, the arrows indicate the direction of the numbering. The 3-D diagrams are viewed down the axis chosen as the basis for numbering; the Schlegel drawings are viewed down one of the C2 axes.
Note: This fullerene is numbered by the system used in CA index nomenclature which has not been accepted by IUPAC; a future report will deal with numbering of fullerenes other than C60-Ih and C70-D5h(6)[5,6]fullerene.
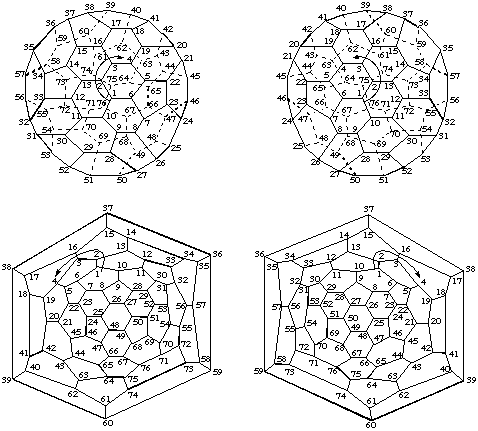
Fig. 7. Three Dimensional and Schlegel Diagrams of the Inherently Chiral (C76-D2)[5,6]Fullerene with Enantiomeric Numbering
Note: In this document, the convention for Schlegel diagrams is that the central polygon is considered to be closer to the viewer.Because of the lack of mirror symmetry, each numbering fits only a single enantiomer. A description of the handedness of the numbering scheme used is sufficient to unambiguously characterize the absolute configuration of the fullerene. The viewer, looking at the polygon in which the numbering starts from outside the fullerene cage, traces a path from C-1 to C-2 to C-3 which are never aligned in a fullerene structure. If this path describes a clockwise direction, the configuration is indicated by the descriptor (f,xC)- , where the superscript 'f' indicates that the descriptor refers to a fullerene, and the superscript 'x' is either 's' for the systematic numbering described in Fu-3.2 or Fu-3.3 or 't' for the trivial numbering described in Fu-3.4. If the path from C-1 to C-2 to C-3 describes an anticlockwise direction, the descriptor is (f,xA)-. Thus, the enantiomer of (C76-D2)[5,6]fullerene shown in the upper half of Fig. 7 is (f,sC)-(C76-D2)[5,6]fullerene and the enantiomer shown in the lower half of Fig. 6 is (f,sA)-(C76-D2)[5,6]fullerene.
Note: As is customary for stereodiscriptors, parentheses are used as enclosing marks.This procedure is also valid for derivatives of inherently chiral fullerenes, because the numbering scheme applies to the parent fullerene as well as to the derivative.
Examples:
Note: These examples are numbered by the system used in CA index nomenclature which has not been accepted by IUPAC; a future report will deal with numbering of fullerenes other than C60-Ih and C70-D5h(6)[5,6]fullerene.
(f,sA)-2H-5-Aza(C76-D2)[5,6]fullerene
(f,sC)-20-tert-Butyl-20,21-dihydro(C76-D2)[5,6]fullerene
Fu-17.4. Achiral parent fullerenes with an inherently chiral substitution pattern.
The fullerene compounds in this category are all substituted achiral fullerenes. They have an inherently chiral substitution pattern if the existence of enantiomeric species is inherent to the geometric arrangement of the substitution sites on the fullerene parent regardless of whether the substituents are identical or different. In these fullerene derivatives there is a unique numbering scheme that leads to the lowest set of locants for the substituents. As above, a description of the handedness of the numbering scheme that leads to the lowest set of locants is sufficient to unambiguously characterize the absolute configuration of the substituted fullerene as f,xC or f,xA.
Example 1.
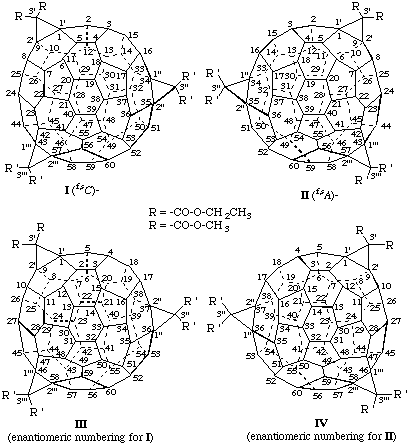
3',3'-Diethyl 3",3",3''',3'''-tetramethyl 3'H,3"H,3'''H-tricyclopropa[1,9:34,35:43,57](C60-Ih)[5,6]fullerene-3',3',3",3",3'",3'"-hexacarboxylate
Explanations:
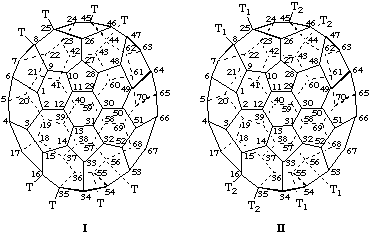
(a) In the enantiomers of the (C60-Ih)[5,6]fullerene derivatives shown above, clockwise (I) and anticlockwise (II) numbering leads to the same lowest set of locants, namely, 1,9:34,35:43,57, respectively. Use of the enantiomeric numbering scheme for each, shown as III and IV, results in the locant set, 1,9:36,37:46,58, a higher locant set that is unacceptable according to the rule of lowest locants in organic nomenclature (ref. 8c).
(b) The parent fullerene, (C60-Ih)[5,6]fullerene, is achiral but the compound is chiral by virtue of its substitution pattern. This compound is inherently chiral because replacement of all of its substituents by the same achiral test substituent, 'T', still results in a chiral structure as shown below.
Example 2.
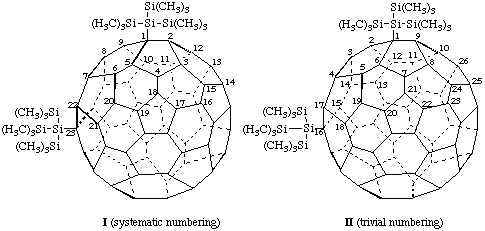
I (f,sC)-1,23-Bis[1,1,1,3,3,3-hexamethyl-2-(trimethylsilyl)trisilan-2-yl]-1,23-dihydro(C60-Ih)[5,6]-fullerene
II (f,tA)-1,16-Bis[1,1,1,3,3,3-hexamethyl-2-(trimethylsilyl)trisilan-2-yl]-1,16-dihydro[60]fullerene
Note: This is a simple compound with an inherently chiral substitution pattern but it serves to illustrate that the configurational descriptor may be different when systematic numbering or trivial numbering is used.Fu-17.5. Achiral parent fullerenes with a noninherently chiral substitution pattern.
The fullerenes in this category are all substituted achiral parent fullerenes in which the chirality is neither inherent to the parent fullerene nor due to an inherently chiral substitution pattern, but is caused by the geometric arrangement of the substituents, all of which cannot be identical.
In all cases of fullerenes with a noninherently chiral substitution pattern, no distinction between enantiomers is possible on the basis of the criteria given above. The same set of lowest locants is obtained with both mirror-symmetric numberings. In order to make a distinction a classification of substituents is necessary. This can be achieved in the most convenient way by using the CIP (Cahn-Ingold-Prelog) method of ranking substituents by atom-by-atom exploration of each attached atom or group (ref. 17). For fullerenes with a noninherently chiral substitution pattern, each substituent is compared by starting the exploration process at the atom located in the fullerene skeleton and moving outward progressively until a priority difference is identified. Of the two mirror-symmetric numbering schemes leading to the same lowest set of locants, the one assigning a lower locant to an atom or group of higher CIP priority at the first point of difference is preferred and confers its descriptor to the enantiomer in question.
Note: In case two enantiomorphic substituted fullerenes would need to be compared according to the CIP exploration procedure, it is suggested that, in addition to the priority of R over S and of M over P in subrule 5 (ref 17), f,xC be given prioriy over f,xA.A classification of substituents based on the principle of alphabetical order provides the same stereodescriptor assignment for fullerenes with noninherently chiral substitution patterns in some cases (see example 1, below) but this is pure coincidence because the two methods are based on fundamentally different principles. Furthermore, alphabetical order cannot be applied in all cases (see example 2, below) and therefore is not broadly applicable.
In considering fullerenes with noninherently chiral substitution patterns, their names must be based on nomenclature principles, such as alphabetical order and lowest locants, but their stereodescriptors are based on purely structural considerations for which the CIP method is clearly the method of choice.
Example 1:
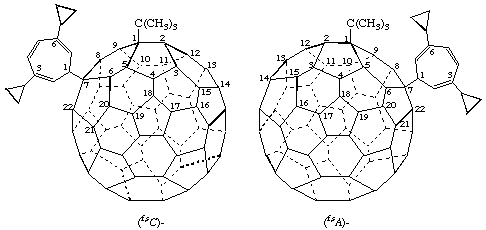
1-tert-Butyl-7-(3,6-dicyclopropylcyclohepta-2,4,6-trien-1-yl)-1,7-dihydro(C60-Ih)[5,6]fullerene
Explanation: This compound is chiral only because the substituents are constitutionally different. The parent fullerene is achiral and it does not have an inherently chiral substitution pattern because replacement of both substituents by the same achiral test substituent, 'T', results in an achiral compound. Therefore it has a noninherently chiral substitution pattern. The tert-butyl group is assigned the locant '1' because its CIP priority is higher than that of the 3,6-dicyclopropylcyclohepta-2,4,6-trien-1-yl group. For this example, using the nomenclature principle of alphabetic order of substituents would give the same result, but this is purely coincidental because the two procedures are based on fundamentally different principles (see example 2, below, for which a different numbering is required in order to use the CIP priority method).
Example 2:
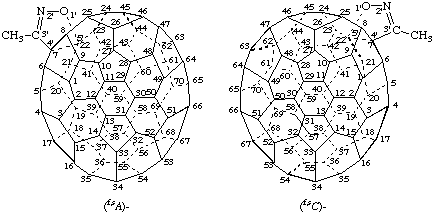
Explanations:
(a) This compound is chiral only because the fused ring is unsymmetrical. The parent fullerene is achiral and it does not have an inherently chiral substitution pattern because replacement of both substituents by the same achiral test substituent, 'T', results in an achiral compound. Therefore it has a noninherently chiral substitution pattern.
(b) In this example, the numbering used to name the compound is based on the nomenclature principle of lowest set of locants (ref. 8c) for the fusion site and is not appropriate for assignment of the stereodescriptor. The numbering scheme to be used for assignment of the stereodescriptor is chosen so that the lower locant '7' of the fullerene is associated with atom of highest priority in the isoxazole ring, i.e., the 'O' atom. This numbering is shown in the structures below which would lead to the incorrect name 3'-Methylisoxazolo[4',5':22,7](C70-D5h(6))[5,6]-fullerene because its locants are higher than those in the name above. Therefore, the stereodescriptors for the enantiomers shown above are (f,sC)- and (f,sA)-, respectively, as shown.
Note: It must be kept clearly in mind that the generation of the stereodescriptor is additional to name formation.
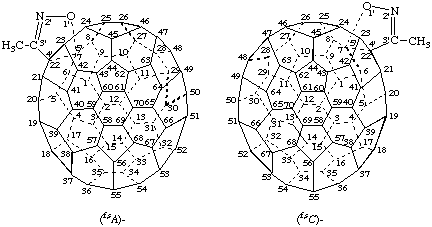
Example 3:
3',3',3''' ',3''' '-Tetra-tert-butyl 3",3",3''',3'''-tetraethyl 3'H,3"H,3'''H,3''' 'H-tetracyclopropa[8,25:16,35:44,45:55,56](C70-D5h(6) )[5,6]fullerene- 3',3',3",3",3''',3''',3''' ',3''' '-octacarboxylate
Explanations:
(a) The parent fullerene for this compound is achiral and replacement of all substituents by the achiral test substituent 'T' (see I, below) still results in an achiral compound.
(b) Accordingly, the second step of the substitution test (see Scheme 1), i.e., replacement of the substituents by the achiral test substituents T1 and T2, which must respect the identities and nonidentities of the original substituents, is applied (see II, above), resulting in a chiral structure because T1 is different from T2. Hence, the compound has a noninherently chiral substitution pattern. The fused cyclopropane rings carrying the tert-butyl ester groups have the highest CIP priority and are therefore assigned the lowest fusion locants.
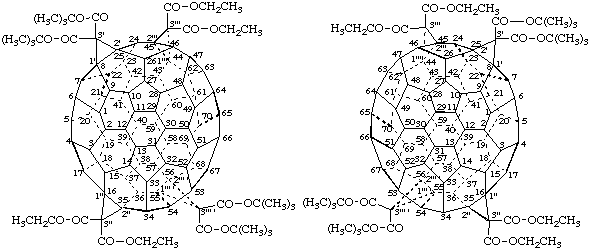
Fu-17.6. Fullerene derivatives with achiral substitution patterns and chiral substituents.
Example:
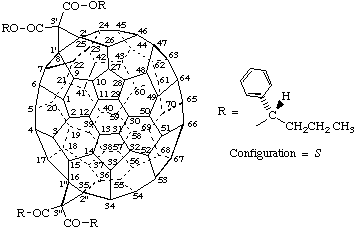
Tetrakis[(1S)-1-phenylbutyl] 3'H,3"H-dicyclopropa[8,25:16,35](C70-D5h(6) )[5,6]fullerene-3',3',3",3"-tetracarboxylate
Explanations:
(a) This fullerene derivative is chiral. The parent fullerene is achiral and the derivative has an achiral substitution pattern. Replacement of all substituents by the achiral test group T (see Scheme 1) results in an achiral structure; and since the original substituents were already identical the second step of the substitution test is not necessary. Step 3 of the substitution test (see Scheme 1) asks if the original compound was chiral. The answer is yes, and thus the stereogenic units occur only in the groups attached to the fullerene core. The configuration of such stereogenic units can be described by the normal descriptors of organic nomenclature (ref. 18).
(b) If the esters of the acid in the above example were different, the structure resulting from step 2 of the substitution test would have the achiral substituents T1 and T2; but the parent fullerene and it substitution pattern would be achiral and therefore any chirality of the original compound would be due to stereogenic centers located in the substituents.
Fu-17.7. Superimposition of stereogenic elements in a fullerene molecule.
If a fullerene derivative with an inherently or noninherently chiral substitution pattern carries chiral substituents, the configuration of both types of stereogenic elements has to be indicated. The two types are independent of each other and the configuration of both must be specified for a full description of the compound.
Example:
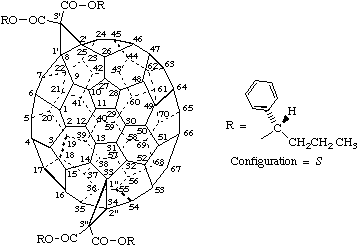
Tetrakis[(1S)-1-phenylbutyl] (f,sC)-3'H,3"H-dicyclopropa[8,25:33,34](C70-D5h(6))[5,6]fullerene-3',3',3",3"-tetracarboxylate
Explanation: The chiralities of the stereogenic centers in the esters are superimposed on the descriptor for the fullerene with the inherently chiral substitution pattern. The enantiomer would be named tetrakis[(1R)-1-phenylbutyl] (f,sA)-3'H,3"H-dicyclopropa[8,25:33,34](C70-D5h(6))[5,6]fullerene-3',3',3", 3"-tetracarboxylate and the same for the various diastereoisomers.
8. International Union of Pure and Applied Chemistry. Division of Organic Chemistry. Commission on Nomenclature of Organic Chemistry, A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford, 1993: (a) Appendix, R-9.3, Table 33, p. 182; (b) R-2.4.4, Ring Assemblies, pp. 53-55; (c) R-0.2.4.2, Lowest set of locants, p. 17.
16. C. Thilgen, A. Herrmann, and F. Diederich, "Configurational Description of Chiral Fullerenes and Fullerene Derivatives with a Chiral Functionalization Pattern". Helv. Chim. Acta 1997, 80, 183-199.
17. R. S. Cahn, C. K. Ingold, V. Prelog, V., "Specification of Molecular Chirality", Angew. Chem., Int. Ed. Engl., 1966, 5, 385-414 (errata: 1966, 5, 511); Angew. Chem. 1966, 78, 413-447; V. Prelog and G. Helmchen, "Basic Principles of the CIP-System and Proposals for a Revision". Angew. Chem., Int. Ed. Engl. 1982, 21, 567-583; Angew. Chem. 1982, 94, 614-631.
18. International Union of Pure and Applied Chemistry. Division of Organic Chemistry. Commission on Nomenclature of Organic Chemistry, Nomenclature of Organic Chemistry, sections A, B, C, D, E, F and H, 1979 edition, Pergamon Press, Oxford, 1979, Section E, pp. 473-490.