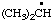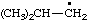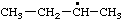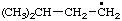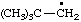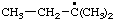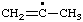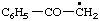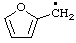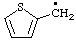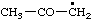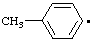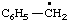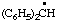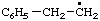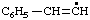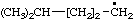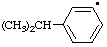Revised Nomenclature for Radicals, Ions, Radical Ions and Related Species
(IUPAC Recommendations 1993)
Appendix
Continued from References
Contents of this section
- Appendix: Trivial and Abbreviated Names for Compounds with Radical Centers on Carbon Atoms
- List A. Names retained in "A Guide to IUPAC Nomenclature of Organic Compounds", Recommendations 1993
- List B. Names not retained in "A Guide to IUPAC Nomenclature of Organic Compounds", Recommendations 1993, but included in the 1979 IUPAC Organic Rules
Appendix: Trivial and Abbreviated Names for Compounds with Radical Centers on Carbon Atoms
List A. Names retained in "A Guide to IUPAC Nomenclature of Organic Compounds", Recommendations 1993 (ref 5)
* Where appropriate, the suffixes "-ylidene" and "-ylidyne" are used with the trivial and abbreviated names given here (see RC-81.1.3.2).
** This name is used for unsubstituted structures only; see Rule A-2.25 (ref 1)
*** This name is included in "A Guide to IUPAC Nomenclature of Organic Compounds", Recommendations 1993 (ref 5), but substitution is not allowed.
**** This name is included in "A Guide to IUPAC Nomenclature of Organic Compounds", Recommendations 1993 (ref 5), but substitution is allowed only on the ring.
List B. Names not retained in "A Guide to IUPAC Nomenclature of Organic Compounds", Recommendations 1993 (ref 5), but included in the 1979 IUPAC Organic Rules (ref 1)
| Structure | Trivial Name* | Systematic Name(s) According to the Recommendations Herein* |
| 1. | 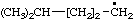 | isohexyl** | 4-methylpentyl (RC-81.1.1)
4-methylpentan-1-yl (RC-81.1.2) |
| 2. | 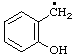 | salicyl
[Rule A-13.3; C-201.4 (ref 1)] | (2-hydroxyphenyl)methyl (RC-81.1.1)
2-hydroxybenzyl (see entry A-17, above) |
| 3. | 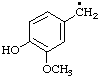 | vanillyl
[cf. Rule A-13.3; C-201.4; C-411.1 (ref 1)] | (4-hydroxy-3-methoxyphenyl)methyl (RC-81.1.1)
(4-hydroxy-3-methoxy)benzyl (see entry A-17, above) |
| 4. | 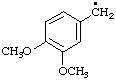 | veratryl
[cf. Rules A-13.3; C-201.4; C-411.1 (ref 1)] | (3,4-dimethoxyphenyl)methyl (RC-81.1.1)
3,4-dimethoxybenzyl (see entry A-17, above) |
| 5. | 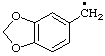 | piperonyl
[cf. Rule C-411.1 (ref 1)] | (benzo[d][1,3]dioxol-5-yl)methyl (RC-81.1.1)
(1,3-benzodioxol-5-yl)methyl (ref 3h) (RC-81.1.1) |
| 6. | 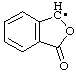 | phthalidyl
[Rule B-5.11; C-473.1 (ref 1)] | 3-oxo-1,3-dihydroisobenzofuran-1-yl (RC-81.1.2) |
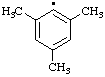
| 7. |  | xylyl
[Rule A-13.1
(ref 1)] (2,3-isomer shown) | dimethylphenyl (RC-81.1.2) (2,3-isomer shown) |
| 8. | 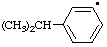 | cumenyl
[Rule A-13.1
(ref 1)] | (3-isomer shown) isopropylphenyl
(RC-81.1.2 and entry A-1 above)
(1-methylethyl)phenyl (RC-81.1.1 and RC-81.1.2) propan-2-ylphenyl (RC-81.1.2) |
| 9. | 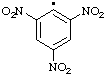 | picryl
[Rule C-202.2
(ref 1)] | 2,4,6-trinitrophenyl (RC-81.1.2) |
* where appropriate, the suffixes "-ylidene" and "-ylidyne" are used with the trivial and abbreviated names given here (see RC-81.1.3.2).
** This name is used for unsubstituted structures only; see Rule A-2.25 (ref 1).
References for this section
1. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, 1979 ed., Pergamon Press, Oxford, 1979, 559 p.
3. Reference 1, Section B, pp. 53-76: [h] Rule B3.5, pp. 67-8.
5. International Union of Pure and Applied Chemistry. Organic Chemistry Division. Commission on Nomenclature of Organic Chemistry, A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford, 1993.
Return to ions and radicals home page.
Return to chemical nomenclature home page.